Abstract
Offenders with a history of opioid dependence are a particularly difficult group to treat. A large proportion of offenders typically relapse shortly after release from prison, commit drug-related crimes and then are arrested and eventually re-incarcerated. Previous research demonstrated that oral naltrexone was effective in reducing opioid use and preventing recidivism among offenders under federal supervision. The 111 opioid-dependent offenders in this study were under various levels of supervision that included county and federal probation/parole, a treatment court, an alternative disposition program, and an intermediate punishment program. Subjects were randomly assigned to receive six months of either 300 mg per week of oral naltrexone plus standard psychosocial treatment as usual (n=56) or standard psychosocial treatment as usual (TAU) without naltrexone (n=55). While the TAU subjects who remained in treatment used more opioids than the naltrexone subjects who remained, the high drop out rate for both groups made it difficult to assess the effectiveness of naltrexone. The study provides limited support for the use of oral naltrexone for offenders who are not closely monitored by the criminal justice system.
Introduction
Treatment for opioid-dependent criminal justice clients has largely been abstinence oriented even though drug-free counseling for these individuals has failed to demonstrate efficacy.1-6 Kinlock et al.3 found that offenders who received methadone while incarcerated or immediately upon release from prison used less opioids than those who received only counseling. Despite the success rate of agonist treatments such as methadone and buprenorphine,7 relatively few probationers/parolees receive these medications. The criminal justice system generally has not been favorable to these treatments because they produce opioid effects similar to heroin and they have the potential for abuse and diversion.4,8 Moreover, many clients encounter barriers to methadone therapy including the restrictions of daily dosing regimens and the fear of detoxification from methadone.9
An alternative treatment is antagonist maintenance with naltrexone. Naltrexone blocks the intoxicating and reinforcing effects of opioids, yet naltrexone has virtually no psychotropic or euphoric effects and is not an addictive drug.5,10,11 When taken regularly, naltrexone extinguishes opioid-taking behavior.5
Among non-offenders, clinical trials have shown the beneficial effects of naltrexone while patients remain in treatment.12-16 However, patients are often reluctant to initiate the medication and, once prescribed, compliance with naltrexone has been a problem.15,17-20 For example, in order to begin naltrexone therapy the patient must be opioid-free and many dependent individuals are unwilling or unable to detoxify. In addition, unlike agonist treatments, there is no physical dependence with naltrexone, therefore patients can drop out of treatment, stop taking the medication and experience no withdrawal effects. Greenstein et al 17 demonstrated that at least 30 days of naltrexone was needed for improvement in treatment outcomes.
Highly motivated individuals, on the other hand, may be more compliant with treatment and hence benefit from the medication. Naltrexone has shown favorable results in uncontrolled studies of “white collar” opioid-dependent substance abusers such as physicians, lawyers, and nurses who face external pressure to complete treatment or risk losing their license.21-25 While opioid-dependent parolees represent a different kind of group, they may be similarly motivated to take naltrexone and remain opioid-free or risk re-incarceration. Since few professionals in charge of the care of probationers/parolees are aware of naltrexone very few offenders have an opportunity to receive the medication.4
A pilot study conducted by our group with 51 federal probationers or parolees with a history of heroin addiction showed efficacy for oral naltrexone treatment.26 After six months of treatment, participants randomly assigned to naltrexone had a re-incarceration rate (26%) less than half that of those in the control group who just received psychosocial treatment as usual (56%). The rate of opioid positive urines was also significantly less in the naltrexone (8%) compared to the control group (30%). Although retention was greater for the naltrexone subjects, the difference was not statistically significant. Overall, there were few side effects reported and there were actually higher levels of distress reported by the control group than among naltrexone subjects.
Given the above findings, we were encouraged to undertake a larger investigation of the efficacy of oral naltrexone in opioid-dependent offenders. The current study compares oral naltrexone to treatment as usual (TAU) among a larger, more diverse sample of offenders than in the prior study of federal probationers/paroles. This study of 111 subjects included federal and county criminal justice clients as well as those assigned to an alternative disposition program, an intermediate punishment program, and a treatment court. It was hypothesized that participants randomly assigned to receive oral naltrexone will have fewer opioid positive urines, commit less crime and will be more likely to complete the six-month treatment protocol than those randomly assigned to the TAU group.
Methods
Subjects
The participants in this study were 111 opioid-dependent individuals who were under legal supervision in the community. All subjects were enrolled in drug treatment for at least six months at either the intensive outpatient program (IOP) offered by the research study or were treated at one of several community-based IOPs.
Subjects were eligible for participation if they: (1) signed an informed consent form agreeing to randomization to one of the two treatment groups; (2) were between the ages of 18 and 55; (3) had a diagnosis of opioid dependence based on DSM-IV criteria and a structured psychiatric interview; (4) were in good general health as determined by a complete physical examination and laboratory tests; (5) had been assigned to probation/parole for a minimum of six months; and (6) had a negative result for urinary opioids and reported being at least 3 days opioid-free prior to randomization.
Subjects were excluded if they had: (1) current severe alcohol dependence that required medical supervision for alcohol withdrawal symptoms; (2) current psychosis, dementia, mental retardation, or history of schizophrenia; (3) clinically significant abnormalities in hematology, chemistry, or urinalysis; (4) clinically significant cardiovascular, neurological, hepatic, renal, pulmonary, metabolic, endocrine, or gastrointestinal disorders; (5) a diagnosis of chronic pain disorder; or (6) taken an opioid antagonist within the prior 6 months. Female subjects who were pregnant or lactating, or women of childbearing potential who were not using birth control were also excluded.
Study Recruitment
Participants were recruited from various sources. Referrals were made by county and federal probation/parole officers, a drug treatment court, the public defenders office, community-based IOPs and two inpatient programs. Referrals were also obtained from an alternative disposition program that offered early parole, if participants agreed to attend a mandated substance abuse treatment program, and an intermediate punishment program (IPP) that provided offenders the option of attending mandated treatment in lieu of incarceration.
Procedures
The project was approved by the Institution Review Boards of the University of Pennsylvania and the City of Philadelphia. The research was also approved by an administrative board consisting of the Chief Federal District Court Judge in Philadelphia and by a research review committee of one of the inpatient hospital programs. Interested participants were given a full description of the study by research staff. Special efforts were made to assure offenders that participation in the study was voluntary. Individuals were instructed that the research study was an additional service they could receive and that choosing to participate or not participate would have no effect on their probation/parole status and would not affect the duration of their probation/parole. Moreover, they could stop participation in this study at any time without any effect on their probation/parole, treatment or services. Subjects who agreed to participate in the study signed an informed consent document at the point of entry into the trial.
Enrolled subjects completed a two to three day screening process to determine eligibility into the study. Physical exams and laboratory tests were assessed to ensure that each subject was in good general health and had normal hepatic function. The Structured Clinical Interview for DSM-IV Axis-I (SCID)27 was administered to ensure each subject had a diagnosis of opioid dependence and to rule out any severe psychiatric disorders. During the screening process participants also completed a series of baseline research assessments. In order to enter the study, all subjects had to self-report that they had not used opioids for three days and had to provide a negative urine result for opioids immediately prior to randomization. Some participants who were unable to provide an opioid-free urine were referred to inpatient detoxification and returned to the study after having successfully completed detoxification. Candidates who passed these evaluations and provided a negative urine result, successfully passed this screening process and were randomly assigned to one of two treatment conditions (naltrexone + TAU or TAU only).
Randomization was balanced using six prognostic variables: gender, current marital status (yes/no), comorbid current alcohol abuse or dependence, comorbid current cocaine abuse or dependence, previous arrests and criminal charges (≤ 5 vs. >5), and previous drug treatments other than self-help groups and detoxification only (≤ 3 vs. > 3). The point of randomization of each subject was the actual starting point for the study. All subsequent scheduled events were calculated from the point of randomization. Subjects were assessed at baseline, twice weekly during the six-month treatment phase and then at six months post-treatment entry.
Participants in both groups were administered a standard side effects questionnaire that assessed any adverse events. Retention in the study was determined by the number of weeks participants remained in compliance with the protocol. Subjects who missed three consecutive weeks were dropped from the study.
Treatment Conditions
Psychosocial Treatment Only (TAU Group)
Participants in the TAU group received six months of psychosocial treatment at one of several community-based treatment programs or at the university-based IOP provided by the research study. The university-based psychosocial treatment consisted of three hours of group therapy, one hour of individual therapy, and one hour of case management for six weeks of intensive outpatient treatment followed by 20 weeks of outpatient treatment (OP) consisting of one hour of individual and one hour of case management per week. The psychosocial therapy provided by the community-based programs was similar in content, but typically included additional hours of group therapy during the IOP phase.
Subjects in the TAU group did not receive a placebo. The rationale for this decision was based on our clinical experience with naltrexone and with placebo-controlled studies involving antagonist medications. It has been our experience that subjects on naltrexone will test the efficacy of the naltrexone one or more times during the early phase of treatment by using opioids and thus break the blind.17,18,28 Moreover, unlike placebo-controlled trials of other medications such as anti-depressants, the subject is easily able to determine if the study medication is active or inactive simply by using an opioid. We did not want subjects to test the study medication and increase the possibility of re-addiction for those receiving placebo. In addition, permission to study a criminal justice population is a very sensitive issue and the court system made it clear that the use of a placebo would be unacceptable. Therefore, for both ethical and practical reasons, our TAU group was not blinded and consisted of psychosocial treatment without additional medication. A total of 55 subjects were assigned to the TAU condition.
Oral Naltrexone Plus Psychosocial Treatment
Subjects randomized to naltrexone received a challenge test consisting of 0.8 mg of naloxone administered intravenously or intramuscularly followed by a 20-mininute observation period during which the subject was evaluated for signs and symptoms of opioid withdrawal. Subjects who failed the naloxone challenge test were re-evaluated to determine if they required additional days of abstinence prior to re-challenge or were referred to a substance abuse detoxification program. Relatively few subjects failed the challenge since all participants had to be opioid-free prior to randomization. Subjects who successfully passed the naloxone challenge test were started immediately on naltrexone. The initial naltrexone dose was 25 mg. During the first week, subjects returned for two more visits, and on the second visit the dose was increased to 50 mg and on the third visit the dose was 100 mg. Beginning in the second week, subjects receive 150 mg of naltrexone twice a week (300 mg per week) for a total of 26 weeks or six months. The dispensing of all medication was directly observed. Participants in the naltrexone group received the same six months of psychosocial treatment as the TAU condition. Fifty-six participants received oral naltrexone plus TAU.
Research Assessments
The instruments listed below were administered in order to obtain measurements of substance abuse, comorbid psychiatric disorders, psychosocial functioning, human immunovirus (HIV) risk behaviors, depression, antisociality, and criminal behavior. Both groups received the same schedule of assessments and were compensated for completing research paperwork at each evaluation time point.
The Addiction Severity Index (ASI) was administered by a trained technician at baseline to assess lifetime and recent (past 30 days) functioning in seven potential problem areas: medical, employment/economic, drug use, alcohol use, legal, family/social and psychiatric.29-31 Composite scores (CS) computed in each of the seven areas provided an indication of overall problem severity. The ASI also yields relevant sociodemographic information. The abbreviated, follow-up version of the ASI was administered six months after treatment entry.
The Structured Clinical Interview for DSM-IV (SCID) was administered during screening to exclude subjects with severe psychiatric disorders and to verify an opioid substance dependence diagnosis.27,32 The Beck Depression Inventory (BDI) was administered at baseline to assess level of depression and measure potential effects of depression on treatment outcome.33
The California Psychological Inventory-Socialization Scale (CPI-So) is a self-report inventory that was administered as an independent instrument (i.e., unembedded in the larger CPI) at the baseline assessment.34-38 The socialization scale is a measure of socialization, social judgment, and normative behaviors during childhood and adolescence that yields one summary measure of a disposition to antisociality. Each of the 46 items is rated true or false (range 0-46) with lower scores reflecting poorer social judgment, less empathy, and less conformance with social norms. A score of 22 and below was considered diagnostic of severe problems in rule-following and norm-accepting behavior.39
The Risk Assessment Battery (RAB) is a self-report measure that was used at baseline and six months to assess both sex and drug risk factors associated with HIV acquisition and transmission such as needle sharing and unsafe sexual practices.40-41
With subject’s informed consent, criminal records data were obtained from the City of Philadelphia and the Pennsylvania Commission on Crime and Delinquency. These data included the total number and type of offenses a participant was charged with prior to and after randomization. Self-report measures of criminal activities from the legal section of the ASI that included months incarcerated in lifetime, days of illegal activity in the last month, and parole/probation status were also obtained.
Monitored urine drug screens were collected by research staff and were analyzed using the Enzyme Multiplied Immunoassay Technique (EMIT) system in our urine toxicology lab. Five drugs were tested: opioids, cocaine, methadone, benzodiazepines, and cannabis. Urine drug screens were collected at baseline, twice weekly during the six-month treatment phase (to coincide with the naltrexone dosing schedule) and at the six-month follow-up.
Location
Subjects who were assigned to the medication group received their naltrexone dose at the research offices at the University of Pennsylvania. Psychosocial treatment for both the TAU and naltrexone groups were provided at either the university research office for those who received treatment as part of the research program, or at the community-based clinic that they were attending. All urine specimens and research data were collected at the research offices at the University of Pennsylvania.
Statistical Analysis
Baseline comparisons between the randomization groups used t-tests for continuous measures and chi-square tests for binary measures. Logistic regression models were used to predict treatment completion from baseline characteristics. Cox Proportional Hazards regression models were used to predict time to dropout from treatment.
Our primary comparisons were on the rates of opioid use across the two groups. Rates of opioid positive urines were compared across the two groups using Generalized Estimating Equations (GEE) methods for repeated binary outcomes. The GEE approach to comparing two sets of repeated measures assumes a ‘working’ correlation matrix for the within subject correlations across time, and yields regression coefficients that are valid even if that assumption is incorrect. Thus, the methods are robust to misspecification of the within subject correlations. The primary factor in these GEE models was treatment group assignment, and the model also included time trends, and some group by time interactions.
There was a great deal of missing data in this trial. While the GEE models are insensitive to model misspecification of the within subject correlation, they may be sensitive to drop out that is related to treatment outcomes. As a result, the conclusions of the GEE models must be very sensitive to what is assumed about the missing data. To determine sensitivity of the results to the high rates of missing data, we performed three versions of the GEE analyses:
First, we assumed that the missing data are ignorable, which in the context of our GEE models means that we assumed that they were missing completely at random. This is a stronger assumption than simply assuming that the missing data can be predicted from observed variables and prior outcomes, which are referred to as missing at random. In our initial analyses, we found no significant predictors of completion or of time to drop out (except for age) or first use, so the distinction between the two types of missing at random is of no practical importance for this dataset.
Our second analysis imputed missing urine drug screen responses as being positive for opioid use. This is a common strategy in analyzing data from substance abuse studies, and can be regarded as defining a new outcome variable: a treatment visit is defined to be successful if a patient makes the visit and provides an opioid negative urine, otherwise the visit is regarded as a failure. Thus, our second analysis can be regarded as a GEE analysis of the repeated binary outcomes indicating successful visit, where our definition of response yields complete data for all subjects.
Our third strategy used a pattern mixture approach42 and extended the first analysis approach by including variables describing the dropout process as main effects and as interactions with treatment group in the model. A significant interaction between treatment and these variables would indicate that treatment efficacy was different at different levels of dropout, which would suggest that the missing visits are non-ignorable, and would yield different inference for completers compared to non-completers.
Results
Baseline Data
The 111 research subjects reported an average age of 34 years and about 11 years of education (Table 1). The majority of participants (82%) were male; nearly one-half (47%) were Caucasian, 26% were African American, and 27% were Hispanic. Twenty-three percent of subjects were employed at least one day in the month prior to the baseline interview and one quarter (25%) were married.
Table 1.
Characteristics of the Sample at Baseline
| Naltrexone (n=56) | TAU (n=55) | Total (N=111) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Average Age | 33.1 | 33.9 | 33.5 | ns |
| Average Education | 11.3 | 11.2 | 11.2 | ns |
| % Male | 82 | 82 | 82 | ns |
| Race | ns | |||
| % African American | 20 | 33 | 26 | |
| % Caucasian | 54 | 40 | 47 | |
| % Hispanic | 27 | 27 | 27 | |
| % Employed | 28 | 19 | 23 | ns |
| % Married | 24 | 27 | 25 | ns |
| Drug Use/Treatment | ||||
| Average Years of Lifetime Use | ||||
| Heroin | 6.3 | 9.0 | 7.7 | .059 |
| Other opioids | 1.5 | 1.7 | 1.6 | ns |
| Cocaine | 3.6 | 3.2 | 3.4 | ns |
| Alcohol | 5.8 | 5.6 | 5.7 | ns |
| Average Use Last 30 Days | ||||
| Heroin | 5.8 | 1.9 | 3.8 | .010 |
| Other opioids | 0.8 | 0.4 | 0.6 | ns |
| Cocaine | 1.7 | 0.1 | 0.9 | .051 |
| Alcohol | 1.4 | 0.5 | 0.9 | ns |
| Average # of Lifetime Drug Treatments Episodes | 5.3 | 5.5 | 5.4 | ns |
| Prior Charges | ||||
| % Drug charges | 86 | 92 | 89 | ns |
| % Parole/probation violations | 65 | 77 | 71 | ns |
| % Shoplifting/vandalism | 26 | 25 | 25 | ns |
| % Assault | 24 | 25 | 24 | ns |
| % Robbery | 22 | 19 | 20 | ns |
| % Burglary, larceny, breaking & entering | 16 | 23 | 19 | ns |
| % Weapons offenses | 20 | 17 | 18 | ns |
| % Contempt of court | 4 | 17 | 11 | .028 |
| % Prostitution | 8 | 10 | 9 | ns |
| % Forgery | 8 | 6 | 7 | ns |
| % Arson | 0 | 6 | 3 | ns |
| % Homicide, manslaughter | 0 | 2 | 1 | ns |
| % Other | 16 | 8 | 12 | ns |
| Average # Prior Charges | 7.6 | 10.1 | 8.9 | ns |
| Average # Prior Convictions | 4.8 | 5.2 | 5.0 | ns |
| HIV Risk Behaviors Past 6 Months | ||||
| % IV Drug Users | 25 | 28 | 26 | ns |
| % Shared Needles | 4 | 6 | 5 | ns |
| % Not Using Condoms | 33 | 36 | 34 | ns |
| Average Beck Score (BDI) | 10.6 | 9.7 | 10.1 | ns |
| California Psychological Inventory Socialization Score (CPI-So): % ≤ 22 | 58 | 64 | 61 | ns |
Participants reported using heroin regularly for nearly eight years and in the 30 days prior to the interview had used an average of 3.8 days. On average, subjects reported using other opiates less than one day in the last 30 days and 1.6 years of regular use. About six years of regular alcohol use and three years of regular cocaine use were also reported. Participants reported a history of about five prior drug treatment episodes.
As Table 1 demonstrates, the majority of prior criminal charges reported on the ASI were for drug offenses (89%) followed by parole violations (71%). Analysis of criminal records data showed similar findings with regard to drug charges (90%) and 56% of participants had prior parole violations. Criminal records data showed an average of 28.5 prior charges (24.4 naltrexone vs. 32.8 TAU) compared to the average of about nine prior charges reported on the ASI (7.6 naltrexone vs. 10.1 TAU).
Approximately one-quarter of the subjects (26%) reported intravenous (IV) drug use, 5% shared needles and about one-third (34%) did not use a condom in the six months prior to the baseline interview.
The average BDI score was 10.1, indicating mild depression and 61% of the subjects scored 22 or below on the CPI-So scale demonstrating that the majority of subjects reported significant child/adolescent problems with norm compliance behaviors.
Whereas subjects in the naltrexone condition reported more days of heroin (F=6.9, df=102, p=.010) and cocaine (F=3.9, df=102, p=.051) use in the last 30 days, there was a trend for the TAU group to report a longer history of heroin use (F=3.6, df=102, p=.059). The TAU group also reported more prior contempt of court charges on the ASI compared to the naltrexone group (χ2=4.8, df=1, p=.028). There were no other significant differences between participants in the two treatment conditions at baseline including no differences in ASI composite scores or prior criminal records.
Referral Sources
One-third of the subjects (33%) were referred to the study from one of two inpatient programs, 21% were from a drug treatment court, 12% were alternative sentencing referrals, 11% were from an intermediate punishment program (IPP), 10% were federal referrals, 7% were from Philadelphia County probation/parole and the remaining 7% were from other sources (Table 2). There were no group differences in referral source.
Table 2.
Referral Sources*
| Naltrexone (n=56) | TAU (n=55) | Total (N=111) | |
|---|---|---|---|
| % | % | % | |
| Inpatient Treatment | 36 | 31 | 33 |
| Treatment Court | 25 | 16 | 21 |
| Alternative Disposition Program | 7 | 16 | 12 |
| Intermediate Punishment Program (IPP) | 9 | 13 | 11 |
| Federal Probation/Parole | 7 | 13 | 10 |
| Philadelphia County Probation/Parole | 7 | 7 | 7 |
| Outpatient Drug Treatment | 5 | 0 | 3 |
| Public Defenders | 2 | 2 | 2 |
| Other | 2 | 2 | 2 |
No significant group differences.
Treatment Completion and Attendance
Figure 1 shows the available subjects in each group at each visit. The high dropout in the first week is apparent. About one-third (32%) of naltrexone subjects competed the six months of treatment compared to 29% of TAU subjects. On average, the naltrexone group completed 101.4 days of treatment compared to 109.2 days for the TAU group. There were no significant group differences in treatment completion or attendance. Sixty-three percent of subjects were treated by the university-based IOP compared to 37% who were treated by community-based IOPs. There were no significant differences in treatment completion by type of IOP. However, there was a significant difference with treatment court referrals. Specifically, 57% of the 14 naltrexone subjects who were participating in treatment court completed the six months of treatment compared to the none of the nine treatment court participants randomly assigned to the TAU group (χ2=7.9, df=1, p=.005).
Figure 1.
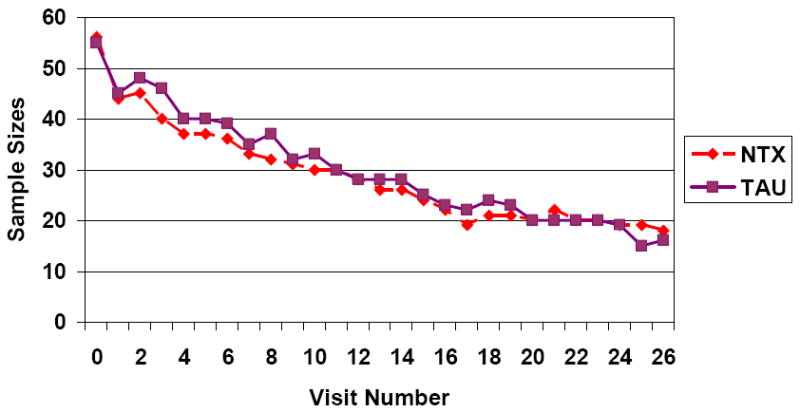
Sample Sizes at each Visit
Correlates of Treatment Completion
Those who completed the six months of treatment were compared to non-completers on demographic characteristics, drug use, inpatient treatment prior to randomization, prior charges, HIV risk behaviors, BDI and CPI-So scores (Table 3). Older subjects were more likely to complete treatment than were younger subjects (F=6.0, df=102, p=.016). While it appears that African American and Hispanic subjects were more likely to complete treatment compared to Caucasian (χ2=4.1, df=1, p=.042), this difference may actually be due to age since the Caucasian subjects were significantly younger than were the African Americans and Hispanics (F=18.3, df=102, p <.001). The older age of the completers may also explain the longer history of heroin use (F=4.6, df=102, p=.035) and greater number of prior drug charges (χ2=5.8, df=1, p=.016) among the completers compared to non-completers. Subjects who completed treatment also reported fewer days of alcohol use at baseline than did non-completers (F=3.8, df=102, p=.054). Finally, there was a trend for a greater proportion of non-completers (67%) to score 22 or below on the CPI-So scale than completers (48%) (χ2=2.9, df=1, p=.087).
Table 3.
Treatment Completion By Baseline Characteristics
| Completers (n=34) | Non-completers (n=77) | Total (N=111) | p value | |
|---|---|---|---|---|
| Demographics | ||||
| Average Age | 36.8 | 32.0 | 33.5 | .016 |
| Average Education | 11.4 | 11.2 | 11.2 | ns |
| % Male | 77 | 84 | 82 | ns |
| Race | ||||
| % Caucasian | 32 | 53 | 47 | .042 |
| % Non-Caucasian | 68 | 47 | 53 | |
| % Employed | 21 | 24 | 23 | ns |
| % Married | 36 | 20 | 25 | ns |
| Drug Use/Treatment | ||||
| Average Years of Lifetime Use | ||||
| Heroin | 9.9 | 6.6 | 7.7 | .035 |
| Other opioids | 1.3 | 1.7 | 1.6 | ns |
| Cocaine | 2.9 | 3.6 | 3.4 | ns |
| Alcohol | 6.0 | 5.5 | 5.7 | ns |
| Average Use Last 30 Days | ||||
| Heroin | 3.2 | 4.1 | 3.8 | ns |
| Other opioids | 0.5 | 0.7 | 0.6 | ns |
| Cocaine | 0.1 | 1.2 | 0.9 | ns |
| Alcohol | 0.1 | 1.3 | 0.9 | .054 |
| Average # of Lifetime Drug Treatments Episodes | 6.7 | 4.8 | 5.4 | .ns |
| % Prior Inpatient | 32 | 34 | 33 | ns |
| Prior Charges | ||||
| % Drug charges | 100 | 84 | 89 | .016 |
| % Parole/probation violations | 73 | 70 | 71 | ns |
| % Shoplifting/vandalism | 27 | 24 | 25 | ns |
| % Assault | 18 | 27 | 24 | ns |
| % Robbery | 21 | 20 | 20 | ns |
| % Burglary, larceny, breaking & entering | 21 | 19 | 19 | ns |
| % Weapons offenses | 18 | 19 | 18 | ns |
| % Contempt of court | 12 | 10 | 11 | ns |
| % Prostitution | 12 | 7 | 9 | ns |
| % Forgery | 0 | 10 | 7 | ns |
| % Arson | 6 | 1 | 3 | ns |
| % Homicide, manslaughter | 0 | 1 | 1 | ns |
| % Other | 3 | 16 | 12 | ns |
| Average # Prior Charges | 9.3 | 8.6 | 8.9 | ns |
| Average # Prior Convictions | 5.5 | 4.8 | 5.0 | ns |
| HIV Risk Behaviors Past 6 Months | ||||
| % IV Drug Users | 22 | 28 | 26 | ns |
| % Shared Needles | 6 | 4 | 5 | ns |
| % Not Using Condoms | 41 | 31 | 34 | ns |
| Average Beck Score (BDI) | 9.4 | 10.4 | 10.1 | ns |
| California Psychological Inventory Socialization Score (CPI-So): % ≤ 22 | 48 | 67 | 61 | .087 |
Predictors of Treatment Completion
All variables that were independently related to treatment completion (p < .10) were then entered into a logistic regression model that included group assignment to determine the contribution of factors predicting treatment completion. These nine variables included age; race; lifetime heroin use; recent use of heroin, cocaine and alcohol; CPI-So score; treatment court referral; and group assignment. The model found that none of these variables were predictive of treatment completion (Table 4). We also conducted a survival analysis examining time to drop out or time to first use and found no statistically significant group differences. Older participants were retained in treatment longer and there was a trend for females to be more likely to complete treatment.
Table 4.
Predictors of Treatment Completion
| Variable | Odds Ratio | 95% CI | p value |
|---|---|---|---|
| Age | 1.0 | 1.0-1.1 | .203 |
| Race | |||
| Non-Caucasian | 1.5 | 0.5-4.9 | .470 |
| Caucasian | 1.0 | ||
| Lifetime heroin use | 1.0 | 0.9-1.1 | .868 |
| Heroin use last 30 days | 1.0 | 0.9-1.1 | .646 |
| Cocaine use last 30 days | 1.0 | 0.4-2.7 | .993 |
| Alcohol use last 30 days | 0.5 | 0.2-1.3 | .137 |
| CPI-So Score | |||
| > 23 | 2.1 | 0.7-6.3 | .199 |
| ≤ 22 | 1.0 | ||
| Treatment Court | |||
| Yes | 1.4 | 0.4-5.3 | .602 |
| No | 1.0 | ||
| Group | |||
| Naltrexone | 1.5 | 0.5-4.5 | .464 |
| TAU | 1.0 |
N=83; model χ2=16.8, df=9, p=.051.
Repeated Measures of Urine Drug Screen Results
Analysis 1: Missing Data Ignored
Figure 2 shows the group proportions using over time, when missing data are ignored. It is clear that the rate of opioid positive urines was practically zero in the naltrexone group, while the TAU group fluctuates between about a 5% rate and a 15% rate for the middle part of the treatment period.
Figure 2.
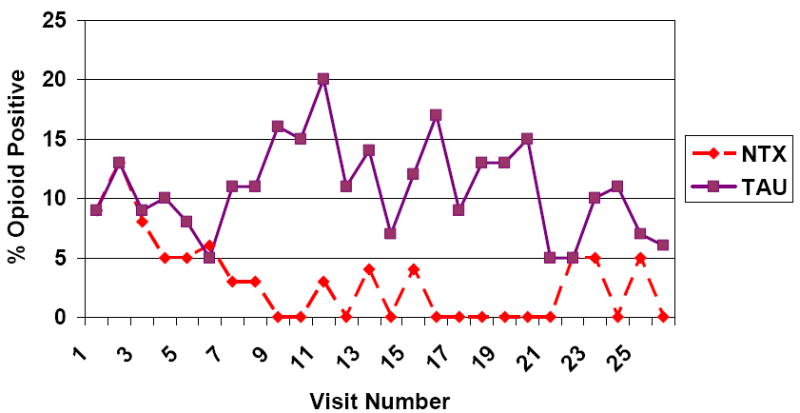
UDS Rates of Opioid Use: Missing UDS ignored
The corresponding GEE analyses showed significant group by linear (Wald c2(1)=7.18, p = 0.01) and group by quadratic (Wald c2(1)=3.92, p = 0.05) time effects. Within-timepoint contrasts showed that the groups were not significantly different during the first few weeks of the study, or during the last four weeks. Between weeks 4 and 20, the naltrexone group had significantly lower use than the TAU group.
Analysis 2 - Missing Data Imputed as Positive for Opioid Use
Figure 3 shows the group proportions using over time, when missing data are imputed as positive for opioid use. The two groups vary between rates of about 15% and 50%, with the naltrexone group having slightly lower rates of use (between 5% and 10% lower) for the later weeks of the treatment period.
Figure 3.

UDS Rates of Opioid Use: Missing UDS imputed as positive for opioid use
The corresponding GEE analyses showed no significant effects. This reflects the fact that drop out rates were large and comparable between the two groups, so that the difference in rates of positive urine drug screens among available urine drug screen tests was overwhelmed by the number of missed visits imputed as opioid positive.
Analysis 3 – Pattern Mixture Model
We included a binary indicator of completer (versus non-completer) as a pattern mixture variable. Quadratic trends were not significant, so we reduced the model allowing intercept and linear time trends to vary across the four treatment by completer groups. There was a significant interaction between completer status and treatment group (Wald c2(1)=5.20, p = 0.02), suggesting that the effects of naltrexone relative to TAU varied with drop out status, and that the missing data are not ignorable.
Figure 4 shows the rates of opioid use for the four groups defined by medication and completer status. The figure should be interpreted with caution, as the sample sizes diminish to zero in the two non-completer groups. For example, the 60% rate of positive urines in the TAU:Dropout group at week 20 reflects the fact that three of the remaining five people in that group gave positive urine tests at that week.
Figure 4.
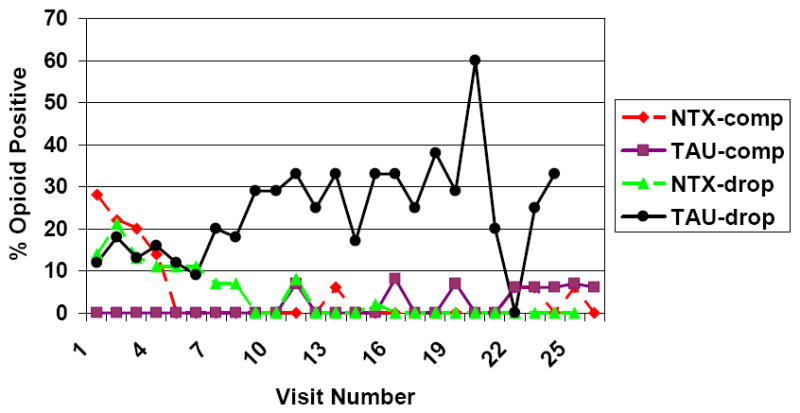
UDS Rates of Opioid Use for Medication and Completion groups: Missing ignored
Note: The decrease in the rates of use in the naltrexone non-completer group was due to users dropping out, rather than to individuals decreasing their use.
However, the figure illustrates the nature of the significant interactions, and also provides insight into the results of the study. The two groups of completers had practically no opioid positive urines through the entire treatment period. Non-completers in the naltrexone and TAU groups had similar rates of use early in the study. As the study progressed, the rates of use in the naltrexone non-completers diminished, while those in the TAU non-completers stayed at about 25% to 20%. More detailed analysis suggested that the decrease in the rates of use in the naltrexone non-completer group was due to users dropping out, rather than to individuals decreasing their use.
Six-Month Outcomes
Table 5 displays the six-month outcomes (six months after study entry) by treatment condition.
Table 5.
Six-Month Outcomes
| Naltrexone (n=31) | TAU (n=32) | Total (N=63) | p value | |
|---|---|---|---|---|
| % Positive Urine Drug Screens | ||||
| Opioids | 18 | 27 | 22 | ns |
| Cocaine | 18 | 8 | 13 | ns |
| Average Use Last 30 Days | ||||
| Heroin | 4.0 | 2.7 | 3.3 | ns |
| Other opioids | 0.4 | 0.4 | 0.4 | ns |
| Cocaine | 0.3 | 1.0 | 0.6 | ns |
| Alcohol | 0.6 | 0.5 | 0.6 | ns |
| Post-Baseline Charges/Incarcerations | ||||
| % Drug charges | 10 | 3 | 7 | ns |
| % Parole/probation violations | 7 | 27 | 17 | .043 |
| % Shoplifting/vandalism | 0 | 7 | 3 | ns |
| % Assault | 0 | 0 | 0 | ns |
| % Robbery | 0 | 0 | 0 | ns |
| % Burglary, larceny, breaking & entering | 0 | 3 | 2 | ns |
| % Weapons offenses | 0 | 0 | 0 | ns |
| % Contempt of court | 0 | 3 | 2 | ns |
| % Prostitution | 3 | 0 | 2 | ns |
| % Forgery | 0 | 3 | 2 | ns |
| % Arson | 0 | 0 | 0 | ns |
| % Homicide, manslaughter | 0 | 0 | 0 | ns |
| % Other | 0 | 0 | 0 | ns |
| Average # Charges | 0.2 | 0.5 | 0.4 | ns |
| Average # Convictions | 0.1 | 0.1 | 0.1 | ns |
| Average # Months Incarcerated | 0.5 | 0.8 | 0.7 | ns |
| % Employed | 66 | 52 | 58 | ns |
| HIV Risk Behaviors Past 6 Months | ||||
| % IV Drug Use | 14 | 22 | 18 | ns |
| % Shared Needles | 7 | 16 | 12 | ns |
| % Not Use Condoms | 43 | 22 | 32 | ns |
Six-Month Follow-up Rate
A total of 63 out of 111 (57%) subjects completed a six-month follow-up evaluation; 55% of the naltrexone group completed the six-month follow-up compared to 58% of the TAU group. Of the participants who did not complete follow-up, 63% could not be located and another 21% were either incarcerated or were receiving inpatient treatment and could not be interviewed. There were no significant group differences in six-month follow-up rates. Criminal records data were obtained for 106 out of the 111 (95%) participants.
Drug Use
At the six-month follow-up, 22% of subjects had positive urine drug screen results for opioids and participants reported using an average of three days of heroin and less than 1 day of other opioids in the last month. There were no differences in self-reported opioid use or urine drug screen results for opioids between the two treatment conditions.
Criminal Behavior
In terms of self-reported criminal activity, the TAU group (27%) reported more parole violations at six months compared to the naltrexone group (7%) (χ2=4.1, df=1, p=043). Criminal records data were also analyzed, but showed no differences between the two treatment conditions in parole violations, drug charges or total number of charges post-randomization. Crime records showed an average of 4.3 charges post-randomization compared to 0.4 charges reported on the ASI.
Employment
While 66% of the naltrexone group was employed at six months compared to 52% of the TAU group, this difference was not statistically significant. However, there was a significant improvement in total days paid for working (F=11.9, df=1/54, p=.001) and employment income (F=15.5, df=1/48, p <.001) for both groups from baseline to six months.
HIV Risk Behaviors
At six months, 18% of respondents reported IV drug use, 12% shared needles and about one-third (32%) did not use a condom in the past six months. There were no significant differences between the two treatment conditions in drug, sex or total HIV risk behaviors at the six-month follow-up.
Discussion
The urine drug screen results demonstrate that there is limited support for the use of oral naltrexone in the context of opioid-dependent parolee populations. The very high dropout rate from the study makes it difficult to interpret the results, as three different approaches to handling missing data yield three different sets of conclusions. It seems clear that the TAU participants who remained in treatment used more opioids than the naltrexone group who remained, but it is difficult to separate the effect of naltrexone versus TAU from the selection effects of the dropout process.
Contrary to the earlier pilot study of federal probationers,26 the findings from this current study conducted among a larger sample of more diverse offenders did not find any differences in criminal behavior between the naltrexone and TAU groups. Although it did show some support for less opioid use among the naltrexone completers, this finding was not consistent due to the high drop out rate. The major difference between the two studies lies in the supervision available to all participants in the original federal study. One of the federal parole officers was so enthusiastic about the study that he became a member of the team. Supervision and follow-up was much closer in that setting than in the more diverse settings of city and federal programs five to ten years later. The results of this second study have convinced us that in order to be successful, oral naltrexone in probationers and parolees requires more supervision than is typically available in the criminal justice system.
The one setting in which the naltrexone group did perform better than TAU in terms of treatment completion was among subjects who were attending treatment court. While 57% of treatment court participants who received naltrexone completed treatment, none in the TAU group completed. Treatment court participants were more closely monitored compared to offenders in other levels of supervision. Treatment court clients appeared before a judge once a month and urine specimens were typically collected three times a week during IOP treatment. This supports our belief that oral naltrexone might require more criminal justice supervision. However, caution is noted due to the small numbers of treatment court clients and the lack of any significant findings in a survival analysis of time to drop out.
It is anticipated that long-acting injectable or depot naltrexone will be substantially more effective than oral naltrexone despite variation in levels of supervision. This depot formulation can provide effective blood levels for 30 days or more following a single injection. Since the depot injection eliminates the need for daily dosing it increases medication compliance and improves retention, thus reducing the likelihood of relapse. A recently completed pilot study of a six-month treatment protocol of depot naltrexone demonstrated a better retention rate with depot naltrexone and better opioid and recidivism outcomes among treatment completers compared to non-completers. Moreover, while many of the subjects in the oral study dropped out early, nearly 60% of the depot participants completed at least four monthly injections. The use of depot naltrexone, which may improve retention rates in this population, may be a promising alternative to oral naltrexone.
A major limitation of the oral naltrexone study was the low treatment retention and six-month follow-up rates. In general substance abusing offenders are a very difficult group to treat and follow.43-46 Despite our intensive follow-up efforts, most of the respondents (63%) did not return for the follow-up evaluation because they were not able to be located. Some of these individuals had warrants out for their arrest and did not want to be located. Moreover, if they were receiving psychosocial treatment as part of our research study, we were required to report the progress of the offender’s treatment including urine drug screen results to the criminal justice system. In the follow-up phase of the study, no outcome information was conveyed to parole officers. Although we conveyed the confidentiality of the information to the patients, it is likely that those using drugs at follow-up might perceive a risk of re-incarceration and not show for the follow-up interview. It should be noted, that while the treatment retention rates are low, they are comparable to rates found among opioid-dependent patients in outpatient settings.47,48
The findings from this study provide some support to earlier work showing the benefits of naltrexone if patients remain in treatment.12-16 However, due to the low retention rate for both groups, the study does not provide conclusive evidence regarding the efficacy of oral naltrexone in this offender sample. In summary, the findings provide only limited support for use of oral naltrexone among offenders who are not closely monitored by the criminal justice system.
Acknowledgments
The study was supported by grant R01-DA-012268 from the National Institute on Drug Abuse, Bethesda, MD (Dr. Cornish).
The authors would like to thank the following Philadelphia area agencies for their participation in the project: NorthEast Treatment Centers, Eagleville Hospital, Riverside Care Inc., the Federal Probation Department and the City of Philadelphia criminal justice system.
Footnotes
Declaration of Interest In the past 3 years, Dr. O’Brien has served as a consultant on one occasion to Alkermes, a company that makes a version of depot naltrexone. He is also conducting an NIH funded study of this medication in opioid addiction.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Cropsey KL, Villalobos GC, St Clair CL. Pharmacotherapy treatment in substance dependent correctional populations: A review. Subst Use Misuse. 2005;40:1983–1999. doi: 10.1080/10826080500294866. [DOI] [PubMed] [Google Scholar]
- 2.Keen J, Oliver P, Rowse G, Mathers N. Residential rehabilitation for drug users: A review of 13 months’ intake to a therapeutic community. Fam Pract. 2001;18:545–548. doi: 10.1093/fampra/18.5.545. [DOI] [PubMed] [Google Scholar]
- 3.Kinlock TW, Gordon MS, Schwartz RP, O’Grady KO, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: Results at 1-month post-release. Drug Alcohol Depend. 2007;91:220–227. doi: 10.1016/j.drugalcdep.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prendergast ML. Interventions to promote successful re-entry among drug-abusing parolees. Addiction Science and Clinical Practice. 2009;5:4–13. doi: 10.1151/ascp09514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawson RA, Glazer M, Callahan EJ, Liberman RP. Naltrexone and behavior therapy for heroin addiction. NIDA Research Monograph. 1979:26–43. doi: 10.1037/e497382006-005. [DOI] [PubMed] [Google Scholar]
- 6.Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. Lancet. 1999;353:221–226. doi: 10.1016/S0140-6736(98)05356-2. [DOI] [PubMed] [Google Scholar]
- 7.Kreek MJ. Rational for maintenance pharmacotherapy of opiate dependence. In: O’Brien CP, Jaffe JH, editors. Addictive States. New York: Raven Press; 1992. pp. 205–230. [PubMed] [Google Scholar]
- 8.Kinlock TW, Battjes RJ, Schwartz RP. The MTC Project Team. A novel opioid maintenance program for prisoners: Report of post- release outcomes. Am J Drug Alcohol Abuse. 2005;31:433–454. doi: 10.1081/ada-200056804. [DOI] [PubMed] [Google Scholar]
- 9.Coviello DM, Zanis DA, Wesnoski SA, Alterman AI. The effectiveness of outreach case management in re-enrolling discharged methadone patients. Drug Alcohol Depend. 2006;85:56–65. doi: 10.1016/j.drugalcdep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Brahen L, Capone T, Wiechert V, Desiderio D. Naltrexone and cyclazocine. Arch Gen Psychiatry. 1977;34:1181–1184. doi: 10.1001/archpsyc.1977.01770220063007. [DOI] [PubMed] [Google Scholar]
- 11.Brahen L, Henderson R, Capone T, Kordal N. Naltrexone treatment in a jail work-release program. J Clin Psychiatry. 1984;45:49–52. [PubMed] [Google Scholar]
- 12.Greenstein RA, Amdt LC, McLellan AT, O’Brien CP, Evans B. Naltrexone: A clinical perspective. J Clin Psychiatry. 1984;45:25–28. [PubMed] [Google Scholar]
- 13.Judson BA, Camey TM, Goldstein A. Naltrexone treatment of heroin addiction: Efficacy and safety in a double-blind dosage comparison. Drug Alcohol Depend. 1981;7:325–346. doi: 10.1016/0376-8716(81)90049-1. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DC, Mayer J, Hersch RG, Black R. Narcotic antagonist treatment: Clinical experience with naltrexone. Int J Addict. 1978;13:961–973. doi: 10.3109/10826087809039316. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien CP, Greenstein RA, Mintz J, Woody GE. Clinical experience with naltrexone. Am J Drug Alcohol Abuse. 1975;2:365–377. doi: 10.3109/00952997509005662. [DOI] [PubMed] [Google Scholar]
- 16.Shufman EN, Porat S, Witztum E, Gandacu D, Bar-Hamburger R, Ginath Y. The efficacy of naltrexone in preventing reabuse of heroin after detoxification. Biol Psychiatry. 1994;35:935–945. doi: 10.1016/0006-3223(94)91240-8. [DOI] [PubMed] [Google Scholar]
- 17.Greenstein RA, Evans BD, McLellan AT. Predictors of favorable outcome following naltrexone treatment. Drug Alcohol Depend. 1983;12:173–180. doi: 10.1016/0376-8716(83)90042-x. [DOI] [PubMed] [Google Scholar]
- 18.Hollister L. Clinical evaluation of naltrexone treatment of opiate dependence: Report of the NRC committee on clinical evaluation of narcotic antagonists. Arch Gen Psychiatry. 1978;35:335–340. [PubMed] [Google Scholar]
- 19.Judson BA, Goldstein A. Naltrexone treatment of heroin addiction: One-year follow-up. Drug Alcohol Depend. 1984;13:357–365. doi: 10.1016/0376-8716(84)90003-6. [DOI] [PubMed] [Google Scholar]
- 20.Kleber HD, Kosten TR. Naltrexone induction: Psychologic and pharmacologic strategies. J Clin Psychiatry. 1984;45:29–38. [PubMed] [Google Scholar]
- 21.Ling W, Wesson DR. Naltrexone treatment for addicted health-care professionals: A collaborative private practice experience. J Clin Psychiatry. 1984;45:46–48. [PubMed] [Google Scholar]
- 22.O’Brien CP, Woody GE, McLellan AT. A new tool in the treatment of impaired physicians. Philadelphia Medicine. 1986;82:442–446. [Google Scholar]
- 23.Roth A, Hogan I, Farren C. Naltrexone plus group therapy for the treatment of opiate-abusing health-care professionals. J Subst Abuse Treat. 1997;14:19–22. doi: 10.1016/s0740-5472(96)00164-x. [DOI] [PubMed] [Google Scholar]
- 24.Tennant FS, Jr, Rawson RA, Cohen AJ, Mann A. Clinical experience with naltrexone in suburban opioid addicts. J Clin Psychiatry. 1984;45:42–45. [PubMed] [Google Scholar]
- 25.Washton AM, Pottash AC, Gold MS. Naltrexone in addicted business executives and physicians. J Clin Psychiatry. 1984;45:39–41. [PubMed] [Google Scholar]
- 26.Cornish JW, Metzger D, Woody GE, et al. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treat. 1997;14:529–534. doi: 10.1016/s0740-5472(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 27.Spitzer RL, Williams J. Structured Clinical Interview for DSM-III-R. New York, NY: New York State Psychiatric Institute, Biometrics Research Department; 1987. [Google Scholar]
- 28.Resnick RB, Volavka J, Freedman AM, Thomas M. Studies of EN-1639A (naltrexone): A new narcotic antagonist. Am J Psychiatry. 1974;131:646–650. doi: 10.1176/ajp.131.6.646. [DOI] [PubMed] [Google Scholar]
- 29.McLellan AT, Luborsky L, O’Brien CP, Woody GE. An improved diagnostic evaluation instrument for substance abuse patients. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 30.McLellan AT, Luborsky L, Cacciola J, et al. New data from the Addiction Severity Index: Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Woody GE, McLellan AT, Luborsky L, O’Brien CP. 12-month follow-up of psychotherapy for opiate dependence. Am J Psychiatry. 1987;144:590–596. doi: 10.1176/ajp.144.5.590. [DOI] [PubMed] [Google Scholar]
- 32.Williams JB, Gibbon M, First MB, Spitzer RL, Davis M, Borus J. The Structured Clinical Interview for the DSM-III-R (SCID): Multi-site test retest reliability. Arch Gen Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 33.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 34.Gough HG. The California Psychological Inventory administrator’s guide. Palo Alto: Consulting Psychologists Press; 1987. [Google Scholar]
- 35.Gough HG, Bradley P. Delinquent and criminal behavior as assessed by the revised California Psychological Inventory. J Clin Psychol. 1992;48:298–308. doi: 10.1002/1097-4679(199205)48:3<298::aid-jclp2270480306>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 36.Cooney NL, Kadden RM, Litt MD. A comparison of methods for assessing sociopathy in male and female alcoholics. J Stud Alcohol. 1990;51:42–48. doi: 10.15288/jsa.1990.51.42. [DOI] [PubMed] [Google Scholar]
- 37.Alterman AI, McDermott PA, Rutherford M, et al. A typology of antisociality in methadone patients. J Abnorm Psychol. 1998;107:412–422. doi: 10.1037//0021-843x.107.3.412. [DOI] [PubMed] [Google Scholar]
- 38.Kadden RM, Cooney NL, Getter H, Litt MD. Matching alcoholics to coping skills or interactional therapies: Post treatment results. J Consult Clin Psychol. 1989;57:698–704. doi: 10.1037//0022-006x.57.6.698. [DOI] [PubMed] [Google Scholar]
- 39.Kalman D, Longabaugh R, Clifford PR, Beattie M, Maisto SA. Matching alcoholics to treatment: Failure to replicate finding of an earlier study. J Subst Abuse Treat. 2000;9:183–187. doi: 10.1016/s0740-5472(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 40.Metzger DS, Woody GE, Druley P, et al. Psychiatric symptoms, high risk behaviors and HIV positivity among methadone patients. NIDA Research Monograph. 1990;105:490–491. [PubMed] [Google Scholar]
- 41.Metzger DS, Woody GE, McLellan AT, et al. HIV seroconversion among in and out of treatment intravenous drug users: An 18-month prospective follow-up. AIDS. 1993;6:1049–1056. [PubMed] [Google Scholar]
- 42.Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. New York: Springer; 2005. [Google Scholar]
- 43.Dembo R, Williams L, LaVoie L, et al. A longitudinal study of the relationships among alcohol use, marijuana/hashish use, cocaine use, and emotional/psychological functioning problems in a cohort of high-risk youths. Int J Addict. 1990;25:1341–1383. doi: 10.3109/10826089009056224. [DOI] [PubMed] [Google Scholar]
- 44.Dillman D. Mail and Telephone Surveys: The Total Design Method. New York: John Wiley; 1978. [Google Scholar]
- 45.Freeman DM. A note on interviewing Mexican-Americans. Soc Sci Quart. 1998;79:909–918. [Google Scholar]
- 46.Windle M, Miller BA. Problem drinking and depression among DWI offenders: A three-wave longitudinal study. J Consult Clin Psychol. 1990;58:166–174. doi: 10.1037//0022-006x.58.2.166. [DOI] [PubMed] [Google Scholar]
- 47.Bell J, Burrell T, Indig D, Gilmour S. Cycling in and out of treatment; participation in methadone treatment in NSW, 1990-2002. Drug Alcohol Depend. 2006;81:55–61. doi: 10.1016/j.drugalcdep.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien CP. Clinical Crossroad: A 50-year-old woman addicted to heroin. JAMA. 2008;300:314–321. doi: 10.1001/jama.300.1.jrr80005. [DOI] [PMC free article] [PubMed] [Google Scholar]


