Abstract
Background
Polybrominated diphenyl ethers (PBDEs) have been used as flame retardants and are becoming a ubiquitous environmental contaminant. Adverse effects in the developing brain are of great health concern.
Objective
We investigated the effect of PBDEs/hydroxylated PBDEs (OH-PBDEs) on thyroid hormone (TH) receptor (TR)-mediated transcription and on TH-induced dendrite arborization of cerebellar Purkinje cells.
Methods
We examined the effect of PBDEs/OH-PBDEs on TR action using a transient transfection-based reporter gene assay. TR–cofactor binding was studied by the mammalian two-hybrid assay, and TR–DNA [TH response element (TRE)] binding was examined by the liquid chemiluminescent DNA pull-down assay. Chimeric receptors generated from TR and glucocorticoid receptor (GR) were used to identify the functional domain of TR responsible for PBDE action. The change in dendrite arborization of the Purkinje cell in primary culture of newborn rat cerebellum was also examined.
Results
Several PBDE congeners suppressed TR-mediated transcription. The magnitude of suppression correlated with that of TR–TRE dissociation. PBDEs suppressed transcription of chimeric receptors containing the TR DNA binding domain (TR-DBD). We observed no such suppression with chimeras containing GR-DBD. In the cerebellar culture, PBDE significantly suppressed TH-induced Purkinje cell dendrite arborization.
Conclusions
Several PBDE congeners may disrupt the TH system by partial dissociation of TR from TRE acting through TR-DBD and, consequently, may disrupt normal brain development.
Keywords: flame retardants, gene regulation, neurodevelopment, neurogenesis, polybrominated diphenyl ethers (PBDEs), thyroid
Polybrominated diphenyl ethers (PBDEs) have been used as flame retardants in a wide range of consumer and household goods (Hale et al. 2003). Their chemical stability allows PBDEs to be global environmental contaminants (Costa and Giordano 2007; Tseng et al. 2008). An alarming increase in PBDE concentrations in humans has been reported (Hites 2004; Law et al. 2003). Among congeners, 2,2′,4,4′-tetra-BDE (BDE47), 2,2′,4,4′,5-penta-BDE (BDE99), 2,2′,4,4′,6-penta-BDE (BDE100), 2,2′,4,4′,5,5′-hexa-BDE (BDE153), 2,2′,4,4′,5,6-hexa-BDE (BDE154), and 2,2′,3,3′,4,4′,5,5′,6,6′-deca-BDE (BDE209) are predominant in human tissues (Costa et al. 2008; Hites 2004). PBDEs and their hydroxylated metabolites (OH-PBDEs) have been detected in fetal blood and liver, and in maternal breast milk and placenta (Daniels et al. 2010; Gómara et al. 2007; Schecter et al. 2003). Furthermore, PBDEs can cross the blood–brain barrier to accumulate in the central nervous system (Naert et al. 2007). On the whole, the body burden of PBDEs is increasing (Hites 2004; Law et al. 2003; Sjödin et al. 2004).
PBDEs/OH-PBDEs have been implicated in developmental neurotoxicity. Neonatal exposure induces neurobehavioral changes in rodents (Branchi et al. 2003; Eriksson et al. 2001; Gee and Moser 2008). Such exposure also reduces long-term potentiation and postsynaptic protein levels in the hippocampus (Dingemans et al. 2007) and affects intracellular calcium homeostasis and induces oxidative stress (Kodavanti and Ward 2005). However, mechanisms of PBDE action in developing brain are not yet fully understood.
PBDEs/OH-PBDEs are structurally similar to the thyroid hormones (THs) 3,5,3′,5′-tetraiodo-l-thyronine [thyroxine (T4)] and 3,5,3′-triiodo-l-thyronine [triiodothyronine (T3)] (Schriks et al. 2007). Thus, PBDEs may act through the TH system (Birnbaum and Staskal 2004). TH is critical for normal brain development (Porterfield 2000), and TH deficiency during the perinatal critical period causes abnormal brain development known as cretinism in humans (Koibuchi and Chin 2000; Rabié et al. 1977). Decreased serum levels of T4 have been reported after perinatal exposure to various PBDEs in rodents (Costa and Giordano 2007; Rice et al. 2007; Zhou et al. 2001, 2002). In addition, OH-PBDEs have been shown to bind to the human TH receptor (TR) (Kitamura et al. 2008; Kojima et al. 2009; Li et al. 2010). A recent reporter gene assay study showed that BDE206 inhibits TR-mediated transcription (Schriks et al. 2007). These results suggest that PBDEs/OH-PBDEs may affect TH-regulated signal transduction pathways at multiple levels. However, mechanisms of PBDE/OH-PBDE action on TR-mediated transcription have not yet been fully clarified.
To study the mechanisms of TH action in the developing brain, we have used rodent cerebellum as a model system. Because perinatal hypothyroidism is associated with decreased dendrite arborization and synaptogenesis of Purkinje cells (Nicholson and Altman 1972a, 1972b), Purkinje cells are considered a good model to examine various TH actions in developing brain. Using this system, Kimura-Kuroda et al. (2007) observed the modification of TH-induced dendrite arborization by hydroxylated polychlorinated biphenyls (OH-PCBs).
The present study was designed to examine the mechanisms of PBDE action on modulation of TR-mediated gene expression and on morphological alterations in cultured Purkinje cells. Because the structure of PBDE is similar to that of PCB, we hypothesized that PBDE could suppress TR-mediated TH action.
Materials and Methods
Chemicals
We purchased T3, T4, and dexamethasone (Dex) from Sigma Chemical Co. (St. Louis, MO, USA). All PBDE/OH-PBDE congeners were purchased from AccuStandard Chemicals (New Haven, CT, USA). Purity was > 99% for all PBDE congeners and > 98% for all OH-PBDEs. DE-71 contains BDE99 (44%), BDE47 (32%), BDE100 (9%), BDE153 (4%), and other PBDEs (total 11%). Ethanol (Wako, Osaka, Japan) was used as a vehicle. All compounds were completely dissolved in ethanol at concentrations < 10−4 M and stored at −20°C. Dilutions were made from stock solutions immediately before use, and repeated freezing and thawing were avoided. PBDE congeners used in the present study were carefully selected to reflect their abundance in the environment and in humans (Hites 2004; Law et al. 2003).
Plasmids
Expression vectors for TRβ1, TRα1, and glucocorticoid receptor (GR) were described previously (Iwasaki et al. 2001, Koibuchi et al. 1999). The luciferase (LUC) reporter constructs containing chick lysozyme–TH response element (TRE) thymidine kinase (TK)-LUC (F2-TRE), artificial direct repeat TRE–DR4-TK-LUC (DR4-TRE), and 2× palindrome (Pal)-TK-LUC (Pal-TRE) in the PT109 vector were described by Koibuchi et al. (1999). A 5× upstream activating sequence (UAS)-TK-LUC reporter plasmid in the PT109 vector and a mouse mammary tumor virus (MMTV) promoter containing glucocorticoid response element (GRE), which is fused to the LUC promoter (MMTV-LUC), were described by Iwasaki et al. (2001). Expression vectors for Gal4–DNA binding domain (DBD)-fused steroid receptor coactivator-1–nuclear binding domain-1 (SRC-1–NBD-1; aa 595–780) (Takeshita et al. 2002) and VP16-TRβ1–ligand binding domain (LBD) (Miyazaki et al. 2004) were described previously. The Gal4 blank, Gal4-SMRT (silencing mediator for retinoid and thyroid hormone receptors; aa 1669–2507), and Gal4–N-CoR (nuclear receptor corepressor; aa 1579–2454) were described previously by Takeshita et al. (2002). The glutathione S-transferase (GST)-fused TRβ1 was described by Qiu CH et al. (2009). Chimeric receptors were generated from TR and GR as described by Miyazaki et al. (2008).
Clonal cell culture
CV-1 monkey fibroblast-derived cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum deprived of small lipophilic hormone by treating with resin and activated charcoal, at 37°C and 5% CO2.
Transient transfection assay
CV-1 cells were plated in 24-well plates 2 days before transfection using the calcium phosphate coprecipitation method (Miyazaki et al. 2008). The total amount of DNA per well was balanced by adding pcDNA3 plasmids (Invitrogen, San Diego, CA, USA). Cytomegalovirus β-galactosidase plasmid was used as an internal control. Sixteen to 24 hr after transfection, wells were refilled with fresh medium containing the indicated concentrations of ligand and/or PBDEs and incubated for 24 hr. For TH treatment, we used T3 because it is bioactive and because CV-1 cells do not contain deiodinase activity. Cells were then harvested to measure the LUC activities as described elsewhere (Miyazaki et al. 2008). The LUC activities were normalized to β-galactosidase activity and calculated as relative LUC activities. All transfection experiments were repeated at least three times in triplicate, and data represent mean ± SE of one experiment. The method of mammalian two-hybrid assay has been described previously (Baniahmad et al. 1995; Carey et al. 1989).
Liquid chemiluminescent DNA pull-down assay
(LCDPA). The LCDPA was used to measure nuclear receptor–DNA binding in solution (Iwasaki et al. 2008). Briefly, a GST-fused TR (GST-TR) bound to glutathione-Sepharose beads was incubated with a digoxigenin (DIG)-labeled double-stranded DNA fragment containing a TRE, in protein–DNA binding buffer. After extensive washing, protein–DNA binding on beads was detected using anti-DIG antibody conjugated to alkaline phosphatase, which was then measured by a chemiluminescent reaction using a luminometer. The LCDPA was performed at least three times, and data represent the mean ± SE of one experiment.
Primary culture
The animal experimentation protocol was approved by the Animal Care and Experimentation Committee, Gunma University. Pregnant Wistar rats were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Animals were treated humanely and with regard for alleviation of suffering. Newborn rats were decapitated under diethyl ether anesthesia on postnatal day 1. We followed the culture protocol described by Kimura-Kuroda et al. (2007). Briefly, cerebella were digested with 0.2 U/mL papain (Worthington, Lakewood, NJ, USA). Dissociated cells were then suspended in a serum-free medium without THs, plated at a density of 2.0 × 105 cells/0.2 mL in poly-l-lysine–coated wells of chamber slides (8-mm-diameter wells, NUNC Lab-Tek; Nalge Nunc International, Rochester, NY, USA), and cultured overnight in a 5% CO2 incubator. Then, T4 and/or PBDEs were added to the culture medium. Half of the medium was replaced with fresh medium every 3–4 days, and cells were cultured for 17 days. The pH of the culture medium was not altered after PBDE treatment. For TH treatment, T4 was used in primary culture because, physiologically, it is preferentially transported to brain through the blood–brain barrier, and primary culture contains the whole subset of cerebellar cells, including astrocytes. Physiologically, T4 is taken up by astrocytes and deiodinated to T3, which is then transferred to neurons.
Immunocytochemistry for calbindin to analyze Purkinje cell development
Immunocytochemistry of the cultured cells was described previously by Kimura-Kuroda et al. (2007). Briefly, we used a mouse monoclonal anti-calbindin-28K antibody (1:1,000; McAB 300; Swant, Bellinzona, Switzerland) and fluorescein isothiocyanate (FITC)-labeled donkey anti-mouse antibody (1:200; Molecular Probes, Eugene, OR, USA). The morphological changes were examined under a laser confocal scanning microscope (FV1000D spectral-type inverted IX81 microscope; Olympus, Tokyo, Japan). To quantify dendrite arborization, we manually traced the outline of the cell and dendritic branches of 10 randomly selected Purkinje cells from each experiment, and the area was computed using NIH Image software (Vischer 2006). Data shown represent mean ± SE of one experiment. We performed more than three independent experiments and confirmed consistency of the results.
Statistical analysis
We analyzed treatment effects using analysis of variance (ANOVA). Post hoc comparison was made using Bonferroni’s test, and p-values < 0.05 are considered significant.
Results
Congener-specific suppression of TR-mediated transcription by PBDEs/OH-PBDEs
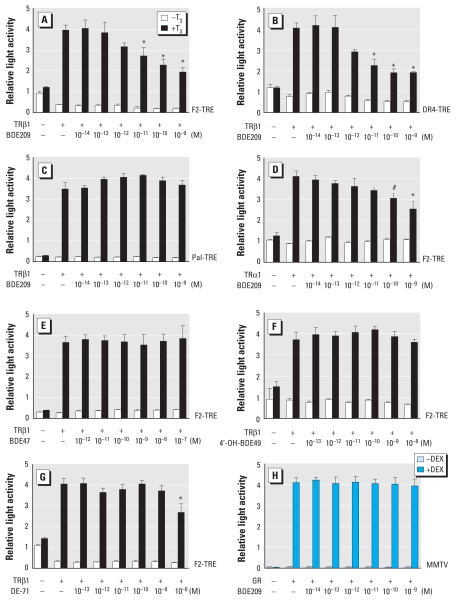
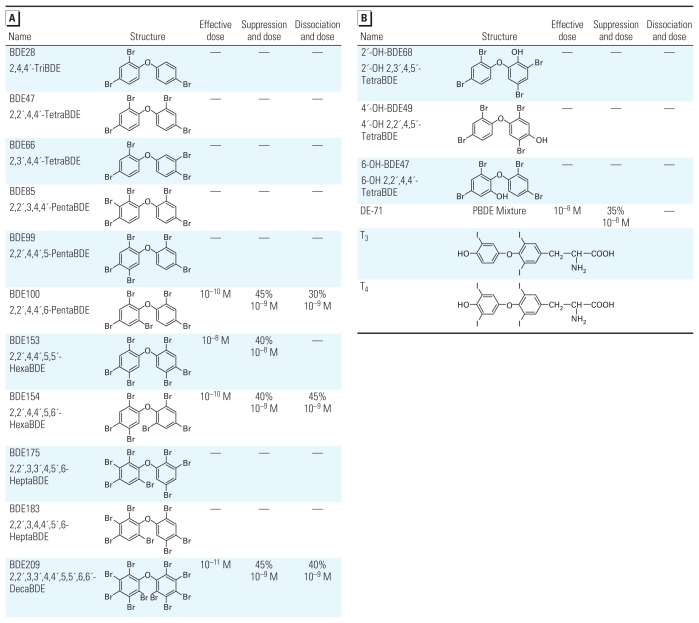
We examined the effect of various PBDE congeners, OH-PBDE metabolites, and PBDE mixtures on TR-mediated transcription in fibroblast-derived CV-1 cells using the transient transfection-based reporter gene assay. Representative results are shown in Figure 1 [see also Supplemental Material, Figures 3 and 4 (doi:10.1289/ehp.1002065)]. The tested compounds are listed in Figure 2. Among the PBDE compounds used, we observed the greatest magnitude of suppression with BDE209 and BDE100 (45% at 10−9 M; Figures 1A and 2A). BDE209 had the lowest effective dose (10−11 M) of the compounds evaluated (Figures 1A and 2). BDE209 suppressed TRβ1-mediated transcription on F2-TRE and DR4-TRE but not on Pal-TRE (Figure 1A–C). PBDEs/OH-PBDEs did not alter transcription levels when TH was absent (Figure 1). BDE209 also suppressed TRα1-mediated transcription (Figure 1D), but the magnitude of suppression was less than that for TRβ1-mediated transcription (Figure 1A). Several congeners (e.g., BDE47) did not suppress TR action (Figure 1E). We also examined the effect of hydroxylated metabolites, because several OH-PCBs suppressed TR action with a greater magnitude than did their parent PCBs (Miyazaki et al. 2004). However, as shown in Figures 1F and 2B [see also Supplemental Material, Figure 4D,E (doi:10.1289/ehp.1002065)], no OH-PBDEs suppressed TR action. DE-71, a commercial PBDE mixture, suppressed TR-mediated transcription to some extent (Figures 1G and 2B), although BDE100 and BDE153 make up < 15% of the mixture. Trypan blue exclusion confirmed that the PBDEs/OH-PBDEs did not induce cell death under the concentrations used in this study (data not shown). Also, we examined the effect of PBDEs/OH-PBDEs on reporter vector expression without TR transfection and observed no significant change (data not shown). On the other hand, BDE209 did not suppress GR-mediated transcription (Figure 1H), suggesting that the suppressive effects may be TR specific.
Figure 1.
Congener-specific suppression of TR-mediated transcription by PBDEs/OH-PBDEs. Expression plasmids encoding TRβ1 (A–C, E–G) or TRα1 (D; 10 ng) were cotransfected with F2-TRE (A, D–G), DR4-TRE (B), or Pal-TRE (C; 100 ng) into CV-1 cells. Cells were incubated with or without 10−7 M T3 and indicated amounts of PBDEs/OH-PBDEs. (H) Expression plasmids encoding GR (10 ng) were cotransfected with MMTV (GRE)-LUC reporter plasmids (100 ng) into CV-1 cells. Cells were cultured in the absence or presence of Dex (10−7 M) and indicated concentrations of BDE209. Total amounts of DNA for each well were balanced by adding vector pcDNA3. Data are mean ± SE of experiments performed in triplicate.
*p < 0.01, and #p < 0.05 by ANOVA, compared with TRβ1 (+), T3 (+), and BDE209 (−) in A, B, and D and DE-71 (−) in G.
Figure 2.
Effects of PBDE congeners (A) or OH-PBDEs and a PBDE mixture (B) on TR-mediated transcription and TR–TRE binding. Transient transfection-based reporter gene assays (Figure 1A) and LCDPA (Figure 3E) were carried out using each chemical. Results of minimum effective dose, maximum suppression (%) and dose, and maximum dissociation (%) and dose are listed. —, no effect.
PBDEs did not alter ligand-dependent cofactor recruitment to TRβ1 in CV-1 cells
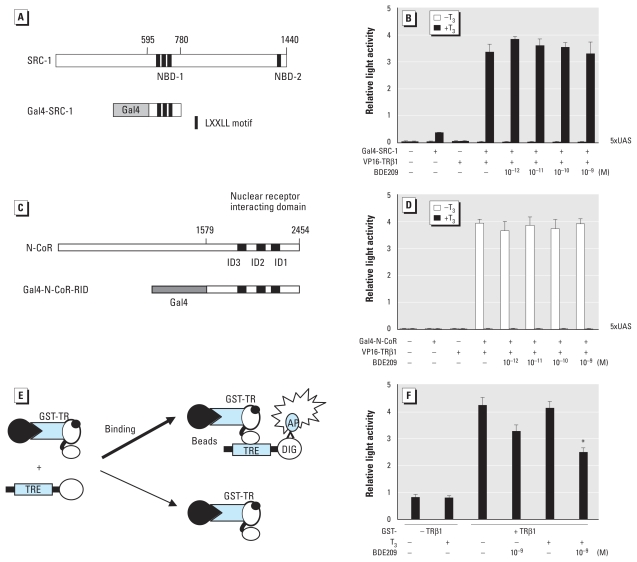
We investigated the effect of PBDEs on binding between TR and coactivator using the mammalian two-hybrid assay with Gal4–SRC-1– NBD-1 and VP16-TRβ1-LBD (Figure 3A). Transcriptional activation by Gal4–SRC-1–NBD-1 and VP16-TRβ1-LBD with T3 was not affected by BDE209 or any other compounds used in the present study (Figure 3B), indicating that these compounds do not dissociate TR–coactivator binding.
Figure 3.
The effect of PBDE on TR cofactor or TR–TRE binding. (A) Schematic diagram of Gal4–SRC-1–NBD-1. Functionally active LXXLL motifs (Leu-Xaa-Xaa-Leu-Leu) are located in central (residues 633–637, 690–694, and 749–753) and C-terminal (residues 1434–1438) regions termed NBD-1 and NBD-2, respectively. Gal4–SRC-1–NBD-1 contains amino acid residues 595–780 of SRC-1. (B) BDE209 did not affect SRC-1 binding to liganded TR in CV-1 cells. Expression plasmids encoding Gal4–DBD–fused SRC-1–NBD-1 (10 ng) were cotransfected with VP16 constructs (50 ng) and 5× UAS-TK-LUC reporter plasmids (170 ng) into CV-1 cells. Cells were incubated with or without T3 (10−7 M) and indicated concentrations of BDE209. (C) Schematic diagram of Gal4–N-CoR, which contains amino acid residues 1579–2454 of N-CoR. (D) BDE209 did not recruit N-CoR to TR in CV-1 cells. Expression plasmids harboring Gal4-DBD-fused N-CoR (100 ng) were cotransfected with VP16-TRβ1-LBD (50 ng) and 5× UAS-TK-LUC (100 ng) into CV-1 cells. Cells were incubated with or without T3 (10−7 M) and indicated concentrations of BDE209. (E) Schematic diagram showing the LCDPA method. GST-TRβ1 bound to Sepharose beads was incubated with DIG-labeled F2-TRE in protein–DNA binding buffer with or without T3 (10−6 M) and indicated concentrations of PBDE. (F) The effect of BDE209 on TRβ1–TRE binding using LCDPA. In B, D, and F, data represent mean ± SE of experiments performed in triplicate.
*p < 0.01 by ANOVA, compared with GST-TRβ1 (+), T3 (+), and PBDE (−).
Another possibility of PBDE action may be recruitment of corepressor complexes to liganded TR. Thus, we performed a mammalian two-hybrid assay using Gal4–N-CoR (Figure 3C) or Gal4-SMRT [see Supplemental Material, Figure 1A (doi:10.1289/ehp.1002065)], and VP16-TRβ1. Without T3, Gal4–N-CoR and VP16-TRβ1 activated transcription (Figure 3D), whereas we observed no activation with T3. Activation was not altered by BDE209 or any other compounds used in the present study (Figure 3D). PBDEs also did not recruit SMRT without T3 (see Supplemental Material, Figure 1B). These results indicate that PBDEs do not recruit corepressors to TR.
PBDEs partially dissociate TR from TRE
To examine whether PBDEs dissociate TR from TRE in vitro, we performed LCDPA (Figure 3E). BDE209 (10−9 M) partially dissociated TR from TRE (40%) in the presence of T3 (10−6 M; Figure 3F). Together with mammalian two-hybrid studies, these results indicate that the suppression of TR-mediated transcription by BDE209 was due to partial dissociation of TR from TRE. On the other hand, BDE47, which did not suppress TR-mediated transcription, did not dissociate TR from TRE [see Supplemental Material, Figure 2 (doi:10.1289/ehp.1002065)]. However, BDE153, which suppresses TR-mediated transcription with a higher dose (10−8 M) than other compounds, did not dissociate TR–TRE binding, although BDE153 did not alter TR–cofactor binding either (data not shown). The results of LCDPA are summarized in Figure 2.
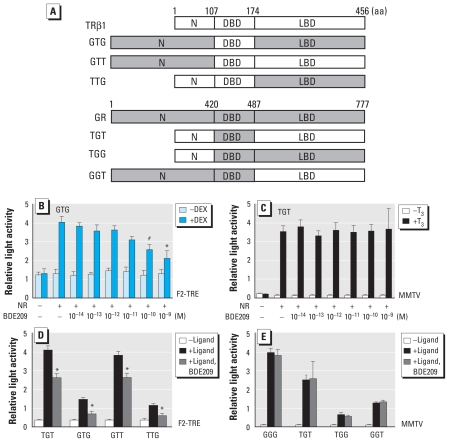
TR-DBD may be responsible for the effect of PBDEs on TR action
Next, we attempted to identify the functional domains of TR responsible for PBDE action using chimeric receptors (Figure 4A). Although all chimeric receptors harboring TR-DBD show suppression of transcription by BDE209 regardless of the difference in LBD (Figure 4B,D), we observed no such suppression with receptors harboring DBD of GR (Figure 4C,E), indicating that the site of PBDE action may be DBD of TR.
Figure 4.
TR-mediated transcription is altered by PBDE through TR-DBD. (A) Schematic diagram of chimeric protein used in the present study. Abbreviations: G, GR; T, TR; N, N-terminal domain. (B,C) Representative examples of PBDE actions on chimeric receptor-induced transcription (B, GTG; C, TGT). (D,E) Effects of BDE209 on transcription through chimeric receptors containing TR-DBD (D) or GR-DBD (E). For B–E, chimeric receptors (10 ng) were cotransfected with F2-TRE (B,D) or MMTV (GRE)-LUC (C,E) reporter plasmid (100 ng) into CV-1 cells and incubated with or without Dex (10−7 M) or T3 (10−7 M; C), and 10−14 to 10−9 M BDE209. Data represent mean ± SE of experiments performed in triplicate.
*p < 0.01, and #p < 0.05 by ANOVA, compared with GTG (+), Dex (+), and BDE209 (−) in B, and compared with chimeric receptors (+), ligand (+), and BDE209 (−) in D.
PBDEs disrupt TH-dependent dendrite arborization of cerebellar Purkinje cells
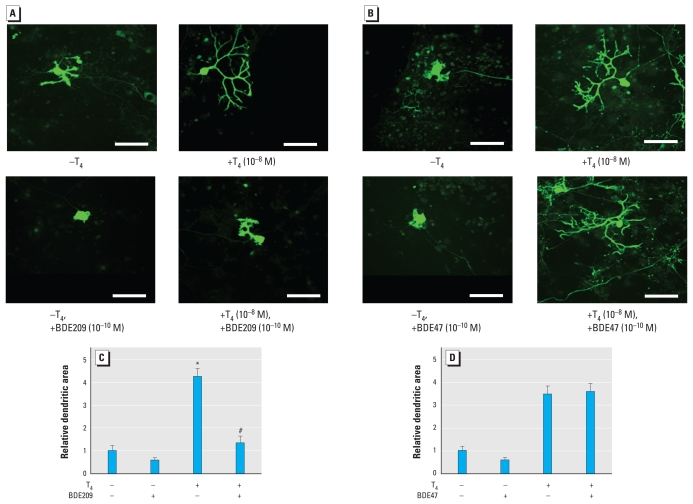
We studied the effect of several PBDE/OH-PBDE compounds on T4-induced dendrite arborization of cerebellar Purkinje cells in primary culture. Seventeen days after the onset of culture, cells were fixed and immunostained with anti-calbindin antibody. Addition of BDE209 (10−10 M) to the culture together with T4 inhibited development of Purkinje cell dendrites. The dendrites showed very poor growth, and the secondary branches were especially small (Figure 5A); the area of these Purkinje cell dendrites was significantly reduced (Figure 5A,C). In the absence of T4, BDE209-treated Purkinje cells showed almost complete absence of dendrites (Figure 5A). On the other hand, addition of BDE47 (10−10 M), which does not suppress TR action, did not inhibit Purkinje cell dendrite development (Figure 5B). The dendritic area was not different from that without BDE47 (Figure 5D). These data indicate that the PBDE congener that suppresses TR-mediated transcription also inhibits TH-mediated Purkinje cell dendrite arborization.
Figure 5.
Effect of PBDEs on dendrite arborization of Purkinje cell (17 days in culture). (A,B) Photomicrographs showing the effect of PBDEs on Purkinje cell morphology. BDE209 (10−10 M; A) or BDE47 (10−10 M; B) was added to the culture in the absence or presence of T4 (10−8 M), and immunocytochemistry was performed using anti-calbindin antibody to visualize Purkinje cells. Bars = 50 μm. (C,D) Change in dendritic areas of Purkinje cells by BDE209 (C)or BDE47 (D). In C and D, data are expressed as mean ± SE (n = 10 determinations) from one experiment and represent at least three independent experiments.
*p < 0.01 by ANOVA, for T4 (+)/BDE209 (−) compared with T4 (−)/BDE209 (−). #p < 0.01 by ANOVA, for T4 (+)/BDE209 (+) compared with T4 (+)/BDE209 (−).
Increasing the concentration of T4 could not fully rescue the suppression of T4-mediated Purkinje cell dendrite arborization by PBDEs
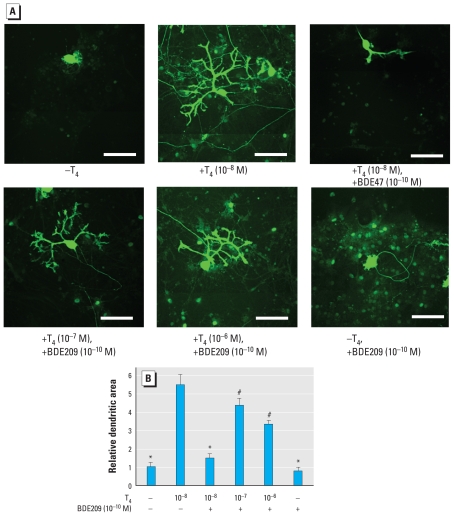
When we increased the T4 concentration to investigate the change in PBDE-mediated suppression of Purkinje cell dendrite arborization, increasing amounts of T4 significantly ameliorated the effect of 10−10 M BDE209. However, even at 10−6 M, T4 could not fully rescue the suppression (Figure 6). Purkinje cells had fewer secondary branches and bifurcations and less dendrite area than those cultured with 10−8 M T4 alone (Figure 6), indicating that T4 can only partially rescue BDE209 action.
Figure 6.
Increasing the concentration of T4 partially reverses the suppressive effects of BDE209 on Purkinje cell dendrite arborization (17 days in culture). (A) Photomicrographs showing the effect of BDE209 and T4 on Purkinje cell morphology. BDE209 (10−10 M) was added to the culture in the absence or presence of T4 (10−8 M to 10−6 M), and immunocytochemistry was performed using anti-calbindin antibody to visualize Purkinje cells. Bars = 50 μm. (B) Effect of BDE209 and T4 on dendritic area of Purkinje cells. Data are mean ± SE (n = 10 determinations) from one experiment and represent at least three independent experiments.
*p < 0.01, and #p < 0.05 by ANOVA, compared with T4 (10−8 M)/BDE209 (−).
Discussion
In the present study, PBDE induced congener-specific suppression of TR-mediated transcription. Of the congeners evaluated, BDE209 (deca-BDE) and BDE100 showed the greatest suppression (45% at 10−9 M), and BDE209 had the lowest effective dose (10−11 M). Such effects may be partially induced by dissociation of TR from TRE. The site of PBDE action may be on TR-DBD. Furthermore, PBDE disrupted Purkinje cell dendrite arborization.
Because of structural similarity, we initially hypothesized that the suppression of TR-mediated transcription by PBDEs could be due to competitive inhibition of T3 binding to TR or alteration of TR–cofactor binding. However, the mechanism of action is completely different from our initial hypothesis. Our experiments, involving the mammalian two-hybrid assay, transient transfection assays using chimeric receptors, and LCDPA, clearly demonstrated that the mechanism of action of PBDEs is similar to that of PCBs that we previously reported (Miyazaki et al. 2008). However, the BDE153 congener did not cause dissociation of the binding by LCDPA, although it significantly suppressed TR action. It should be noted that BDE153 did not alter TR–cofactor binding (data not shown). Because a greater amount of BDE153 (10−8 M) is required to induce suppression in CV-1 cells, its effect may be too weak to detect by in vitro assay.
In the chimeric receptor experiments, all chimeric receptors containing TR-DBD showed suppression of transcription by PBDE regardless of the difference in N- or C-terminus (Figure 4B,D), whereas no such suppression was observed with receptors harboring GR-DBD (Figure 4C,E), indicating that the site of BDE209 action may be TR-DBD. These findings are similar to those of our previous studies showing that PCBs may disrupt TR action by partial dissociation of TR–TRE binding, acting via TR-DBD (Miyazaki et al. 2008). Thus, PBDEs and PCBs could affect TR-mediated gene expression via a common pathway. PBDEs and PCBs have also been shown to affect signal transduction pathways, particularly calcium homeostasis and protein kinase C activity (Kodavanti and Ward 2005; Madia et al. 2004). In contrast, although hydroxylated PCB metabolites have been shown to effectively suppress TR-mediated gene expression (Miyazaki et al. 2008), the present study clearly revealed that only parent PBDE congeners effectively suppressed TR-mediated transcription. Thus, the site of PBDE action in the TR-DBD molecule could be different from that of PCBs because of differences in molecular structure and hydrophilicity. None of the OH-PBDE congeners used in our study significantly suppressed TR-mediated transcription (Figure 2B), although previous reports have suggested that other OH-PBDE congeners, whose concentration in humans is much lower, bound to TR (Kitamura et al. 2008; Qiu X et al. 2009). Recently, Li et al. (2010) showed binding between TRβ1 and some OH-PBDE compounds used in our study. However, they failed to show transcriptional activation or suppression with such compounds. Similar tendencies have been reported, showing that some OH-PBDE compounds—at low doses used in the present study—bind to TR without altering transcriptional activities (Kitamura et al. 2008; Kojima et al. 2009).
Although TH markedly promoted Purkinje cell dendrite arborization, BDE209 inhibited TH-induced dendrite development of Purkinje cells (Figure 5A,C). The effective dose, 10−10 M, is similar to that observed in our reporter gene assay. TRs are ubiquitously expressed in most cerebellar cells, including Purkinje cells, during development (Bradley et al. 1992), and previous studies have shown that TH induces Purkinje cell dendrite development via TR (Bradley et al. 1992; Strait et al. 1991). Thus, the inhibitory effects of PBDEs observed in our study could be due to their action on TR-mediated gene expression in Purkinje cells and may consequently disrupt normal brain development. However, increased concentrations of TH could not fully reverse the suppressive effects of BDE209 on TH-mediated Purkinje cell dendrite arborization (Figure 6). Purkinje cells cultured in the presence of 10−10 M BDE209 and 10−7 M or 10−6 M T4 showed less arborized dendrites than did those cultured in the presence of 10−8 M T4 alone (Figure 6), indicating the existence of additional pathways, such as disruption of intracellular signaling pathways that rely on Ca2+ homeostasis (Dingemans et al. 2010; Kodavanti and Ward 2005; Londoño et al. 2010; Madia et al. 2004) or induction of cytochrome P450 activities (Zhou et al. 2002). Further studies are required to investigate the synergistic effect of PBDEs on multiple signal transduction pathways. Nevertheless, the present study has provided an important clue to clarify the mechanisms of PBDE action in developing brain.
Because TH also tightly regulates fundamental gene expression both directly and indirectly, not only in the cerebellum but also in other brain regions (Koibuchi et al. 1999, 2001), the inhibitory effects of PBDEs on TR-mediated gene expression may widely disrupt normal brain development via TH-dependent gene regulations. Because PBDEs can be transferred to the developing brain through placenta or breast milk, continuous investigation is absolutely required for further clarification of PBDE action in other brain regions.
Conclusions
Our study shows that several PBDE congeners suppress TR-mediated gene expression by partial dissociation of TR from TRE through the DBD. At least partly through such a mechanism, PBDEs may inhibit TH-dependent dendrite arborization of cerebellar Purkinje cells. We hope that our study will provide significant clarifications on the mechanisms of PBDE action in the developing brain.
Footnotes
Supplemental Material is available online (doi:10.1289/ehp.1002065 via http://dx.doi.org/).
We thank M. Londoño, T. Aoki, K. Takata, K. Hanamura, and T. Shirao for helpful assistance and A. Takeshita for kindly providing plasmids.
This project was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17510039 to T.I., 17390060 to N.K.) and a grant from Ministry of the Environment of Japan to N.K.
References
- Baniahmad A, Leng X, Burris TP, Tsai SY, Tsai MJ, O’Malley BW. The tau 4 activation domain of the thyroid hormone receptor is required for release of a putative corepressor(s) necessary for transcriptional silencing. Mol Cell Biol. 1995;15:76–86. doi: 10.1128/mcb.15.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ, Towle HC, Young WS. Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β2 subtype, in the developing mammalian nervous system. J Neurosci. 1992;12:2288–2302. doi: 10.1523/JNEUROSCI.12-06-02288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Carey M, Kakidani H, Leatherwood J, Mostashari F, Ptashne M. An amino-terminal fragment of GAL4 binds DNA as a dimer. J Mol Biol. 1989;209:423–432. doi: 10.1016/0022-2836(89)90007-7. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenylether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79:172–183. [PubMed] [Google Scholar]
- Daniels JL, Jen-Pan I, Jones R, Anderson S, Patterson DG, Jr, Needham LL, et al. Individual characteristics associated with PBDE levels in U.S. human milk samples. Environ Health Perspect. 2010;118:155–160. doi: 10.1289/ehp.0900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, Ramakers GMJ, Gardoni F, van Kleef RGDM, Bergman A, Di Luca M, et al. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect. 2007;115:865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MML, van den Berg M, Bergman A, Westerink HS. Calcium-related processes involved in the inhibition of depolarization-evoked calcium increase by hydroxylated PBDEs in PC12 cells. Toxicol Sci. 2010;114:302–309. doi: 10.1093/toxsci/kfp310. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardant: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect. 2001;109:903–908. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee JR, Moser VC. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol Teratol. 2008;30:79–87. doi: 10.1016/j.ntt.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Gómara B, Herrero L, Ramos JJ, Mateo JR, Fernández MA, Garcia JF, et al. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol. 2007;41:6961–6968. doi: 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- Hale R, Alaee M, Manchester-Neesvig JB, Stapleton HM, Ikonomou MG. Polybrominated diphenyl ether flame retardants in the North American environment. Environ Int. 2003;29:771–779. doi: 10.1016/S0160-4120(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Chin WW, Ko L. Identification and characterization of RRM-containing coactivator activator (CoAA) as TRBP-interacting protein and its splice variant as a coactivator modulator (CoAM) J Biol Chem. 2001;276:33375–33383. doi: 10.1074/jbc.M101517200. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Miyazaki W, Rokutanda N, Koibuchi N. Liquid chemiluminescent DNA pull-down assay to measure nuclear receptor-DNA binding in solution. BioTechniques. 2008;45:445–448. doi: 10.2144/000112915. [DOI] [PubMed] [Google Scholar]
- Kimura-Kuroda J, Nagata I, Kuroda Y. Disrupting effects of hydroxy-polychlorinated biphenyl (PCB) congeners on neuronal development of cerebellar Purkinje cells: a possible causal factor for developmental brain disorders? Chemosphere. 2007;67:412–420. doi: 10.1016/j.chemosphere.2006.05.137. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Shinohara S, Iwase E, Sugihara K, Uramaru N, Shigematsu H, et al. Affinity for thyroid hormone and estrogen receptors of hydroxylated polybrominated diphenyl ethers. J Health Sci. 2008;54:607–614. [Google Scholar]
- Kodavanti PRS, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol Sci. 2005;85:952–962. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Chin WW. Thyroid hormone action and brain development. Trends Endocrinol Metab. 2000;11:123–128. doi: 10.1016/s1043-2760(00)00238-1. [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Liu Y, Fukuda H, Takeshita A, Yen PM, Chin WW. ROR alpha augments thyroid hormone receptor-mediated transcriptional activation. Endocrinology. 1999;140:1356–1364. doi: 10.1210/endo.140.3.6562. [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Yamaoka S, Chin WW. Effect of altered thyroid status on neurotrophin gene expression during postnatal development of the mouse cerebellum. Thyroid. 2001;11:205–210. doi: 10.1089/105072501750159534. [DOI] [PubMed] [Google Scholar]
- Kojima H, Takeuchi S, Uramaru N, Sugihara K, Yoshida T, Kitamura S. Nuclear hormone receptor activity of polybrominated diphenyl ethers and their hydroxylated and methoxylated metabolites in transactivation assays using Chinese hamster ovary cells. Environ Health Perspect. 2009;117:1210–1218. doi: 10.1289/ehp.0900753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RJ, Alaee M, Allchin CR, Boon JP, Lebeuf M, Lepom P, et al. Levels and trends of polybrominated diphenyl ethers and other brominated flame retardants in wild life. Environ Int. 2003;29:757–770. doi: 10.1016/S0160-4120(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Li F, Xie Q, Li X, Li N, Chi P, Chen J, et al. Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-β: in vitro and in silico investigations. Environ Health Perspect. 2010;118:602–606. doi: 10.1289/ehp.0901457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño M, Shimokawa N, Miyazaki W, Iwasaki T, Koibuchi N. Hydroxylated PCB induces Ca2+ oscillations and alterations of membrane potential in cultured cortical cells. J Appl Toxicol. 2010;30:334–342. doi: 10.1002/jat.1501. [DOI] [PubMed] [Google Scholar]
- Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, et al. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol Lett. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Miyazaki W, Iwasaki T, Takeshita A, Kuroda Y, Koibuchi N. Polychlorinated biphenyls suppress thyroid hormone receptor-mediated transcription through a novel mechanism. J Biol Chem. 2004;279:18195–18202. doi: 10.1074/jbc.M310531200. [DOI] [PubMed] [Google Scholar]
- Miyazaki W, Iwasaki T, Takeshita A, Tohyama C, Koibuchi N. Identification of the functional domain of thyroid hormone receptor responsible for polychlorinated biphenyl-mediated suppression of its action in vitro. Environ Health Perspect. 2008;116:1231–1236. doi: 10.1289/ehp.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert C, Van Peteqhem C, Kupper J, Jenni L, Naeqeli H. Distribution of polychlorinated biphenyls and polybrominated diphenyl ethers in birds of prey from Switzerland. Chemosphere. 2007;68:977–987. doi: 10.1016/j.chemosphere.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Nicholson JL, Altman J. The effects of early hypo- and hyperthyroidism on the development of the rat cerebellar cortex. II. Synaptogenesis in the molecular layer. Brain Res. 1972a;44:25–36. doi: 10.1016/0006-8993(72)90363-0. [DOI] [PubMed] [Google Scholar]
- Nicholson JL, Altman J. Synaptogenesis in the rat cerebellum: effects of early hypo- and hyperthyroidism. Science. 1972b;176:530–532. doi: 10.1126/science.176.4034.530. [DOI] [PubMed] [Google Scholar]
- Porterfield SP. Thyroid dysfunction and environmental chemicals-potential impact on brain development. Environ Health Perspect. 2000;108:433–438. doi: 10.1289/ehp.00108s3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu CH, Miyazaki W, Iwasaki T, Londoño M, Ibhazehiebo K, Shimokawa N, et al. Retinoic acid receptor-related orphan receptor alpha-enhanced thyroid hormone receptor-mediated transcription requires its ligand binding domain which is not, by itself, sufficient: possible direct interaction of two receptors. Thyroid. 2009;19:893–898. doi: 10.1089/thy.2008.0336. [DOI] [PubMed] [Google Scholar]
- Qiu X, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ Health Perspect. 2009;117:93–98. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabié A, Favre C, Clavel MC, Legrand J. Effects of thyroid dysfunction on the development of the rat cerebellum, with special reference to cell death within the internal granular layer. Brain Res. 1977;120:521–531. doi: 10.1016/0006-8993(77)90405-x. [DOI] [PubMed] [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zweller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29:511–520. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Päpke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ Health Perspect. 2003;111:1723–1729. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriks M, Roessig JM, Murk AJ, Furlow JD. Thyroid hormone receptor isoform selectivity of thyroid hormone disrupting compounds quantified with an in vitro reporter gene assay. Environ Toxicol Pharmacol. 2007;23:302–307. doi: 10.1016/j.etap.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, et al. Retrospective time trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyls in human serum from United States. Environ Health Perspect. 2004;112:654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait KA, Schwartz HL, Seybold VS, Ling NC, Oppenheimer JH. Immunofluorescence localization of thyroid hormone receptor protein β1 and variant α2 in selected tissues cerebellar Purkinje cells as a model for β1 receptor-mediated developmental effects of thyroid hormone in brain. Proc Natl Acad Sci USA. 1991;88:3887–3891. doi: 10.1073/pnas.88.9.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita A, Taguchi M, Koibuchi N, Ozawa Y. Putative role of the orphan nuclear receptor SXR (steroid and xenobiotic receptor) in the mechanism of CYP3A4 inhibition by xenobiotics. J Biol Chem. 2002;277:32453–32458. doi: 10.1074/jbc.M111245200. [DOI] [PubMed] [Google Scholar]
- Tseng L, Li M, Tsai S, Lee C, Pan M, Yao W, et al. Developmental exposure to decabromodiphenyl ether (PBDE 209): effects on thyroid hormone and hepatic enzyme activity in male mouse offspring. Chemosphere. 2008;70:640–647. doi: 10.1016/j.chemosphere.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Vischer N. Object-Image2.21. 2006. [accessed 4 Januaary 2011]. Available: http://simon.bio.uva.nl/Object-Image/object-image.html.
- Zhou T, Ross DG, Devito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormone and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, Devito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]