Abstract
Fatty acid oxidation in mitochondrial matrix is a major source of energy in muscle, especially when physiological energy demand is increased and exceeds what can be provided through glycolysis. Not surprisingly, a group of muscle disorders due to defects in this system usually leads to the development of acute rhabdomyolysis in conditions such as infection, fasting and prolonged exercise. This group includes β-oxidation cycle defects and deficiencies of carnitine palmitoyltransferase II (CPTII) and very-long-chain acyl-CoA dehydrogenase (VLCAD). Muscle pathology is usually not very helpful for the diagnosis but immunohistochemistry may be useful for screening VLCAD deficiency. Another group of lipid dysmetablolism is lipid storage myopathy (LSM) that is pathologically characterized by increased lipid droplets both in number and size in muscle fibers. So far, causative genes have been identified in four different LSMs, comprising primary carnitine deficiency, multiple acyl-CoA dehydrogenase deficiency or glutaric aciduria type II, neutral lipid storage disease with ichthyosis, and neutral lipid storage disease with myopathy. Clinically, the LSM patients show slowly progressive muscle weakness unlike the former group. Final diagnosis is usually made by specific biochemical assays with mutation analyses. As some effective drugs have been widely used and some promising therapies are under certified, comprehensive understanding of these diseases from clinical, pathological and molecular aspects would be of much help for the patients.
Key words: Lipid storage myopathy, primary carnitine deficiency, multiple acyl-coenzyme A dehydrogenase deficiency, neutrolipid storage disease, carnitine palmitoyltransferase II, very long-chain acyl-CoA dehydrogenase
Introduction
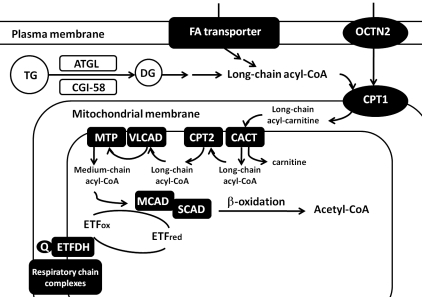
Lipid consists of two types of molecules: fatty acid and its derivatives including triglycerides (TG), and sterol- containing metabolites such as cholesterol. Fatty acids are catabolized through β-oxidation cycle in mitochondrial matrix and thus ATP is produced. Short- and medium- chain fatty acids can enter cells and then mitochondria by diffusion but long-chain fatty acids require fatty acid transporters at the plasma membrane and carnitine palmitoyltransferase (CPT) system at the mitochondrial membranes.
Lipid dysmetabolism, involving intracellular TG catabolism, the transport of long-chain fatty acids and carnitine, or β-oxidation, often causes different extent of lipid accumulation in skeletal muscle fibers and in other organs. Among the disorders of lipid metabolism, primary carnitine deficiency (PCD), multiple acyl-coenzyme A dehydrogenase deficiency (MADD) and neutral lipid storage disease with ichthyosis (NLSDI) or myopathy (NLSDM) usually show markedly increased lipid droplets in muscle fibers which are ordinarily termed lipid storage myopathy (LSM). On the other hand, lipid storage could be mild or even absent in the defects of intramitochondrial fatty acid transport and β-oxidation.
The phenotype of lipid metabolism disorders is heterogeneous but can generally be divided into two major categories (1), especially in late onset patients. Constant or progressive muscle weakness associated with or without metabolic crisis, is often seen in LSM patients while recurrent rhabdomyolysis triggered by infections, fasting or vigorous exercise usually occur in the patients with disorders affecting intramitochondrial fatty acid transport and β-oxidation, such as deficiencies of carnitine palmitoyltransferase II (CPTII), mitochondrial trifunctional protein (MTP) and very-long-chain acyl-CoA dehydrogenase (VLCAD). However, in infantile onset patients, the clinical manifestations are somehow similar among all types of lipid dysmetabolism, including hypotonia, hypoketotic hypoglycemic encephalopathy, hepatomegaly and cardiomyopathy.
In this review, we would like to go through CPTII and VLCAD deficiencies briefly but mainly focus on four LSMs with known causative genes, PCD, MADD, NLSDI and NLSDM.
Carnitine palmitoyltransferase II deficiency (CPTII deficiency)
CPTII, located at the inner mitochondrial membrane, is responsible for the transfer of long-chain acyl-CoA (Fig. 1), thus the defects in CPTII would apparently affect the access of long-chain acyl-CoA to β-oxidation. CPTII deficiency caused by the mutations in the CPT2 gene is the first inherited defect of fatty acid oxidation to be identified (2). Three clinical subtypes, neonatal, infantile and mild late-onset forms, have been described but muscular symptoms including recurrent rhabdomyolysis and muscle pain after long-term exercise were mainly associated with the late-onset form (3). Infantile cases usually present recurrent attacks of acute liver failure with hypoketotic hypoglycemia, cardiomyopathy and sudden death while neonatal-onset patients demonstrate a more severe phenotype with dysmorphic features. There is a good correlation between genotype, metabolic dysfunction and phenotype as null or truncated mutations often cause absent enzyme activity and earlier-onset phenotype (2). A common mutation, p.S113L, has been found in more than 50% of mutant alleles in mild late-onset patients.
Figure 1. Scheme of selected metabolic pathways of lipid. (OCTN2: plasma membrane sodium-dependent carnitine transporter; TG: triglycerides; DG: diglycerides; ATGL: adipose triglyceride lipase; CGI-58: comparative gene identification-58; CPTI: carnitine palmitoyltransferase I; CACT: carnitine-acylcarnitine translocase; CPTII: carnitine palmitoyltransferase II; VLCAD: very long-chain acyl-CoA dehydrogenase; MTP: mitochondrial trifunctional protein; SCAD/MCAD: short-chain/medium-chain acyl-CoA dehydrogenases; ETF: electron-transfer flavoprotein; ETFDH: ETF-dehydrogenase; Q: coenzyme Q; C: cytochrome c).
Metabolic profiles in CPTII deficiency patients usually show increased long-chain acylcarnitines. Creatine kinase (CK) level is markedly elevated after prolonged fasting or exercise. Muscle pathology is typically characterized by nonspecific changes without increased lipid droplets. Therefore, enzymatic assay in leukocyte, cultured fibroblasts or biopsied muscles may be the most reliable diagnostic test, as well as the mutation analysis for CPT2. The treatment for CPTII deficiency is mainly dependent on restricting the diet and avoiding fasting. Long-chain fat –restricted diet with medium-chain triglycerides (MCT) supplementation is recommended (4). Recently, bezafibrate, a commonly used hypolipidemic drug, has shown to restore the capacity for normal fatty acid oxidation in muscle cells from patients with a mild form of CPTII deficiency (5). Though one open-labeled pilot study was just reported to improve the oxidation rate of long-chain fatty acid and the physical activity of the CPTII patients (6), further clinical trials and prolonged clinical follow-up are necessary to confirm its efficacy.
Very-long-chain acyl-coenzyme A dehydrogenase deficiency (VLCAD deficiency)
VLCAD localizes in the inner mitochondrial membrane and catalyzes the long-chain fatty acyl-CoA which is just incorporated into mitochondrial matrix by CPTII. Therefore, VLCAD is immediately downstream to CPTII in long-chain fatty acyl-CoA oxidation pathway. Not surprisingly, the clinicopathological manifestations of VLCAD deficiency in myopathic or adult-onset form are very similar to CPTII deficiency which typically presents recurrent rhabdomyolysis triggered by exercise or fasting. There are also early-onset patients showing mainly cardiac and hepatic involvement. The mutations in the ACADVL gene was first identified in 1995 (7) and a clear genotype-phenotype correlation has been reported (8).
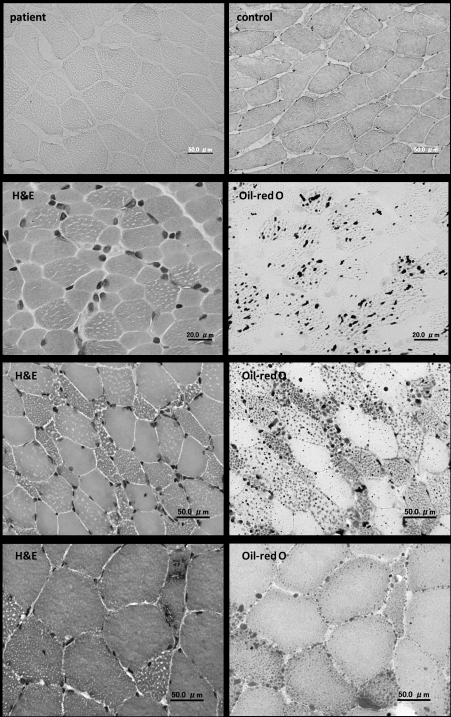
The diagnosis of VLCAD deficiency relies on the measurement of metabolic profile, showing abnormal elevation of long-chain acylcarnitines. Muscle pathology usually reveals only nonspecific findings but sometimes a variable degree of necrotic and regenerating changes in muscles fibers reflecting recent episodes of rhabdomyolysis. Lipid droplets are usually not increased. In addition, immunohistochemistry has demonstrated as an effective and useful diagnostic method to detect VLCAD deficiency (Fig. 2A) (9). The therapeutic strategy is also similar to CPTII deficiency. However, some reports showed MCT supplementation did not benefit the patients and even impaired hepatic lipid metabolism in VLCADknockout mice (10, 11). And as well as CPTII deficiency, bezafibrate was also used to treat the cultured fibroblasts from VLCAD deficiency patient and demonstrated the increases of both mRNA expression and protein level (12). Thus, further in vivo studies are needed to prove its benefit in VLCAD deficiency.
Figure 2. Muscle pathology. (H&E: hematoxylin-eosin) (A) Immunohistochemistry shows negative staining of VLCAD in the patient with VLCAD deficiency. (B) In PCD, lipid droplets are markedly increased in both number and size in muscle fibers, especially type 1 fibers. (C) In MADD, similar features of increased lipid accumulation are seen as those in PCD. (D) In NLSDM, except for increased lipid droplets, rimmed vacuoles are seen in muscle fibers.
Primary carnitine deficiency (PCD)
PCD, caused by impaired function of plasma membrane sodium-dependent carnitine transporter (OCTN2), is possibly the second most frequent disorder affecting fatty acid oxidation following medium chain acyl-CoA dehydrogenase deficiency with the carrier frequency of about 1% (13). The function of OCTN2 is to transfer carnitine across the plasma membrane. As carnitine is essential for the transfer of long-chain fatty acids from the cytoplasm to the mitochondrial matrix for following oxidation (Fig. 1), the defect of OCTN2, leading to urinary loss of carnitine and the failure of intracellular accumulation, would culminate in deficient fatty acid oxidation.
PCD is an autosomal recessive disorder, caused by the mutations in SLC22A5 which encodes OCTN2 (14). The clinical manifestations of PCD are widely variable, ranging from asymptomatic feature, isolated cardiomyopathy to lethal metabolic decompensation. As mentioned above, the infantile onset patients with PCD principally present hypotonia, Reye-like syndrome and cardiomyopathy. However, the cardiomyopathy may develop solely or with a milder metabolic presentation during childhood or even older age (15). Since skeletal muscle uses fatty acid as a major energy source, muscle weakness can also be observed in PCD patients. Some patients, who have been asymptomatic for their whole life, may be identified because of their affected children or siblings (16, 17). There is no clear correlation between genotype and either clinical or biochemical phenotype yet reported, suggesting that the wide phenotypic variability may be related to epigenetic or exogenous factors which exacerbate carnitine deficiency (18). Common blood tests may reveal increased levels of hepatic enzymes and CK.
As for the diagnosis of PCD, the measurement of free carnitine and all acylcarnitine species is essential and both extremely low levels are indicative of PCD. Secondary carnitine deficiency should be carefully excluded which may show decreased free carnitine level but elevated specific species of acylcarnitine. However, as plasma carnitine level can occasionally be normal in PCD, carnitine transport study in fibroblasts may also be used to confirm the diagnosis. On muscle pathology, markedly increased lipid droplets in both number and size in muscle fibers are seen, especially in type 1 fibers (Fig. 2B). Ultrastructural study often shows that lipid droplets are present next to mitochondria which are usually enlarged but structurally normal. Moreover, as PCD is caused by the defect of OCTN2, searching for the mutations in SLC22A5 is another way to establish the diagnosis of PCD.
PCD patients are well responsive to carnitine supplementation (100-400 mg/kg per day). Early carnitine therapy has been believed to prevent the occurrence of cardiomyopathy and other irreversible organ damage (18). In recent years, activation of peroxisome proliferator-activated receptor α (PPARα) has been proved to cause an up-regulation of OCTN2, leading to an increase of intracellular carnitine concentration in animal models (19, 20). Therefore, PPARα agonists may be potential candidates for treating PCD patients in addition to carnitine supplementation.
Multiple acyl-coenzyme A dehydrogenase deficiency (MADD)
MADD, also known as glutaric aciduria type II, is caused by the defects in electron transfer flavoprotein (ETF), ETF dehydrogenase (ETFDH) (also called ETFubiquinone oxidoreductase), or an unidentified abnormality in flavin metabolism or transport. In mitochondria, ETF, which is located in the matrix, receives electron from several dehydrogenases involved in fatty acid oxidation. Electrons are then transferred to ETFDH, located in the inner mitochondrial membrane, and subsequently, are passed to ubiquinone in the respiratory chain (Fig. 1). ETF, ETFDH, and most mitochondrial enzymes associated with electron transfer system are flavoproteins, which contain flavin adenine dinucleotide prosthetic groups (FAD). Therefore, defective ETF or ETFDH, disturbing the electron transfer, would finally result in accumulation of various intramitochondrial acyl-CoA esters, decrease of intramitochondrial flavin, and probable mitochondrial dysfunction.
Homozygous or compound heterozygous mutations in ETFA, ETFB and ETFDH, which encode α– and β–subunits of ETF and ETFDH, respectively (21), have been identified in MADD patients. Importantly, ETFDH mutations were reported to be major causes of riboflavin-responsive MADD (RR-MADD) (22). About the same time, ETFDH mutations were also found to cause the myopathic form of coenzyme Q10 (CoQ10) deficiency (23). However, the relationships between riboflavin responsiveness, CoQ10 levels and ETFDH gene mutations are not well-defined. Intriguingly, a probable founder mutation, c.250G > A (p.A84T) in ETFDH, was recently reported in southern Chinese population with an estimated carrier frequency of about 0.8% in Taiwanese (24, 25). To date, all 20 reported MADD patients with ETFDH mutations from southern Chinese population harbor this mutation (25-27). Compared to Japan with more than 5 times population of Taiwan, the similar number of reported MADD patients with ETFDH mutations indicates that the incidence of MADD may be much higher than previously estimated and many MADD patients may actually be underdiagnosed at least in Taiwan.
The clinical phenotype of MADD is quite heterogeneous and has been classified as neonatal onset forms with or without congenital anomalies, and mild and/or later onset form. Patients with neonatal onset forms usually present with hypotonia, hepatomegaly, nonketotic hypoglycemia, metabolic acidosis and the patients usually died early in infancy. Later onset patients manifest proximal myopathy often with hepatomegaly and episodic metabolic crisis; these episodes can be lethal (28). Cardiomyopathy has also been reported in both neonatal and later onset MADD patients (29). Though no clear genotype-phenotype correlation for each gene has been described, there seems a tendency that the mutations in ETFA and ETFB cause neonatal onset forms while the patients with ETFDH mutations often present the clinical course as later onset form (30, 31). However, the disease severity is correlated to not only the nature of the gene defect but also the cellular factors that may modulate the enzymatic activity (32). Mildly to moderately elevated CK levels are often found in routine biochemical test, especially during the episodes of metabolic decompensation.
The key to diagnosis is the measurement of urinary organic acid profiles, plasma carnitine, and acylcarnitines. Urine organic acid analysis typically shows C5 to C10 dicarboxylic aciduria and acylglycine derivatives. Plasma free carnitine level is decreased but sometimes retains normal in some cases. Blood acylcarnitine analysis usually displays elevated concentrations of mainly medium- and long-chain acylcarnitines. In addition, reduced biochemical activities of other mitochondrial enzymes including flavin-dependent and respiratory chain enzymes, have been reported (22, 33, 34) in MADD, though it is still unknown if this mitochondrial dysfunction is directly caused by ETFDH mutations or other factors. As the variable clinical manifestations may not be correlated to the impression of MADD, increased lipid deposition in muscles is sometimes the first sign guiding the subsequent biochemical studies for lipid dysmetabolism. Noteworthily, the biochemical assays occasionally show normal results between each episodes of metabolic decompensation, thus mutation analyses of ETFA, ETFB and ETFDH may be the most confirmative diagnostic method for MADD. The characteristic features of muscle pathology are similar to those seen in PCD (Fig. 2C).
Although the molecular mechanism of MADD is still unclear, riboflavin supplementation (100-400 mg/day) has been known to markedly improve the clinical symptoms and metabolic profiles of many MADD patients, particularly with ETFDH mutations and later onset form, as mentioned previously. Several studies have shown that FAD, comprised in many mitochondrial enzymes related to electron transfer, may modulate the enzymatic phenotype of mutations in the electron transfer proteins. It has been observed that FAD level affects folding and maintenance of the native structure of these proteins and could improve their conformation and generate a more stable and active enzyme in vitro (35). Accordingly, riboflavin should be tried in all types of MADD patients. There is still a controversy about the combination therapy with carnitine. It could be helpful when secondary carnitine deficiency is present. CoQ10 supplementation has also been reported to improve muscle weakness in MADD patients with CoQ10 deficiency (23), together with riboflavin use. However, as the CoQ10 level is not always decreased in MADD patients (24), its supplementation should be considered only when secondary CoQ10 deficiency is present.
Neutral lipid storage disease with ichthyosis (NLSDI) or myopathy (NLSDM)
Neutral lipid storage disease (NLSD) is a rare lipid storage disorder caused by defects in two TG-associated proteins, adipose triglyceride lipase (ATGL) and alpha/beta-hydrolase domain-containing protein 5 (ABHD5) (also called comparative gene identification-58 [CGI- 58)]). ATGL catalyzes TG and releases the first fatty acid from the glycerol backbone and CGI-58 activates ATGL and acylates lysophosphatidic acid. Activation of ATGL initiates the hydrolytic catabolism of cellular TG stores to glycerol and nonesterified fatty acids (Fig. 1). Therefore, dysfunction of these two proteins apparently would affect the degradation of TG, and then cause its accumulation.
Mutations in alpha/beta-hydrolase domain-containing protein 5 (ABHD5) have been identified to cause NLSDI, also known as Chanarin-Dorfman syndrome (CDS) (36). However, some patients present atypical CDS features, with myopathy and cardiomyopathy but without ichthyosis and mutation in ABHD5. This phenotype was called NLSDM and further facilitated the discovery of new causative gene, patatin-like phospholipase domain containing 2 (PNPLA2), encoding ATGL (37). So far, the mutations in ABHD5 have been reported to cause protein truncations but not absence, suggesting that the mutant protein is not completely deficient but functionally impaired (33). In NLSDM, almost all mutations in PNPLA2 are located on C-terminal region, preserving the proposed active site of the enzyme. These truncated enzyme variants have later been shown impaired in their ability to bind to cellular lipid droplets in vitro (38).
NLSD is characterized by systemic TG deposition in multiple tissues, including skin, muscle, liver, central nervous system, and blood leukocytes. Clinically, it is often present with ichthyosis, mild myopathy, hepatomegaly and, variably, ophthalmologic symptoms, neurosensory hearing loss, mental retardation and short stature (39-41). In NLSDI, the ichthyosis represents nonbullous congenital ichthyosiform erythroderma and the weakness is usually mild and predominant proximal. However, in NLSDM, no ichthyosis is observed and the slowly progressive muscle involvement could be in both proximal and distal limbs, with a predominant distal weakness in some patients. Importantly, the cardiomyopathy is exclusively seen in almost half of the patients with NLSDM (41), but not NLSDI while neurosensory defects and mental retardation are commonly seen in NLSDI but not NLSDM. CK level is usually mildly to moderately elevated.
The detection of lipid accumulation in leukocytes, muscle fibers and fibroblasts is critical for the diagnosis of NLSD as the clinical manifestations of NLSD are inconstant and the biochemical investigations for lipid dysmetabolism usually do not show any abnormality. The intracytoplasmic lipid storage in leukocytes is visible on peripheral blood smear, which is called Jordan’s anomaly. In skeletal muscles, increased lipid droplets could be observed even in presymptomatic period. Noteworthily, rimmed vacuoles in the muscles were reported in some NLSDM patients (Fig. 2D) (42, 43), suggesting a different pathomechanism from other LSMs, which might be associated with membrane phospholipid abnormalities caused by decreased DG availability.
So far, there is still no effective treatment in NLSD. The functions of ATGL and CGI-58 are still largely unknown. However, the production of the mice lacking ATGL has provided an opportunity to prompt the understanding of NSLDM (44), though unfortunately, the phenotype of CGI-58-deficient mice has not been reported yet. The elucidation of the biological role of CGI-58 in lipid dysmetabolism and further development of therapeutic strategy may be dependent on more detailed genetic and clinical characterization of NSLDI patients and the establishment of appropriate animal models.
Conclusions
Muscle lipid disease is phenotypically and genotypically heterogeneous. The detailed observation of clinical features combined with the distinct results of biochemical assays is required. In addition, mutation analyses are usually helpful for making the final diagnosis especially when clinical phenotype and laboratory tests show indistinguishable and nonspecific findings. Prompt diagnosis is important for subsequent treatment of patients especially as carnitine and riboflavin have shown excellent efficacy in the patients with PCD and RR-MADD. Moreover, in many patients with lipid dysmetabolism, the causative genes remain unknown. Thus, to discover the novel causative genes and then further explore the pathomechanism would be important missions in the future studies on muscle lipid diseases.
References
- 1.Bruno C, Dimauro S. Lipid storage myopathies. Curr Opin Neurol. 2008;21:601–606. doi: 10.1097/WCO.0b013e32830dd5a6. [DOI] [PubMed] [Google Scholar]
- 2.DiMauro S, DiMauro PM. Muscle carnitine palmityltransferease deficiency and myoglobinuria. Science. 1973;182:929–931. doi: 10.1126/science.182.4115.929. [DOI] [PubMed] [Google Scholar]
- 3.Bonnefont JP, Djouadi F, Prip-Buus C, et al. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25:495–520. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Spiekerkoetter U, Lindner M, Santer R, et al. Treatment recommendations in long-chain fatty acid oxidation defects: consensus from a workshop. J Inherit Metab Dis. 2009;32:498–505. doi: 10.1007/s10545-009-1126-8. [DOI] [PubMed] [Google Scholar]
- 5.Bonnefont JP, Bastin J, Behin A, et al. Bezafibrate for an inborn mitochondrial beta-oxidation defect. N Engl J Med. 2009;360:838–840. doi: 10.1056/NEJMc0806334. [DOI] [PubMed] [Google Scholar]
- 6.Bonnefont JP, Bastin J, Laforêt P, et al. Long-term follow-up of bezafibrate treatment in patients with the myopathic form of carnitine palmityltransferease 2 deficiency. Clin Pharmacol Ther. 2010;88:101–108. doi: 10.1038/clpt.2010.55. [DOI] [PubMed] [Google Scholar]
- 7.Strauss AW, Powell CK, Hale DE, et al. Molecular basis of human mitochondrial very-long-chain acyl-CoA dehydrogenase deficiency causing cardiomyopathy and sudden death in childhood. Proc Natl Acad Sci USA. 1995;92:10496–10500. doi: 10.1073/pnas.92.23.10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson BS, Olpin S, Poorthuis BJHM, et al. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am J Hum Genet. 1999;64:478–494. doi: 10.1086/302261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohashi Y, Hasegawa Y, Murayama K, et al. A new diagnostic test for VLCAD deficiency using immunohistochemistry. Neurology. 2004;62:2209–2213. doi: 10.1212/01.wnl.0000130486.54839.15. [DOI] [PubMed] [Google Scholar]
- 10.Ømgreen MC, Nørgaard MG, Engelen BG, et al. Effects of IV glucose and oral medium-chain triglyceride in patients with VLCAD deficiency. Neurology. 2007;69:313–315. doi: 10.1212/01.wnl.0000265854.41013.84. [DOI] [PubMed] [Google Scholar]
- 11.Tucci S, Primassin S, Veld F, et al. Medium-chain triglycerides impair lipid metabolism and induce hepatic steatosis in very-longchain acyl-CoA dehydrogenase (VLCAD)-deficient mice. Mol Genet Metab. 2010;101:40–47. doi: 10.1016/j.ymgme.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Djouadi F, Aubey F, Schlemmer D, et al. Bezafibrate increases very-long-chain acyl-CoA dehydrogenase protein and mRNA expression in deficient fibroblasts and is a potential therapy for fatty acid oxidation disorders. Hum Mol Genet. 2005;18:2695–2703. doi: 10.1093/hmg/ddi303. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi A, Nozaki J, Ohura T, et al. Genetic epidemiology of the carnitine transporter OCTN2 gene in a Japanese population and phenotypic characterization in Japanese pedigrees with primary systemic carnitine deficiency. Hum Mol Genet. 1999;8:2247–2254. doi: 10.1093/hmg/8.12.2247. [DOI] [PubMed] [Google Scholar]
- 14.Nezu J, Tamai I, Oku A, et al. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat Genet. 1999;21:91–94. doi: 10.1038/5030. [DOI] [PubMed] [Google Scholar]
- 15.Yamak AA, Bitar F, Karam P, et al. Exclusive cardiac dysfunction in familial primary carnitine deficiency cases: a genotype-phenotype correlation. Clin Genet. 2007;72:59–62. doi: 10.1111/j.1399-0004.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Korman SH, Ye J, et al. Phenotype and genotype variation in primary carnitine deficiency. Genet Med. 2001;3:387–392. doi: 10.1097/00125817-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 17.El-Hattab AW, Li FY, Shen J, et al. Maternal systemic primary carnitine deficiency uncovered by newborn screening: clinical, biochemical, and molecular aspects. Genet Med. 2010;12:19–24. doi: 10.1097/GIM.0b013e3181c5e6f7. [DOI] [PubMed] [Google Scholar]
- 18.Lamhonwah AM, Olpin SE, Pollitt RJ, et al. Novel OCTN2 mutations: no genotype-phenotype correlations: early carnitine therapy prevents cardiomyopathy. Am J Med Genet. 2002;111:271–284. doi: 10.1002/ajmg.10585. [DOI] [PubMed] [Google Scholar]
- 19.Ringseis R, Ludi S, Hirche F, et al. Treatment with pharmacological peroxisome proliferator-activated receptor alpha agonist clofibrate increases intestinal carnitine absorption in rats. Pharmacol Res. 2008;58:58–64. doi: 10.1016/j.phrs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Wen G, Ringseis R, Eder K, et al. Mouse OCTN2 is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha) via a PPRE located in the first intron. Biochem Pharmacol. 2010;79:768–776. doi: 10.1016/j.bcp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Schiff M, Froissart R, Olsen RK, et al. Electron transfer flavoprotein deficiency: functional and molecular aspects. Mol Genet Metab. 2006;88:153–158. doi: 10.1016/j.ymgme.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Olsen RK, Olpin SE, Andresen BS, et al. ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain. 2007;130:2045–2054. doi: 10.1093/brain/awm135. [DOI] [PubMed] [Google Scholar]
- 23.Gempel K, Topaloglu H, Talim B, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electrontransferring- flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130:2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang WC, Ohkuma A, Hayashi YK, et al. ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin- responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord. 2009;19:212–216. doi: 10.1016/j.nmd.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Er TK, Liang WC, Chang JG, et al. High resolution melting analysis facilitates mutation screening of ETFDH gene: applications in riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Clin Chim Acta. 2010;411:690–699. doi: 10.1016/j.cca.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Law LK, Tang NL, Hui J, et al. Novel mutations in ETFDH gene in Chinese patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Clin Chim Acta. 2009;404:95–99. doi: 10.1016/j.cca.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Lan MY, Fu MH, Liu YF, et al. High frequency of ETFDH c.250G > A mutation in Taiwanese patients with late-onset lipid storage myopathy. Clin Genet. 2010;78:565–569. doi: 10.1111/j.1399-0004.2010.01421.x. [DOI] [PubMed] [Google Scholar]
- 28.Angle B, Burton BK, et al. Risk of sudden death and acute life-threatening events in patients with glutaric acidemia type II. Mol Genet Metab. 2008;93:36–39. doi: 10.1016/j.ymgme.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Singla M, Guzman G, Griffin AJ, et al. Cardiomyopathy in multiple Acyl-CoA dehydrogenase deficiency: a clinico-pathological correlation and review of literature. Pediatr Cardiol. 2008;29:446–451. doi: 10.1007/s00246-007-9119-6. [DOI] [PubMed] [Google Scholar]
- 30.Olsen RK, Andresen BS, Christensen E, et al. Clear relationship between ETF/ETFDH genotype and phenotype in patients with multiple acyl-CoA dehydrogenation deficiency. Hum Mutat. 2003;22:12–23. doi: 10.1002/humu.10226. [DOI] [PubMed] [Google Scholar]
- 31.Yotsumoto Y, Hasegawa Y, Fukuda S, et al. Clinical and molecular investigations of Japanese cases of glutaric acidemia type 2. Mol Genet Metab. 2008;94:61–67. doi: 10.1016/j.ymgme.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Henriques BJ, Rodrigues JV, Olsen RK, et al. Role of flavinylation in a mild variant of multiple acyl-CoA dehydrogenation deficiency: a molecular rationale for the effects of riboflavin supplementation. J Biol Chem. 2009;284:4222–4229. doi: 10.1074/jbc.M805719200. [DOI] [PubMed] [Google Scholar]
- 33.Antozzi C, Garavaglia B, Mora M, et al. Late-onset riboflavinresponsive myopathy with combined multiple acyl coenzyme A dehydrogenase and respiratory chain deficiency. Neurology. 1994;44:2153–2158. doi: 10.1212/wnl.44.11.2153. [DOI] [PubMed] [Google Scholar]
- 34.Gianazza E, Vergani L, Wait R, et al. Coordinated and reversible reduction of enzymes involved in terminal oxidative metabolism in skeletal muscle mitochondria from a riboflavin-responsive, multiple acyl-CoA dehydrogenase deficiency patient. Electrophoresis. 2006;27:1182–1198. doi: 10.1002/elps.200500687. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Nishina Y, Shiga K, et al. In vitro refolding and unfolding of subunits of electron-transferring flavoprotein: characterization of the folding intermediates and the effects of FAD and AMP on the folding reaction. J Biochem. 1996;120:276–285. doi: 10.1093/oxfordjournals.jbchem.a021410. [DOI] [PubMed] [Google Scholar]
- 36.Lefevre C, Jobard F, Caux F, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer J, Lefevre C, Morava E, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 38.Schweiger M, Schoiswohl G, Lass A, et al. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- 39.Dorfman ML, Hershko C, Eisenberg S, et al. Ichthyosiform dermatosis with systemic lipidosis. Arch Dermatol. 1974;110:261–266. [PubMed] [Google Scholar]
- 40.Chanarin I, Patel A, Slavin G, et al. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975;1:553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Igal RA, Rhoads JM, Coleman RA, et al. Neutral lipid storage disease with fatty liver and cholestasis. J Pediatr Gastroenterol Nutr. 1997;25:541–547. doi: 10.1097/00005176-199711000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Ohkuma A, Nonaka I, Malicdan MC, et al. Distal lipid storage myopathy due to PNPLA2 mutation. Neuromuscul Disord. 2008;18:671–674. doi: 10.1016/j.nmd.2008.06.382. [DOI] [PubMed] [Google Scholar]
- 43.Ohkuma A, Noguchi S, Sugie H, et al. Clinical and genetic analysis of lipid storage myopathies. Muscle Nerve. 2009;39:333–342. doi: 10.1002/mus.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]