Abstract
Oxidative stress and mitochondrial dysfunction have been closely associated in many subcellular, cellular, animal, and human studies of both acute brain injury and neurodegenerative diseases. Our animal models of brain injury caused by cardiac arrest illustrate this relationship and demonstrate that both oxidative molecular modifications and mitochondrial metabolic impairment are exacerbated by reoxygenation of the brain using 100% ventilatory O2 compared to lower levels that maintain normoxemia. Numerous molecular mechanisms may be responsible for mitochondrial dysfunction caused by oxidative stress, including oxidation and inactivation of mitochondrial proteins, promotion of the mitochondrial membrane permeability transition, and consumption of metabolic cofactors and intermediates, e.g., NAD(H). Moreover, the relative contribution of these mechanisms to cell injury and death is likely different among different types of brain cells, e.g., neurons and astrocytes. In order to better understand these oxidative stress mechanisms and their relevance to neurologic disorders, we have undertaken studies with primary cultures of astrocytes and neurons exposed to O2 and glucose deprivation and reoxygenation and compared the results of these studies to those using a rat model of neonatal asphyxic brain injury. These results support the hypothesis that release and or consumption of mitochondrial NAD(H) is at least partially responsible for respiratory inhibition, particularly in neurons.
Keywords: pyruvate dehydrogenase, respiration, nicotinamide adenine dinucleotide
Mitochondrial Dysfunction in Ischemic Brain Injury
A large body of evidence indicates that mitochondrial dysfunction plays a critical role in the pathophysiology of ischemic brain injury 1-6. Consequences of mitochondrial dysfunction are numerous and include oxidative stress, loss of cellular Ca2+ homeostasis, promotion of apoptosis, and metabolic failure.
There are many possible causes of mitochondrial metabolic impairment and most involve oxidative modifications to proteins, lipids, or DNA. Identification of the sites at which oxidative stress impairs respiration can guide the development of counteractive interventions with neuroprotective potential. Complex I of the electron transport chain (ETC), which catalyzes the oxidation of NADH and the reduction of ubiquinone, is particularly sensitive to inhibition by both oxidative stress and ischemia/reperfusion and is generally considered to be the rate-limiting component of the ETC 7-10. Another cause of impaired ETC activity is the release of cytochrome c through the outer mitochondrial membrane into the cytosol, an event that is also often followed by caspase-dependent apoptosis 11. Oxidative stress promotes cytochrome c release by several mechanisms, including those promoting translocation of Bax and Bak to the outer membrane where they form pores that allow for passive efflux of cytochrome c and other intermembrane proteins 12,13. Oxidation of the mitochondria-specific phospholipid, cardiolipin, can also promote release of mitochondrial cytochrome c 14,15.
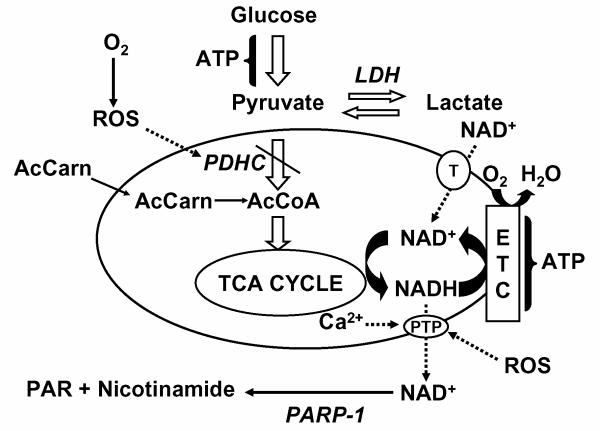
In addition to impaired ETC activities, oxidative phosphorylation can also be obstructed by inhibition of other mitochondrial enzymes and membrane transporters (Fig. 1). Thus, oxidative inactivation of mitochondrial matrix enzymes, e.g., pyruvate and α-ketoglutarate dehydrogenases and aconitase, are implicated in metabolic failure 16-18. Evidence also suggests that mitochondrial oxidative stress impairs the adenine nucleotide translocase, necessary for influx of ADP and efflux of ATP19. In addition, much interest is currently focused on the availability of the metabolic cofactor NAD+, necessary for dehydrogenases present within the mitochondrial matrix20. NAD+ in its oxidized or reduced form (NADH) can be lost from the mitochondrial matrix following opening of the inner membrane permeability transition pore (PTP), which results in transmembrane equilibration of small ions and molecules of up to approximately 1500 Da21,22 . The PTP is activated by abnormally high concentrations of Ca2+ and by oxidative stress23. Contribution of PTP opening to ischemic brain injury is supported by the neuroprotection observed with PTP inhibitors, e.g., cyclosporin drugs24-27, that bind to cyclophilin D, the one well-established protein associated with pore opening. Cyclophilin D knock-out mice are also resistant to ischemic brain injury 28. It is not known, however, if the PTP promotes ischemic neural cell death through chronic mitochondrial depolarization, and therefore uncoupling of oxidative phosphorylation, or through transient pore opening that releases sufficient NAD(H) to inhibit respiration and ATP synthesis by limiting the kinetics of dehydrogenase reactions.
Fig. 1. Loss of pyruvate dehydrogenase activity and matrix NAD(H) as mitochondrial mechanisms of metabolic failure.
Reactive O2 and N2 species can inhibit aerobic energy metabolism and promote lactate formation in several ways, including direct inhibition of PDHC and activation of the PTP, resulting in respiratory uncoupling and loss of NAD(H), that can then be consumed by PARP-1. Acetyl-carnitine can bypass the metabolic block at PDHC. Exogenous NAD+ can enter intact mitochondria through a transporter (T), compensating for lost NAD(H), and providing the electron shuttle between dehydrogenases and the electron transport chain (ETC).
NAD(H) Metabolism in Brain Injury
In addition to the multitude of redox reactions that either reduce or oxidize NAD(H), there are many reactions that consume NAD+, including those catalyzed by poly(ADP-ribose) polymerases (PARPs) 29. Under physiological conditions, the level of NAD+ in the brain is primarily controlled by the NADase enzyme CD38 30. Under pathological conditions, e.g, during cerebral ischemia/reperfusion, oxidative stress, hypoglycemia, and glutamate excitotoxicity, PARP-1 appears to be the most potent NAD+ consuming enzyme 31-35. PARP-1 becomes activated due to its role in facilitating repair of DNA strand breaks, particularly those caused by oxidative modification 36. Activated PARP-1 hydrolyzes NAD+ and transfers the ADP-ribose moieties to form poly(ADP-ribose) on acceptor proteins 37. This activity can result in a dramatic decline in cellular NAD+, particularly under conditions where a decline in cellular ATP limits ATP-dependent NAD+ biosynthesis. Once the NAD+ concentration falls below the approximately 1 mM level necessary to sustain glycolysis in the cytosol or the TCA cycle in the mitochondrial matrix, ATP production is impaired, resulting in a vicious cycle that if not reversed, eventually results in permanent metabolic failure and necrotic cell death 38,39. Reduced levels of ATP can also lead to impaired biosynthesis of NAD(H), at least in the cytosol 40, further accelerating the decline in energy metabolism. In addition to the effects of NAD+ depletion on ATP production, loss of NAD(H) can induce necrosis and apoptosis by additional mechanisms, including promotion of PTP opening and modulation of NAD-dependent sirtuins, which regulate genetic cell death programs 41-43.
Mitochondrial NAD(H) Metabolism in Cellular and Animal Models of Brain Injury
Our interest in studying the effects of anoxia and reoxygenation on cellular and mitochondrial NAD(P)H fluorescence in vitro within neurons and astrocytes arose originally from observations made in vivo by Univ. of Miami investigators demonstrating a decrease in intrinsic NAD(P)H light absorbance on the surface of the cerebral cortex below baseline following global cerebral ischemia and reperfusion 44. This decrease and associated electrophysiologic abnormalities were ameliorated by the presence of antioxidants 45, and by perfusion with normoxic compared to hyperoxic gas-equilibrated media 46, suggesting that reactive oxygen species (ROS) mediate the decrease in light absorbance by NAD(P)H. While an apparent oxidized shift in the NAD(P)H redox state can be due to many factors, e.g., increased NADH utilization by stimulated respiration or impaired production of NADH from NAD+, studies demonstrating net catabolism of NAD(H) in oxidative stress paradigms suggest an alternative explanation 47,48.
Experiments in our lab performed with primary cultures of rat cortical neurons exposed to glucose deprivation and either O2 deprivation or chemical hypoxia (cyanide) support the concept that ischemia/reperfusion can result in rapid and extensive catabolism of cellular NAD(H), including that present in mitochondria, that is promoted by oxidative stress 49. Moreover, partial inhibition of NAD(H) catabolism under these conditions by cyclosporin A or by the PARP-1 inhibitor, 3,4-dihydro-5-[4-(1-piperidinyl)butoxy]-1(2H)-isoquinolinone (DPQ), suggests that opening of the PTP triggers the release of mitochondrial NAD(H), allowing for its degradation by PARP-1 located in the nucleus or cytosol (Fig. 1) 50. Parallel experiments performed with primary cultures of cortical astrocytes indicated a relative resistance of these cells to NAD(H) catabolism induced by O2 and glucose deprivation 49. While this resistance was attributed to the elevated reducing power afforded by glycogen present within astrocytes but not neurons, other factors, e.g., relative PARP activities, could also contribute to cell-selective vulnerability to NAD(H) degradation and metabolic failure.
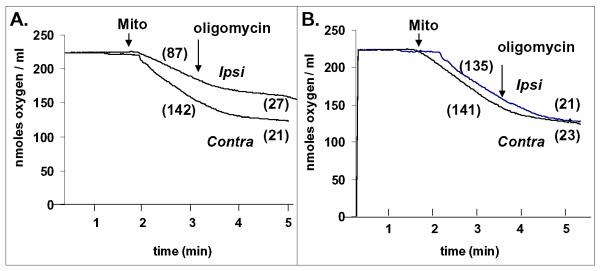
Depletion of the total pool of NAD(H) has been reported for experiments performed in vivo using a rat model of cerebral ischemia/reperfusion 51. Our lab has extended such measurements using a neonatal rat hypoxic ischemia model and found that the mitochondrial pool of NAD(H) is partially depleted within 20 min of reperfusion after 75 min unilateral carotid arterial occlusion under 8% atmospheric O2 52. Mitochondria from the ipsilateral hemispheres that were exposed to both ischemia and hypoxia contained 25% less NAD(H) compared to that present in mitochondria from the contralateral hemispheres that were exposed to hypoxia but no ischemia, as measured on perchloric acid extracts using an enzyme-linked fluorimetric assay 52. Furthermore, the loss of mitochondrial NAD(H) was accompanied by impairment of ADP-stimulated (state 3) O2 consumption, observed in the presence of NAD(H)-dependent oxidizable substrates (malate plus glutamate) (Fig. 2A) but not with the NAD(H)-independent substrate succinate (not shown). Parallel experiments were performed under identical conditions measuring mitochondrial production of 14CO2 over 60 min in the presence of 10 mM 14C-glutamate. These experiments demonstrated a significant reduction of 14CO2 generation by mitochondria from the hypoxic/ischemic hemisphere compared to the hypoxia alone hemisphere (25.7 ± 5.0 SEM vs 34.1 ± 6.7 nmol 14CO2/mg protein. n = 11; p < 0.05 by two-tailed t test) 52. Most importantly, the depressed rate of state 3 respiration was completely reversed by adding 2.5 mM NAD+ to the mitochondrial suspension, indicating that the loss of NAD(H) was responsible for the respiratory inhibition (Fig. 2B). Although not common knowledge, a few studies indicate that the mitochondrial inner membrane contains a transporter that mediates the uptake specifically of NAD+ rather than NADH (Fig. 1)53-55. The additional finding that NADH did not reverse the respiratory inhibition is consistent with this transport mechanism being responsible for mitochondrial NAD+ uptake and provides an additional rationale for using extracellular or systemic addition of NAD+ for neuroprotection, as shown in other models 36,56-59. While the mechanism of mitochondrial NAD(H) depletion is at this juncture unknown, the cyclosporin A-sensitive PTP appears responsible for this phenomenon in the heart following cardiac ischemia/reperfusion 60. In the neonatal cerebral hypoxic ischemia model, respiration by the isolated mitochondria remains well-coupled, i.e., low state 4 (resting) respiration, despite the loss of NAD(H) and reduced state 3 respiration (Fig. 2). Thus it appears that if opening of the PTP was responsible for loss of NAD(H), the pore must have closed, either in vivo or during the mitochondrial isolation procedure. Another possible explanation for depletion of mitochondrial NAD(H) is direct consumption of NAD+ by PARP-1 present within the mitochondrial matrix 61.
Fig. 2. Inhibition of respiration by brain mitochondria after neonatal hypoxic/ischemia and reversal of inhibition by NAD+.
Respiration was measured as described previously 7 at 37°C in medium containing 5 mM glutamate and 5 mM malate as substrates in the presence of 0.8 mM ADP and the absence (A) or presence (B) of 2.5 mM NAD+. Numbers in parentheses represent rates of respiration in nmol O2/min.mg protein. ADP-stimulated (state 3) respiration was lower for mitochondria from the ipsilateral, hypoxic/ischemic hemisphere (ipsi) compared to the contralateral, hypoxia only hemisphere (contra) (A), whereas both were very similar in the presence of NAD+. Resting respiration (state 4) observed after addition of 1 μg/ml oligomycin was similar in all conditions. These tracings are representative of 7 different experiments.
Promotion of Postischemic Oxidative Stress, Metabolic Dysfunction, and Neuronal Death by Hyperoxia
Our lab uses a clinically relevant canine cardiac arrest (CA) and resuscitation model to study molecular mechanisms of cell death and to identify targets for neuroprotection in global cerebral ischemia. A number of observations have been made using this model that illustrate the role of oxidative stress in postischemic metabolic dysfunction and demonstrate how both can be alleviated by simply avoiding abnormally high blood O2 levels. One key observation was that the enzyme activity and immunoreactivity of the pyruvate dehydrogenase complex (PDHC) are both reduced in the brain as early as 2 hr of reperfusion, possibly explaining persistently high brain lactate levels 16,18,62. If decreased metabolic flux through PDHC were responsible for elevated lactate, the presence of an alternative source of fuel for aerobic energy metabolism might correct this abnormality. Indeed, postischemic intravenous administration of acetyl-L-carnitine reduces brain lactate levels and improves neurologic outcome 63. Since acetyl-L-carnitine can donate its acetyl group to coenzyme A, forming acetylCoA via the carnitine acetyltransferase reaction, neuroprotection by early postischemic infusion of this agent may be due to its aerobic metabolism and generation of reducing power to drive both ATP production and ROS detoxification.
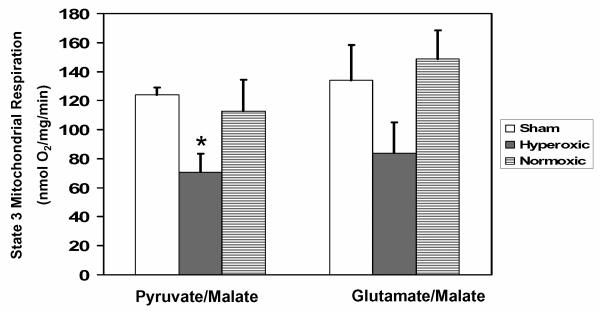
When outcome measures for animals resuscitated for the first hour after CA using hyperoxic ventilation (100% O2) were compared to those for animals resuscitated using normoxic ventilation, protein nitration, loss of PDHC, tissue lactic acidosis, impaired aerobic brain glucose metabolism, delayed neuronal death, and neurologic impairment are all significantly worse in the hyperoxic animals 16-18,64,64-66. Moreover, the differences in oxidative stress, PDHC, and aerobic energy metabolism observed within the first few hours after hyperoxic vs. normoxic resuscitation are evident in the hippocampus but not the frontal cortex, corresponding to the much greater vulnerability of the hippocampus to delayed neuronal cell death 18. While these results suggest that oxidative damage to PDHC may be responsible for impaired cerebral energy metabolism, measurements of respiration by isolated hippocampal mitochondria demonstrate a strong trend in respiratory inhibition by hyperoxic resuscitation using PDHC-independent oxidizable substrates (glutamate plus malate) as well as a significant inhibition using PDHC-dependent substrates (pyruvate plus malate) (Fig. 3) This finding implies that a mechanism in addition to, or other than inhibition of PDHC could be responsible for impaired postischemic cerebral energy metabolism. Based on the findings obtained with the neuronal O2/glucose deprivation model and with the neonatal hypoxic ischemia model, we hypothesize that loss of mitochondrial NAD+ is the additional or alternative mechanism.
Fig. 3. Hyperoxic reperfusion after experimental cardiac arrest worsens mitochondrial respiration.
Mitochondria were isolated from the hippocampi of dogs 2 hr after 10 min of cardiac arrest, using 1 hr of post-resuscitative mechanical ventilation on either 100% O2 (hyperoxic) or room air (21% O2; normoxic) 17. State 3 respiration was measured at 37°C in medium containing 0.1 mM malate plus either 5 mM pyruvate or 5 mM glutamate, as described previously6. Values represent the means ± SEM for 4 separate experiments. * Significantly different than non-ischemic, sham-operated controls; 1-way ANOVA with Tukey post hoc analysis, p < 0.05.
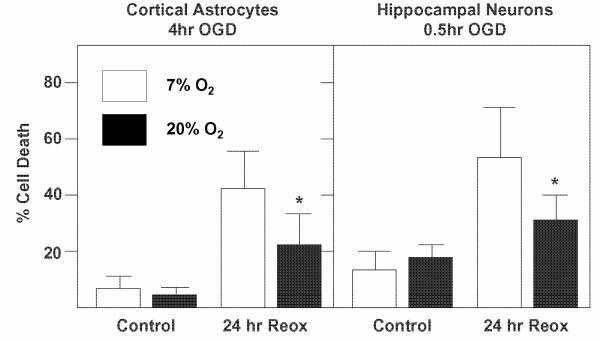
Exacerbation of brain cell death by exposure to abnormally high O2 concentrations after deprivation of O2 and glucose has also been observed with primary cultures of neurons and astrocytes (Fig. 4) 67. Cells were placed in glucose free medium in an anaerobic chamber where the O2 concentration in the medium was less than 1 μM, which effectively blocks the ability of cells to respire. Astrocytes and neurons were exposed to different periods of O2 and glucose deprivation (4 hr and 30 min, respectively) that caused no immediate cell death but did result in significant delayed cell death following 24 hr reoxygenation. This pattern models the temporal course of cell death observed in the canine CA model. During reoxygenation in medium containing glucose, both cell types were exposed to either 20% O2 (149 mm Hg), i.e., the normal 95% air / 5% CO2 used in cell culture, or to 7% O2 (52 mm Hg), which is far closer to the normal brain O2 tension of around 30 mm Hg 68. The death of both astrocytes and neurons was significantly greater at 20% compared to 7% O2. As expected, markers of oxidative stress, like protein nitration and DNA base oxidation, were elevated in cells under 20% compared to 7% O2 within a few hours of reoxygenation67. Experiments using this model are in progress to determine how different reoxygenation O2 tensions influence astrocyte and neuronal energy metabolism and whether changes in NAD(H) levels, PDHC activity, or other factors are responsible.
Fig. 4. Hyperoxia promotes death of astrocytes and neurons during rexoygenation after exposure to O2 and glucose deprivation (OGD).
Rat cortical astrocytes (10 DIV) or murine hippocampal neurons (6 DIV) were exposed to OGD 4 hr and 0.5 hr, respectively, in serum-free “ischemic salts solution”, as described previously 72. The medium was then replaced with serum-free normal growth medium and the cells exposed to either 20% or 7% ambient O2. Cell death was measured 24 hr later using the Hoescht/propidium iodide fluorescent assay for astrocytes and the Live/Dead Assay (Invitrogen) for neurons. See Danilov and Fiskum 67 for additional details. Values represent the means ± SEM for 6 - 8 separate experiments. * Significantly different from 20% O2; t test, p < 0.05.
Development of Metabolism-Based Neuroprotective Interventions
Despite the explosion of available new information on how signal transduction mechanisms can promote cell survival or trigger pathologic apoptosis, mechanisms of metabolic failure and necrotic cell death continue to be critical targets for therapeutic intervention in global cerebral ischemia and other forms of neurodegeneration. The fact that neuroprotection has been observed with agents like creatine and ketone bodies or ketogenic diets in animal models of acute and chronic neurodegeneration 69-71 and that clinical trials based on these results are in progress are testaments to the therapeutic potential of correcting abnormal cerebral energy metabolism. Studies that identify the factor(s) that limit cerebral energy production and that delineate the time-course of metabolic disruption are necessary for strategic development of metabolic therapies. For instance, documentation that impaired PDHC activity limits aerobic energy metabolism at specific periods after global cerebral ischemia would provide the rationale for administering alternative metabolic fuels, e.g., acetyl-L-carnitine or ketone bodies, during these periods. Alternatively, should loss or catabolism of NAD+ prove to be a limiting factor, this would support the pharmacologic use of exogenous NAD+ or nicotinamide, an NAD+ precursor, during the periods when they are needed. For instance, intranasal administration of NAD+ at 10 mg/kg two hr after ischemic onset was recently shown to decrease infarct volume in a rat stroke model 56. Considering the heterogenous response of astrocytes, neurons, and neuronal subtypes to ischemia/reperfusion, it is likely that the most effective metabolic therapies will involve combinations of agents, possibly designed selectively for gender and age. While these approaches are in development, further comparisons of metabolic outcome measures using hyperoxic and normoxic resuscitated animals may provide more support for avoiding hyperoxia early during reperfusion specifically after global cerebral ischemia.
Acknowledgments
The authors and the work described in this article were supported by NIH grants NS34152, HD16596, and DOD grant DAMD 17-03-1-0745.
References
- 1.BLOMGREN K, HAGBERG H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic. Biol. Med. 2006;40:388–397. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 2.FISKUM G, MURPHY AN, BEAL MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J. Cereb. Blood Flow Metab. 1999;19:351–369. doi: 10.1097/00004647-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 3.RIZZUTO R, SIMPSON AW, BRINI M, POZZAN T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [published erratum appears in Nature 1992 Dec 24- 31;360(6406):768] [DOI] [PubMed] [Google Scholar]
- 4.CHANG LH, SHIMIZU H, ABIKO H, SWANSON RA, FADEN AI, JAMES TL, WEINSTEIN PR. Effect of dichloroacetate on recovery of brain lactate, phosphorus energy metabolites, and glutamate during reperfusion after complete cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1992;12:1030–1038. doi: 10.1038/jcbfm.1992.140. [DOI] [PubMed] [Google Scholar]
- 5.KURODA S, KATSURA KI, TSUCHIDATE R, SIESJO BK. Secondary bioenergetic failure after transient focal ischaemia is due to mitochondrial injury. Acta Physiol. Scand. 1996;156:149–150. doi: 10.1046/j.1365-201X.1996.449170000.x. [DOI] [PubMed] [Google Scholar]
- 6.STARKOV AA, CHINOPOULOS C, FISKUM G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 7.ROSENTHAL RE, HAMUD F, FISKUM G, VARGHESE PJ, SHARPE S. Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J. Cereb. Blood Flow Metab. 1987;7:752–758. doi: 10.1038/jcbfm.1987.130. [DOI] [PubMed] [Google Scholar]
- 8.SIMS NR. Selective impairment of respiration in mitochondria isolated from brain subregions following transient forebrain ischemia in the rat. J. Neurochem. 1991;56:1836–1844. doi: 10.1111/j.1471-4159.1991.tb03438.x. [DOI] [PubMed] [Google Scholar]
- 9.HILLERED L, ERNSTER L. Respiratory activity of isolated rat brain mitochondria following in vitro exposure to oxygen radicals. J. Cereb. Blood Flow Metab. 1983;3:207–214. doi: 10.1038/jcbfm.1983.28. [DOI] [PubMed] [Google Scholar]
- 10.ROSENTHAL RE, FISKUM G. Brain mitochondrial function in cerebral ischemia and resuscitation. In: Schurr A, editor. Cerebral Ischemia and Resuscitation. CRC Press; New York: 1990. pp. 289–300. in press. [Google Scholar]
- 11.POLSTER BM, KINNALLY KW, FISKUM G. Bh3 death domain peptide induces cell type-selective mitochondrial outer membrane permeability. J. Biol. Chem. 2001;276:37887–37894. doi: 10.1074/jbc.M104552200. [DOI] [PubMed] [Google Scholar]
- 12.CASTINO R, BELLIO N, NICOTRA G, FOLLO C, TRINCHERI NF, ISIDORO C. Cathepsin D-Bax death pathway in oxidative stressed neuroblastoma cells. Free Radic. Biol. Med. 2007;42:1305–1316. doi: 10.1016/j.freeradbiomed.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 13.PERIER C, TIEU K, GUEGAN C, CASPERSEN C, JACKSON-LEWIS V, CARELLI V, MARTINUZZI A, HIRANO M, PRZEDBORSKI S, VILA M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ORRENIUS S, ZHIVOTOVSKY B. Cardiolipin oxidation sets cytochrome c free. Nat. Chem. Biol. 2005;1:188–189. doi: 10.1038/nchembio0905-188. [DOI] [PubMed] [Google Scholar]
- 15.KAGAN VE, TYURIN VA, JIANG J, TYURINA YY, RITOV VB, AMOSCATO AA, OSIPOV AN, BELIKOVA NA, KAPRALOV AA, KINI V, VLASOVA II, ZHAO Q, ZOU M, DI P, SVISTUNENKO DA, KURNIKOV IV, BORISENKO GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 16.BOGAERT YE, ROSENTHAL RE, FISKUM G. Postischemic inhibition of cerebral cortex pyruvate dehydrogenase. Free Radical Biology & Medicine. 1994;16(6):811–20. doi: 10.1016/0891-5849(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 17.VERECZKI V, MARTIN E, ROSENTHAL RE, HOF PR, HOFFMAN GE, FISKUM G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J. Cereb. Blood Flow Metab. 2006;26:821–835. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RICHARDS EM, ROSENTHAL RE, KRISTIAN T, FISKUM G. Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic. Biol. Med. 2006;40:1960–1970. doi: 10.1016/j.freeradbiomed.2006.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VESCE S, JEKABSONS MB, JOHNSON-CADWELL LI, NICHOLLS DG. Acute glutathione depletion restricts mitochondrial ATP export in cerebellar granule neurons. J. Biol. Chem. 2005;280:38720–38728. doi: 10.1074/jbc.M506575200. [DOI] [PubMed] [Google Scholar]
- 20.YING W. NAD+ and NADH in ischemic brain injury. Front Biosci. 2008;13:1141–1151. doi: 10.2741/2751. [DOI] [PubMed] [Google Scholar]
- 21.CROMPTON M, BARKSBY E, JOHNSON N, CAPANO M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie. 2002;84:143–152. doi: 10.1016/s0300-9084(02)01368-8. [DOI] [PubMed] [Google Scholar]
- 22.HALESTRAP AP, MCSTAY GP, CLARKE SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 23.BERNARDI P, SCORRANO L, COLONNA R, PETRONILLI V, DI LISA F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 24.UCHINO H, MINAMIKAWA-TACHINO R, KRISTIAN T, PERKINS G, NARAZAKI M, SIESJO BK, SHIBASAKI F. Differential neuroprotection by cyclosporin A and FK506 following ischemia corresponds with differing abilities to inhibit calcineurin and the mitochondrial permeability transition. Neurobiol. Dis. 2002;10:219–233. doi: 10.1006/nbdi.2002.0514. [DOI] [PubMed] [Google Scholar]
- 25.SULLIVAN PG, KELLER JN, BUSSEN WL, SCHEFF SW. Cytochrome c release and caspase activation after traumatic brain injury. Brain Res. 2002;949:88–96. doi: 10.1016/s0006-8993(02)02968-2. [DOI] [PubMed] [Google Scholar]
- 26.ALESSANDRI B, RICE AC, LEVASSEUR J, DEFORD M, HAMM RJ, BULLOCK MR. Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J. Neurotrauma. 2002;19:829–841. doi: 10.1089/08977150260190429. [DOI] [PubMed] [Google Scholar]
- 27.HANSSON MJ, MATTIASSON G, MANSSON R, KARLSSON J, KEEP MF, WALDMEIER P, RUEGG UT, DUMONT JM, BESSEGHIR K, ELMER E. The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J. Bioenerg. Biomembr. 2004;36:407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- 28.SCHINZEL AC, TAKEUCHI O, HUANG Z, FISHER JK, ZHOU Z, RUBENS J, HETZ C, DANIAL NN, MOSKOWITZ MA, KORSMEYER SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.YING W. NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006;11:3129–3148. doi: 10.2741/2038. [DOI] [PubMed] [Google Scholar]
- 30.AKSOY P, WHITE TA, THOMPSON M, CHINI EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 31.SZABO C, DAWSON VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol. Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- 32.NARASIMHAN P, FUJIMURA M, NOSHITA N, CHAN PH. Role of superoxide in poly(ADP-ribose) polymerase upregulation after transient cerebral ischemia. Brain Res. Mol. Brain Res. 2003;113:28–36. doi: 10.1016/s0169-328x(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 33.ZHANG J, DAWSON VL, DAWSON TM, SNYDER SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263:687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 34.KOSENKO E, MONTOLIU C, GIORDANO G, KAMINSKY Y, VENEDIKTOVA N, BURYANOV Y, FELIPO V. Acute ammonia intoxication induces an NMDA receptor-mediated increase in poly(ADP-ribose) polymerase level and NAD metabolism in nuclei of rat brain cells. J Neurochem. 2004;89:1101–1110. doi: 10.1111/j.1471-4159.2004.02426.x. [DOI] [PubMed] [Google Scholar]
- 35.SUH SW, AOYAMA K, CHEN Y, GARNIER P, MATSUMORI Y, GUM E, LIU J, SWANSON RA. Hypoglycemic neuronal death and cognitive impairment are prevented by poly(ADP-ribose) polymerase inhibitors administered after hypoglycemia. J Neurosci. 2003;23:10681–10690. doi: 10.1523/JNEUROSCI.23-33-10681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.KLAIDMAN LK, MUKHERJEE SK, ADAMS JD., Jr. Oxidative changes in brain pyridine nucleotides and neuroprotection using nicotinamide. Biochim. Biophys. Acta. 2001;1525:136–148. doi: 10.1016/s0304-4165(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 37.SHALL S, DE MURCIA G. Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat. Res. 2000;460:1–15. doi: 10.1016/s0921-8777(00)00016-1. [DOI] [PubMed] [Google Scholar]
- 38.CARSON DA, SETO S, WASSON DB, CARRERA CJ. DNA strand breaks, NAD metabolism, and programmed cell death. Exp. Cell Res. 1986;164:273–281. doi: 10.1016/0014-4827(86)90028-5. [DOI] [PubMed] [Google Scholar]
- 39.BERGER NA. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat. Res. 1985;101:4–15. [PubMed] [Google Scholar]
- 40.DEVIN A, GUERIN B, RIGOULET M. Cytosolic NAD+ content strictly depends on ATP concentration in isolated liver cells. FEBS Lett. 1997;410:329–332. doi: 10.1016/s0014-5793(97)00612-1. [DOI] [PubMed] [Google Scholar]
- 41.MURUGANANDHAM M, ALFIERI AA, MATEI C, CHEN Y, SUKENICK G, SCHEMAINDA I, HASMANN M, SALTZ LB, KOUTCHER JA. Metabolic signatures associated with a NAD synthesis inhibitor-induced tumor apoptosis identified by 1H-decoupled-31P magnetic resonance spectroscopy. Clin. Cancer Res. 2005;11:3503–3513. doi: 10.1158/1078-0432.CCR-04-1399. [DOI] [PubMed] [Google Scholar]
- 42.TANAKA S, TAKEHASHI M, IIDA S, KITAJIMA T, KAMANAKA Y, STEDEFORD T, BANASIK M, UEDA K. Mitochondrial impairment induced by poly(ADP-ribose) polymerase-1 activation in cortical neurons after oxygen and glucose deprivation. J Neurochem. 2005;95:179–190. doi: 10.1111/j.1471-4159.2005.03353.x. [DOI] [PubMed] [Google Scholar]
- 43.YEUNG F, HOBERG JE, RAMSEY CS, KELLER MD, JONES DR, FRYE RA, MAYO MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.PEREZ-PINZON MA, MUMFORD PL, CARRANZA V, SICK TJ. Calcium influx from the extracellular space promotes NADH hyperoxidation and electrical dysfunction after anoxia in hippocampal slices. J Cereb. Blood Flow Metab. 1998;18:215–221. doi: 10.1097/00004647-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 45.PEREZ-PINZON MA, MUMFORD PL, ROSENTHAL M, SICK TJ. Antioxidants, mitochondrial hyperoxidation and electrical recovery after anoxia in hippocampal slices. Brain Res. 1997;754:163–170. doi: 10.1016/s0006-8993(97)00066-8. [DOI] [PubMed] [Google Scholar]
- 46.FENG ZC, SICK TJ, ROSENTHAL M. Oxygen sensitivity of mitochondrial redox status and evoked potential recovery early during reperfusion in post-ischemic rat brain. Resuscitation. 1998;37:33–41. doi: 10.1016/s0300-9572(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 47.ALANO CC, YING W, SWANSON RA. Poly(ADP-ribose) polymerase-1 mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J. Biol. Chem. 2004 doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- 48.ZENG J, YANG GY, YING W, KELLY M, HIRAI K, JAMES TL, SWANSON RA, LITT L. Pyruvate improves recovery after PARP-1-associated energy failure induced by oxidative stress in neonatal rat cerebrocortical slices. J. Cereb. Blood Flow Metab. 2007;27:304–315. doi: 10.1038/sj.jcbfm.9600335. [DOI] [PubMed] [Google Scholar]
- 49.KAHRAMAN S, FISKUM G. Anoxia-induced changes in pyridine nucleotide redox state in cortical neurons and astrocytes. Neurochem. Res. 2007;32:799–806. doi: 10.1007/s11064-006-9206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.KAHRAMAN S, SIEGEL A, FISKUM G. Mechanisms of NAD(H) depletion in cortical neurons during anoxia and glucose deprivation. Society for Neuroscience. 2007:597. [Google Scholar]
- 51.WELSH FA, VANNUCCI RC, BRIERLEY JB. Columnar alterations of NADH fluorescence during hypoxia-ischemia in immature rat brain. J Cereb. Blood Flow Metab. 1982;2:221–228. doi: 10.1038/jcbfm.1982.22. [DOI] [PubMed] [Google Scholar]
- 52.MEHARABYAN Z, LINDAUER S, KRISTIAN T, HOPKINS I, MCKENNA M, FISKUM G. Mitochondrial oxidative stress and NAD-limited respiratory inhibition after neonatal cerebral hypoxic ischemia. Society for Neuroscience. 2007:597. [Google Scholar]
- 53.YANG H, YANG T, BAUR JA, PEREZ E, MATSUI T, CARMONA JJ, LAMMING DW, SOUZA-PINTO NC, BOHR VA, ROSENZWEIG A, DE CR, SAUVE AA, SINCLAIR DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.RUSTIN P, PARFAIT B, CHRETIEN D, BOURGERON T, DJOUADI F, BASTIN J, ROTIG A, MUNNICH A. Fluxes of nicotinamide adenine dinucleotides through mitochondrial membranes in human cultured cells. J. Biol. Chem. 1996;271:14785–14790. doi: 10.1074/jbc.271.25.14785. [DOI] [PubMed] [Google Scholar]
- 55.TODISCO S, AGRIMI G, CASTEGNA A, PALMIERI F. Identification of the mitochondrial NAD+ transporter in Saccharomyces cerevisiae. J. Biol. Chem. 2006;281:1524–1531. doi: 10.1074/jbc.M510425200. [DOI] [PubMed] [Google Scholar]
- 56.YING W, WEI G, WANG D, WANG Q, TANG X, SHI J, ZHANG P, LU H. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- 57.ZHU K, SWANSON RA, YING W. NADH can enter into astrocytes and block poly(ADP-ribose) polymerase-1-mediated astrocyte death. Neuroreport. 2005;16:1209–1212. doi: 10.1097/00001756-200508010-00015. [DOI] [PubMed] [Google Scholar]
- 58.YING W, GARNIER P, SWANSON RA. NAD+ repletion prevents PARP-1-induced glycolytic blockade and cell death in cultured mouse astrocytes. Biochem. Biophys. Res. Commun. 2003;308:809–813. doi: 10.1016/s0006-291x(03)01483-9. [DOI] [PubMed] [Google Scholar]
- 59.DU L, ZHANG X, HAN YY, BURKE NA, KOCHANEK PM, WATKINS SC, GRAHAM SH, CARCILLO JA, SZABO C, CLARK RS. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. J. Biol. Chem. 2003;278:18426–18433. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- 60.DI LISA F, MENABO R, CANTON M, BARILE M, BERNARDI P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J. Biol. Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 61.DU L, ZHANG X, HAN YY, BURKE NA, KOCHANEK PM, WATKINS SC, GRAHAM SH, CARCILLO JA, SZABO C, CLARK RS. Intra-mitochondrial poly(ADP-ribosylation) contributes to NAD+ depletion and cell death induced by oxidative stress. Journal of Biological Chemistry. 2003;278(20):18426–33. doi: 10.1074/jbc.M301295200. [DOI] [PubMed] [Google Scholar]
- 62.BOGAERT YE, SHEU KF, HOF PR, BROWN AM, BLASS JP, ROSENTHAL RE, FISKUM G. Neuronal subclass-selective loss of pyruvate dehydrogenase immunoreactivity following canine cardiac arrest and resuscitation. Exp. Neurol. 2000;161:115–126. doi: 10.1006/exnr.1999.7250. [DOI] [PubMed] [Google Scholar]
- 63.ROSENTHAL RE, WILLIAMS R, BOGAERT YE, GETSON PR, FISKUM G. Prevention of postischemic canine neurological injury through potentiation of brain energy metabolism by acetyl-L-carnitine. Stroke. 1992;23:1312–1317. doi: 10.1161/01.str.23.9.1312. [DOI] [PubMed] [Google Scholar]
- 64.MARTIN E, ROSENTHAL RE, FISKUM G. Pyruvate dehydrogenase complex: Metabolic link to ischemic brain injury and target of oxidative stress. J Neurosci Res. 2004;79:240–247. doi: 10.1002/jnr.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LIU Y, ROSENTHAL RE, HAYWOOD Y, MILJKOVIC-LOLIC M, VANDERHOEK JY, FISKUM G. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke. 1998;29:1679–1686. doi: 10.1161/01.str.29.8.1679. [DOI] [PubMed] [Google Scholar]
- 66.BALAN IS, FISKUM G, HAZELTON J, COTTO-CUMBA C, ROSENTHAL RE. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke. 2006;37(12):3008–13. doi: 10.1161/01.STR.0000248455.73785.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DANILOV CA, FISKUM G. Hyperoxia promotes astrocyte cell death after oxygen and glucose deprivation. Glia. 2008 doi: 10.1002/glia.20655. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ERECINSKA M, SILVER IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir. Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 69.KORZHEVSKII DE, MOUROVETS VO, KOSTKIN VB, IZVARINA N, PERASSO L, GANDOLFO C, LENSMAN M, OTELLIN VA, POLENOV SA, BALESTRINO M. Intracerebroventricular administration of creatine protects against damage by global cerebral ischemia in rat. Brain Res. 2006;1114:187–194. doi: 10.1016/j.brainres.2006.06.103. [DOI] [PubMed] [Google Scholar]
- 70.MAALOUF M, SULLIVAN PG, DAVIS L, KIM DY, RHO JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.GASIOR M, ROGAWSKI MA, HARTMAN AL. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 2006;17:431–439. doi: 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.BONDARENKO A, CHESLER M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001;34:134–142. doi: 10.1002/glia.1048. [DOI] [PubMed] [Google Scholar]