Correct knowledge of the anatomy and physiology of the operated larynx is crucial to the success of functional laryngeal cancer surgery. A fundamental distinction must be made between procedures involving the removal, to a greater or lesser extent 1, of the vocal fold and those that not only alter the endolaryngeal soft tissues, but also entail the reductive remodelling of the laryngeal framework and repositioning of the neolarynx within the neck.
It addition to the morphology of the neolarynx, other pre-existing and/or post-surgical anatomic and functional elements that can prove decisive to the success of the procedure must also be considered. Of these, the most important are the presence of spinal cord disease, laryngopharyngeal reflux (LPR), any upper respiratory and digestive tract disorders following radiotherapy, salivary flow alterations and, last but not least, the patient’s psychological conditions.
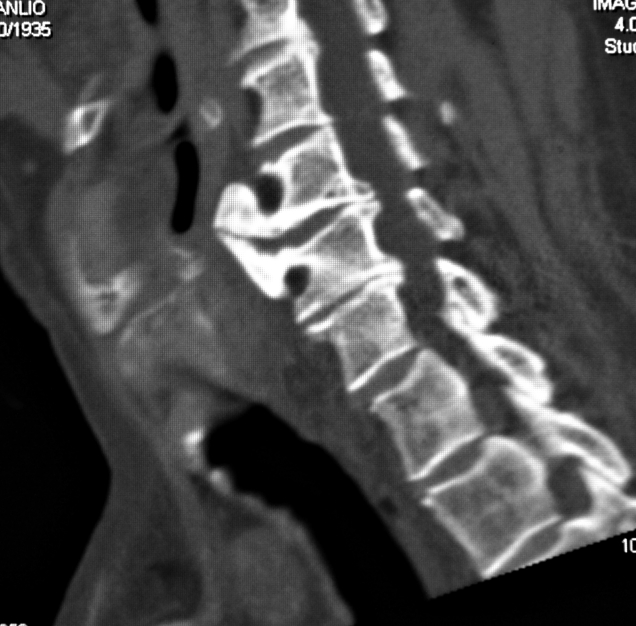
Cervical spinal disease can take the form of cumbersome bone spurs on the vertebral bodies in severe spinal arthritis or concomitant Diffuse Idiopathic Skeletal Hyperostosis (DISH). These conditions must be taken into consideration when planning surgery and be sometimes treated surgically during the laryngeal cancer procedure (Fig. 1). Bruno et al. 2 identified a number of quantitative parameters, visible on pre-operative computed tomography (CT) scans, that can be useful in pinpointing the position of the neolarynx in the neck following crico-hyoido-epiglottopexy (CHEP), of prognostic importance as far as concerns post-operative functional recovery.
Fig. 1. Pre-operative CT: patient with laryngeal cancer (indication to SCLCHEP) and DISH syndrome. Treatment of this latter condition takes place at the same time as the laryngeal cancer operation.
The role of LPR in glottic tissue repair processes and, more generally, in all procedures involving laryngeal and/or laryngotracheal reconstructions, deserves special mention. The negative influence of LPR in glottic repair processes has been analysed in studies on animals and, more recently, in clinical studies on humans. In animal studies 3, irrigation using hydrochloric acid with a pH of 3 and pepsin was administered for 4 or 8 weeks after vocal cord stripping. This group of animals experienced delayed healing, intense inflammation, epithelial erosion and formation of granular tissue, with distant sequelae that evolved into rigid scar tissue, with significant dense collagen deposition. This immediate and delayed tissue damage was evaluated quantitatively and showed a clear statistical significance compared to the control group receiving sterile saline solution irrigations.
In a recent clinical study 4, healing after vocal cord surgery for benign tumours was compared between a control group (50 patients) and a group of 120 patients with LPR, documented with 24-hour dual probe pH monitoring and whose clinical severity was evaluated using subjective parameters, (RSI: Reflux Symptom Index) and objective laryngeal parameters (RFS: Reflux Finding Score). 50% of patients with LPR were randomised to receive pre- and post-operative proton pump inhibitor (PPI) treatment and the anatomical and functional results were evaluated over a one-year follow-up period. The results obtained demonstrated a significant delay in vocal cord re-epithelisation processes and the persistence of high RSI and RFS scores in the untreated patients. This clinical finding confirms the importance of LPR and its pre- and post-operative treatment, with adequate doses of PPI.
The negative impact of LPR on repair processes, following laryngeal surgery, is related to the extent of laryngeal demolition. In one study on rabbits, subject to laryngotracheal reconstruction 5, the Authors observed intense mucosal inflammation, with necrosis of the underlying cartilage in animals receiving hydrochloric acid and pepsin irrigations. These alterations were more marked in the group receiving irrigations with pH of 4 hydrochloric acid compared to those in the group receiving that with a pH of 1.5. Moreover, this latter group of animals was less prone to coughing, when evaluated quantitatively (using the Cough Response Scoring System), compared to those irrigated with HCl with a pH of 4. The pathophysiological basis underlying these events can probably be attributed to the immediate swallowing reflex that is activated when the pharyngo-laryngeal mucosa comes into contact with a strongly acidic solution. This swallowing reflex is so fast and efficacious that it prevents acid micro-aspirations in the lower respiratory tract and restricts the mucosal damage caused when it comes into contact with the areas of the larynx subject to reconstruction. Despite the limits related to the artificiality and complexity of the trial model, this finding has important clinical repercussions. It underlines the detrimental effect of slightly acidic and/or non-acidic LPR and the decisive importance of the sensitive innervation of the hypopharynx and larynx, which is able to activate an effective coughing reflex, the afferent branch of which is the internal branch of the superior laryngeal nerve. Another “extralaryngeal” aspect that can prejudice functional recovery after major laryngeal surgery and that merits closer investigation is the patient’s psychological conditions and related anatomic and functional conditions, represented by the cortical control of laryngeal functions, in general, and deglutition, in particular.
The latest studies using functional magnetic resonance imaging techniques (fMRI ), have confirmed the complexity of neuronal control of deglutition, defining a highly coordinated “swallowing neural sensory-motor network” in which different cortical areas and encephalic and brainstem structures interact to provide a safe and effective transport of the liquids and solid foods from the lips to the stomach. In 2001, Martin et al. published a report on a fundamental study, conducted on healthy volunteers 6, for the definition of the cortical areas activated to promote and coordinate the act of deglutition. The underlying assumption was to make a distinction between “spontaneous” salivary deglutition (automatic swallowing) and deglutition controlled by a voluntary action (volitional swallowing), which, in turn, can be broken down into voluntary salivary deglutition and voluntary swallowing of a bolus (liquid or solid). In the study of Martin et al., healthy volunteers were also evaluated by fMRI-4T in three different swallowing “modes”: 1. N aïve saliva swallowing; 2. V oluntary saliva swallowing: performed with a frequency of one swallow a minute; 3. Water bolus swallowing: swallowing of a fixed quantity (3 ml) of water administered once a minute, through a tube in the mouth. The synchronism of the cortical events and acts of deglutition was guaranteed by recording laryngeal excursions. The still-valid results of this landmark study can be summarised as follows: 1. All swallowing involves cortical activation, even automatic deglutition, which represents the quantitatively predominant event; 2. Both types of deglutition involve several anatomically and functionally separate areas of cortex, with a different pattern during automatic, compared to voluntary, swallowing; 3. V olitional swallowing of both saliva and water boli are associated with a pre-eminent activation of the caudal portion of the cingulate gyrus; 4. There are pre-eminent and more constant foci of cortical activation, which are activated in both types of swallowing, represented by the precentral lateral gyrus (Brodmann areas 4 and 6), the post-central lateral gyrus and the right insula.
Perhaps the most surprising aspect of this study is the documentation of the cortical events that occur at the same time as the most elementary act of deglutition, the automatic swallowing of saliva, termed, on account of its basic nature, “naïve saliva swallowing”. Not only is it invariably associated with cortical activation, but, in this context, it also activates the “nobler” motor areas, such as the premotor cortex (Brodmann area 6) and, above all, the precentral lateral gyrus, area 4, which includes the primary motor cortex, which is, therefore, indicated as M1.
When applied to the clinical setting, these notions allow a broadening of the concept of post-operative dysphagia following major tumour surgery on the upper respiratory tract, intended not merely as an alteration of deglutition for eating and drinking (voluntary bolus swallowing), but also in the broader basic concept of controlling the physiological salivary flow, managed by “naïve saliva swallowing”. Consequently, in laryngeal tumour surgery, a key role is played by all the surgical measures adopted to preserve an adequate “pharyngolaryngeal wall” and the integrity of sensory innervation, as well as the recognition and adequate treatment of post-operative salivary flow disorders 7.
In recent years, a number of studies have been published on the “swallowing cortical network” 8, with the aim of applying this knowledge to clinical practice, both in patients whose swallowing disorders are secondary to neurological damage and whose anatomical “damage” is in the peripheral laryngopharynx, as occurs following major functional laryngeal tumour surgery. In these patients, there is a post-surgical alteration of the laryngopharyngeal structures, with preserved integrity of the central neurological network. Precisely on account of the importance of cortical control of all types of swallowing, this network can be functionally altered due to the patient’s post-operative psychological conditions. A recent study on healthy volunteers, conducted by Palmer et al. 9, compares the dynamics of the oral preparation phase, the oral and pharyngeal stage of solid bolus swallowing, when it takes place automatically or following a voluntary act of deglutition, performed after completion of the oral preparation phase and triggered by a command given by the investigator. The overall dynamics of the initial phases of deglutition are more efficacious when automatic and not commanded, and is slower during controlled swallowing (larger number of masticatory acts, slower propulsion, stoppage of the bolus at the valleculae). The pathophysiological implications of this observation are easily identifiable and explain the organisational complexity of the neuronal network that governs spontaneous deglutition. On a practical level, the points raised previously highlight the importance of early rehabilitation of the swallowing function in patients after major laryngeal surgery, with the triple aim of optimising the dynamics of the neolarynx, obtaining a true reprogramming of the neuronal network through phenomena of neuroplasticity 10 and a minimisation of the effects of volitional control, which can be counterproductive to correcting deglutition dynamics.
If, as previously mentioned, there has been a rapid expansion in the definition of the central neuronal network controlling laryngeal functions, no less significant is the quantitative and qualitative evolution in the knowledge of motor and sensory control of the laryngopharyngeal system, which has led to the definition of the concept of the “neurosensory compartimentalisation” of the larynx. All the areas of intrinsic laryngeal muscle have been defined in relation to their muscle fibre population at structural, ultrastructural and biomolecular levels, intra-muscular distribution of nerve fibres, density of neuromuscular plaques and, consequently, in the amplitude of the motor units. The most extensively studied muscular district is that of the thyroarytenoid muscle, and, specifically, its internal component, or vocal muscle 11.
More recently, the same attention has been dedicated to the definition of the pharyngeal constrictor muscles 12. This activity has led to the identification of a sophisticated “neuromuscular compartimentalisation” that, as for the intrinsic muscles of the larynx, varies significantly with age. The pharyngeal constrictors are divided into two distinct and functionally separate layers: the slow inner layer (SIL), innervated by the glossopharyngeal nerve (IX) and the fast outer layer (FOL), innervated by the vagal nerve (X). This anatomical and functional layering of the constrictor muscles is only present in humans, it appears around two years of age and disappears after the age of 70. The SIL is made up of muscle fibres with myosin heavy chain (MHC) isoforms of the slow-tonic and a-cardiac type. These MH C isoforms are highly specialised in tonic muscle contraction and are linked to the need of controlling deglutition when in an erect position, with a low aerodigestive crossroads, typical of adult. The FOL, with fast tonic MHC and vagal innervation, on the other hand, is specialised in the peristaltic food bolus propulsion. Once again, these considerations lead us to consider the aerodigestive crossroads as an integrated functional structure with synergic, overlapping vagal and glossopharyngeal sensory-motor innervation. On a practical level, this calls for surgical respect of all those structures not involved in the neoplastic process, including all mucosal, muscular, nervous and vascular components.
The other particularly current issue, in the functional anatomy of the larynx, is what we refer to as the “cellular physiology of the larynx” 13. This area focuses on connective cells and the intercellular substance they produce, as concerns both its fibrous (elastin and collagen) and amorphous components. Familiarity with these aspects of cell physiology has allowed a better understanding at molecular level of the repair processes that take place after anatomical cord damage and their “undesired” evolution towards cordal scarring.
Recently, Hirano et al. 14 conducted a study on cord tissue repair processes in patients undergoing vocal cord surgery of various types. The purpose of the study was the molecular quantification of the various components of the extracellular matrix: collagen, elastin, hyaluronic acid, fibronectin and decorin. The results showed a great variability in post-surgical outcomes, inside which different behaviours can be identified for collagen and decorin and for elastin, hyaluronic acid and fibronectin. The postoperative collagen and decorin content is related to the depth of the surgical resection of the cords and subsequent scarring process. The greater the depth of the resection, the greater the deposition of thick, disorganised collagen fibres, especially in cases of post-operative radiotherapy. The opposite occurs for decorin, which is preserved in more superficial cordectomies, but tends to drop in deeper procedures. Decorin is a small-chain proteoglycan that governs the collagen fibrils, preventing them from forming large bundles and thus avoiding the formation of dense scar tissue. Decorin is, physiologically, primarily present in the more superficial layers of the lamina propria, which explains the histological findings reported. Deposition of the other components of the extracellular matrix, such as elastin, fibronectin and, above all, hyaluronic acid, on the other hand, occurs regardless of the depth of vocal cord resection and their content in the post-operative cord tissue is governed by highly variable, individual factors. There are many practical repercussions of the elements that came to light in this study, all of them of great clinical importance, making the indications for phoniatric and/or voice surgery after endoscopic cordectomy, even in the more superficial procedures, an issue of great current interest.
However, there is no doubt that the post-operative redefinition of the operated larynx occurs above all following procedures that reduce the laryngeal framework. At a pathophysiological level, it is correct to define the type of laryngectomy, indicating the most caudal anatomic element above which the neolarynx is reconstructed: hence the definition of supraglottic horizontal laryngectomy (SHL), supracricoid laryngectomy (SCL) (crico-hyoidoepiglottopexy [CHEP], crico-hyoidopexy [CHP]) and supratracheal laryngectomy (STL). It goes without saying that procedures requiring the anatomical and functional redefinition of the operated larynx are those entailing the resection of the glottic level of the cords, the natural sphincter of the larynx, calling for the surgical reconstruction of a “neoglottis”. We will, therefore, describe the basic anatomy and physiology of the neolarynx after SCL and STL procedures.
The anatomical and physiological foundation of this kind of surgery is the cricoarytenoid unit (CAU). This structure has both a “classic” and an “updated” definition.
The classic definition was developed in 1992, by J.J. Piquet et al.,15 the original version of which is provided below:
“L’unité crico-aryténoïdienne se compose d’un squelette fibro-cartilagineux constitué par le cartilage cricoïde ainsi que d’un ou deux cartilages aryténoïdes articulés entre eux. Cette articulation ne peut rester fonctionelle que dans la mesure où les muscles crico-aryténoïdiens posterieur, cricoaryténoïdiens latérals et inter-aryténoïdiens parfois, sont respectés avec leur innervation, leur vascularisation ainsì qu’un plan muqueux de coverture à preserver”. The fundamental aspect of this definition of CAU lies in the specification not so much of its anatomical appearance, but rather its functional appearance that represents the essence of the larynx only if it is perfectly intact as regards to its complex cricoarytenoid joint, its muscular apparatus, sensory-motor innervation and mucosal coating. This “classical” concept of the CAU has been replaced by a more “extreme” version, with a graphic schematisation that graced the cover of the October 2006 issue of Laryngoscope (Fig. 2). Once again, we provide the original definition: “one cricoarytenoid unit (half posterior cricoid plate and one arytenoid)” 16. Reducing the framework makes it all the more urgent to maintain intact the function of all components of the CAU and stresses the second fundamental element of the physiological anatomy of the neolarynx, the ‘position’ element. Here, it becomes necessary to introduce the second “hinge” definition of the issue, the definition of “neoglottis”, which we will borrow, once again, from J.J. Piquet: “La néo-glotte est constituée d’une partie antérieure musculaire basilinguale (à laquelle s’ajoute l’épiglotte dans une CHPE) et d’une partie postérieure correspondant à une ou deux unités crico-aryténoïdiennes… La situation de la néoglotte est particuliére car haute ou additale, située dans le plan de la margelle laryngée”. This defines the concept of the “neoglottis”, a circular structure, the true upkeeper of neolaryngeal functions: respiratory function, speech function and deglutition function. The neoglottis is, therefore, a circular structure in which the rear 180° are, schematically, represented by at least one efficient CAU, whereas the anterior 180° are represented by the base of the tongue, overlapped, when applicable, by the residual suprahyoid epiglottis (Fig. 3). The functional competence of this “ring” stems not so much from the anatomical-functional integrity of each of its components, but rather, to an equally important extent, from the juxtaposition of the front half with the back half. This is what makes “position” the second requisite of an optimised CAU. These elements form the grounds for the success of major functional laryngeal surgery, and are linked to the rehabilitation and/or surgical work performed to correct functional failures.
Fig. 2. CAU: Current concept. Articular, neuromuscular, vascular and mucosal integrity of the cricoarytenoid complex is essential. The continuity of the cricoid cartilage is not necessary.
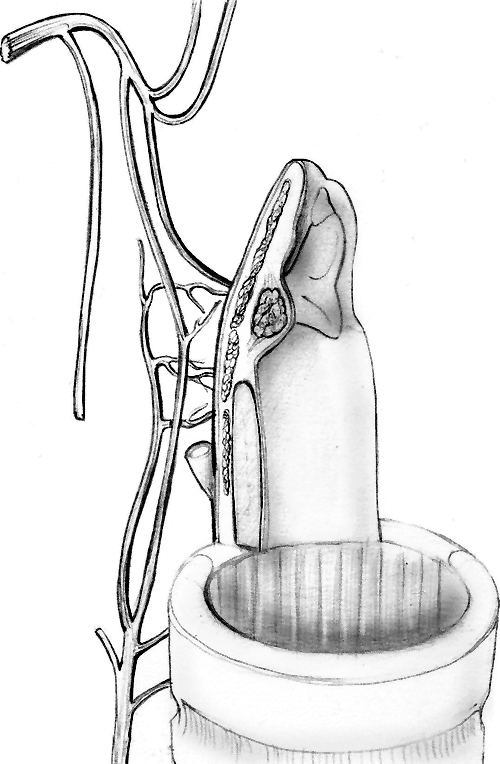
Fig. 3. Diagram of the neoglottis. The front half comprises the base of the tongue, the rear half by at least one efficacious CAU.

The first anatomical element of the “position” of the neoglottis is the lifting of the residual larynx, in a cranial direction, towards the base of the tongue. For this, the reconstruction must be stable, which is obtained by overlapping and positioning the concave portion of the hyoid body on top of the cricoid or, in the case of STL, the upper rings of the trachea. This also guarantees a correct alignment of the reconstruction in relation to the respiratory lumen, the essential condition for natural breathing. Once the structural correctness of the mutual relationships between the components of the neoglottis has been guaranteed, the performance of respiration, speech and deglutition functions will require a specific dynamic pattern for each of the three functions, that is based, as mentioned previously, on a correct neoglottis neuromuscular apparatus and a good degree of cricoarytenoid joint freedom.
Respiratory function requires an adequate lumen along the whole reconstructed respiratory tract and an efficacious opening of the residual larynx. This function is assigned to the posterior cricoarytenoid muscle, innervated by the inferior or recurrent laryngeal nerve. The contraction of this muscle, considering its insertion of the muscular apophysis of the arytenoid and the degrees of freedom of the cricoarytenoid joint, will produce a multiplane arch movement of the body and vocal process of the arytenoid, in an upwards, outwards and backwards direction. This spatially complex movement, more simply defined as abductory, will bring the arytenoid body and vocal process from an inferomedial starting position to a superolateral end position, thus widening the respiratory lumen.
The phonatory and deglutition functions both require the competence of a neoglottic spincter. This neoglottic sphincter will invariably be constituted by the juxtaposition of the CAU to the rear and the base of the tongue to the front. The action of the front half of the neoglottic sphincter will be guaranteed by the retropulsion of the base of the tongue, downwards and backwards. In SCL with CHEP procedures, this sphincter will be assisted by the presence of the residual epiglottis, to give it a correct position, making it possible to follow the movements of the base of the tongue, without, simultaneously representing an obstacle for the respiratory lumen.
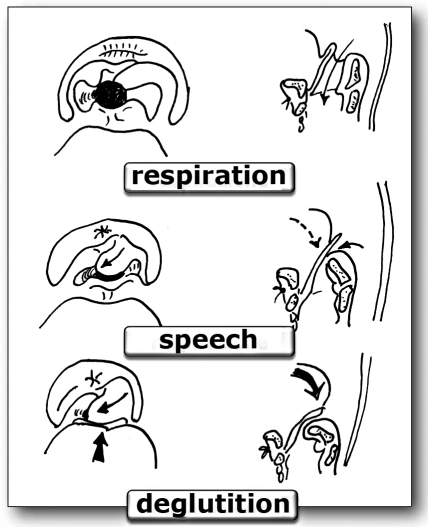
As mentioned previously, the competence of the rear half of the neoglottic sphincter depends on the CAU and is based on a complex cricoarytenoid movement, which occurs with a synergical action, of recorrential competence, of the lateral cricoarytenoid, posterior cricoarytenoid and, when both arytenoids are presence, interarytenoid muscles. The contraction of the lateral cricoarytenoid muscle tends to pull the muscular apophysis downwards and forwards, causing the arytenoid to move over the cricoid so that the vocal apophysis and the arytenoid body draw an arc downwards, inwards and forwards. As the lateral cricoarytenoid muscle contracts, the posterior cricoarytenoid muscle relaxes, tilting the arytenoid body forwards. When present, the simultaneous contraction of the interarytenoid muscle produces a tighter action of the posterior sphincter, thus favouring the meeting of the anterior aspects of the arytenoids. These complex articular and neuromuscular dynamics produce a multiplane movement of the arytenoid that draws a quarter- or semi-circular arc with an internal concavity moving forwards, downwards and inwards. On laryngoscopic observation, this complex dynamic can be schematically split into two essential components, for which the original French names are used: “le salut aryténoïdienne” and “le rideau de scène” (J.J. Piquet) (Fig. 4).
Fig. 4. Dynamics of the neoglottis in the 3 fundamental functions. The arytenoid excursions (“le rideau de scène”) are shown on the right hand side. The dynamics of the neoglottis on the vertical plane: retropulsion of the base of the tongue and “le salut aryténoïdienne” is shown on the left.
“Le salut aryténoïdienne”: describes the vertical component of the arytenoid body, which tilts forwards and downwards, towards the base of the tongue. This causes the posterior cricoarytenoid muscle to relax.
“Le rideau de scène”: describes the horizontal component, favoured by the lateral cricoarytenoid muscle, which brings the arytenoid into medial contact with the contralateral, if present, or up to the contralateral laryngeal wall, in the case of a single residual arytenoid. It should be a true “curtain falling”, with one or two curtains.
Whereas the above description refers to the fundamental mechanism that guarantees neoglottic competence, the dynamics will be different in the occlusion mechanisms for phonation and deglutition.
In phonation, the retropulsion of the base of the tongue has the essential purpose of allowing glottic competence, whilst the active participation of the CAU is predominant. Piquet defines this dynamic action of the neoglottic sphincter as: “mécanisme léger”.
In deglutition, on the contrary, the retropulsion of the base of the tongue is active, to allow a real tightening of the neoglottis. Consequently, it is a “mécanisme lourd”.
Neoglottic vibration: So far, we have described the aspects of the neoglottic “framework” that do not take into consideration the behaviour of the mucosa, the vibration of which is essential in allowing the neoglottic sphincter to produce a “neovoice”. The phonatory vibrations of the mucosa involve the arytenoid hoods and the other elements of the neoglottis, particularly in the case of SCL-CHEP, when the vibratory pattern will also involve the mucosa of the epiglottis and the piriform fossa, as an element of the neo-aryepiglottic folds. Recently, Saito et al. 17 proposed a classification of the mucosal vibratory patterns of the neoglottis after SCL-CHEP. The Authors defined 3 areas of mucosal vibration, defined: Area A (arytenoid/s); Area E (epiglottis); Area S (piriform sinus mucosa). The vibratory patterns encountered are: Type A; Type S; Type AS; Type AE and Type AES.
This proposal responds to the currently particularly urgent need to identify classification systems to evaluate the functional results of functional laryngeal cancer surgery 18, due partly to the enormous progress achieved in video-laryngoscopy techniques.
Conclusions
The topic of the anatomy and physiology of the operated larynx is undoubtedly complex and multifactorial, currently dealt with in the literature of various disciplines and, therefore, “dispersed” but worthy of further speculative and clinical exploration.
These notes illustrate how the functional outcome following laryngeal cancer surgery relies on respecting all the elements in that constellation of factors that permit a minimal neolarynx anatomic and functional dignity.
References
- 1.Remacle M, Haverbeke C, Eckel H, et al. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur Arch Otorhinolaryngol. 2007;264:499–504. doi: 10.1007/s00405-007-0279-z. [DOI] [PubMed] [Google Scholar]
- 2.Bruno E, Napolitano B, Sciuto F, et al. Variations of neck structures after supracricoid partial laryngectomy: A multislice computed tomography evaluation. ORL. 2007;69:265–270. doi: 10.1159/000103869. [DOI] [PubMed] [Google Scholar]
- 3.Jong-Lyel Roh JL, Yoon YH. Effect of acid and pepsin on glottic wound healing - A simulated reflux model. Arch Otolaryngol Head Neck Surg. 2006;132:995–1000. doi: 10.1001/archotol.132.9.995. [DOI] [PubMed] [Google Scholar]
- 4.Kantas I, Balatsouras DG, Kamargianis N, et al. The influence of laryngopharyngeal reflux in the healing of laryngeal trauma. Eur Arch Otorhinolaryngol. 2009;266:253–259. doi: 10.1007/s00405-008-0744-3. [DOI] [PubMed] [Google Scholar]
- 5.Carron JD, Greinwald JH, Oberman JP, et al. Simulated reflux and laryngotracheal reconstruction - a rabbit model. Arch Otolaryngol Head Neck Surg. 2001;127:576–580. doi: 10.1001/archotol.127.5.576. [DOI] [PubMed] [Google Scholar]
- 6.Martin RU, Goodyear BG, Gati J, et al. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol. 2001;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- 7.Bomeli SR, Desai SC, Johnson JT, et al. Management of salivary flow in head and neck cancer patients - A systematic review. Oral Oncol. 2008;44:1000–1008. doi: 10.1016/j.oraloncology.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Michou E, Hamdy S. Cortical input in control of swallowing. Curr Opin Otolaryngol Head Neck Surg. 2009;17:166–171. doi: 10.1097/MOO.0b013e32832b255e. [DOI] [PubMed] [Google Scholar]
- 9.Palmer JB, Hiiemae KM, Matsuo K, et al. Volitional control of food transport and bolus formation during feeding. Physiol Behav. 2007;91:66–70. doi: 10.1016/j.physbeh.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludlow CL, Hoit J, Kent R, et al. Translating principles of neural plasticity into research on speech motor control recovery and rehabilitation. J Speech Lang Hear Res. 2008;51:S240–S258. doi: 10.1044/1092-4388(2008/019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunsolo EM, Marchioni D, Lorenzo G, et al. Attualità in tema di anatomo–fisiologia e biomeccanica della laringe. In: Magnani M, Ricci Maccarini A, Füstös R, editors. La Videolaringoscopia. Relazione Ufficiale XXXII Convegno Nazionale di Aggiornamento AOOI; 16-17 ottobre 2008; Pollenzo (TO). [Google Scholar]
- 12.Mu L, Sanders I. Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol. 2007;116:604–617. doi: 10.1177/000348940711600809. [DOI] [PubMed] [Google Scholar]
- 13.Cunsolo EM, Casolino D, Cenacchi G. La fisiologia cellulare delle corde vocali. Le disfonie: fisiopatologia, clinica ed aspetti medico-legali. In: Casolino G, editor. Relazione Ufficiale del LXXXIX Congresso Nazionale SIO. San Benedetto del Tronto: Pisa: Pacini Editore; 2002. pp. 64–64. 22-25 maggio 2002. [Google Scholar]
- 14.Hirano S, Minamiguchi S, Yamashita M, et al. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Piquet JJ, Chevalier D, Lacau-StGuily J, et al. Aprés exérèse horizontale glottique, sus-glottique, glosso-sus-glottique et hémipharyngolaryngée. In: Traissac L, editor. Réhabilitation de la voix et de la déglutition après chirurgie partielle ou totale du larynx. Socièté Française d’Oto-Rhino-Laryngologie et de Pathologie Cervico-Faciale. Paris: Arnette: 1992. pp. 173–192. [Google Scholar]
- 16.Rizzotto G, Succo G, Lucioni M, et al. Subtotal laryngectomy with tracheohyoidopexy: a possible alternative to total laryngectomy. Laryngoscope. 2006;116:1907–1917. doi: 10.1097/01.mlg.0000236085.85790.d5. [DOI] [PubMed] [Google Scholar]
- 17.Saito K, Araki K, Ogawa K, et al. Laryngeal function after supracricoid laryngectomy. Otolaryngol Head Neck Surg. 2009;140:487–492. doi: 10.1016/j.otohns.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Marioni G, Marchese-Ragona R, Ottaviano G, et al. Supracricoid laryngectomy: is it time to define guidelines to evaluate functional results? A review. Am J Otolaryngol. 2004;25:98–104. doi: 10.1016/j.amjoto.2003.11.008. [DOI] [PubMed] [Google Scholar]