Abstract
Objective
To determine the effect of aging and continence status on the structure and function of the external (EAS) and internal (IAS) anal sphincters
Study Design
Young (YC) and older (OC) continent women were compared to older women with fecal incontinence (OI). Patients completed the FIQOL and FISI and underwent anorectal manometry and transanal ultrasound.
Results
9 YC, 9 OC, and 8 OI women participated. Aging was associated with a thickening of the IAS, while only women with FI had decreased resting pressures. Older incontinent women had a thinner EAS, decreased maximum squeeze pressures, and were hypersensitive to rectal distention with decreased tolerable rectal volumes and urge to defecate at lower volumes.
Conclusion
Thickening of the IAS occurs with aging. Thinning of the EAS and a corresponding drop in squeeze pressure correlated with FI, but not with aging. Rectal hypersensitivity was associated with FI rather than aging and may play a role in the mechanism of FI.
Keywords: external anal sphincter, internal anal sphincter, fecal incontinence, aging
Introduction
Fecal incontinence (FI), defined as the loss of liquid or solid stool, is a frequent condition that can have devastating social, psychological and economic consequences for older adults.1-3 It is a common cause of institutionalization in the elderly4, and epidemiologic studies indicate that aging is a significant risk factor for its development.5-7 While FI in the elderly has long been recognized as a significant medical condition and socially debilitating, little progress has been made in discovering the mechanisms underlying the cause of FI in the elderly. In order to further our understanding, alterations in the individual elements of anorectal structure and function as well as integrated patterns of impairment that develop with age, must be determined.
Fecal continence is maintained by a complex sphincter system involving three anatomical elements: the smooth muscle internal anal sphincter (IAS), the striated external anal sphincter (EAS), and puborectalis muscle. The objective of this study was to utilize an integrative strategy assessing both the structure (using transrectal ultrasound) and function (using anorectal manometry) of the three components of the fecal continence mechanism in order to understand the extent to which age and continence status affect them.
Materials and Methods
Subjects
Between February 2006 and October 2007, 8 older women with weekly fecal incontinence aged 63-85 (OI) as well as 9 young women aged 20-41 years (YC) and 9 older women aged 60-88 years (OC) (representing asymptomatic continent control groups) were recruited to participate. Patients provided consent, and the study was approved by the university's IRB (2005-0294). Subjects were recruited through the university based gynecology clinic and campus-wide advertisements.
Older women who reported loss of solid stool ≥ once per week and had a Wexner score of >8 were considered cases. Women who reported only incontinence of gas/flatus or reported the use of a pad or lifestyle alterations without reporting loss of solid stool were not included. Continent controls (both older and younger) had to have a Wexner score of <4. Exclusion criteria for participation in the study included previous gynecological surgery for pelvic floor disorders and prolapse, previous anal sphincter repair surgery, current treatment for cancer, chronic use of steroids, HIV positive status, sickle cell disease, irritable bowel syndrome, neurological conditions, uncontrolled diabetes, stroke, or Alzheimer's disease. Women who had undergone hysterectomy were eligible if the indication for the surgery was not prolapse and occurred at least 1 year before enrollment.
The frequency and severity of fecal incontinence as well as distress and pad use were determined by use of the Fecal Incontinence Severity Index (FISI)8, Fecal Incontinence Quality of Life Scale (FI-QOL)9, and Wexner questionnaires. 10 Information on comorbid conditions was obtained using a standard patient health questionnaire. All women underwent Pelvic Organ Prolapse Quantification (POP-Q) measurements in the semi recumbent position at a 45 degree angle.
Anorectal Manometry
Anorectal pressures
Anal sphincter function was assessed by performing anorectal manometry (ARM) using a micro-tipped transducer ARM system with Laborie software and a 4-channel Gaeltec cathether (Laborie Electronic, Unisensor, Inc.). Resting and maximum squeeze pressures at 1, 2, 3, 4, 5, and 6 centimeters proximal to the anal verge were recorded in the semi-recumbent position. Maximum squeeze pressure was defined as the highest pressure recorded above the baseline (zero) at any level of the anal canal during maximum squeeze effort by the patient. All pressures were measured three times at each of the 1-cm intervals in the anal canal. Mean resting and squeeze pressures were calculated at all levels.
Rectal distention
Anal pressures were recorded during serial inflation of a rectal balloon with increasing volumes of air (to a maximum of 300 cc). Rectal volumes were increased in 10 cc increments, and the volume/sensory threshold at which subjects experienced first sensation, urge to defecate and maximum tolerance was recorded. The volume at which the recto-anal inhibitory reflex (RAIR) occurred was also recorded. Measurements were recorded and reviewed by a blinded investigator to determine maximal resting and squeeze pressures.
Transrectal Ultrasound
Endoanal ultrasound (EAUS) was performed with the patient in the semirecumbent position in a urodynamics chair. A trans-rectal probe (B-K Medical Systems, Ultrasound Scanner 2101/2102, Model 1850) was used to image the internal and external sphincters. Internal and external anal sphincters were imaged starting at 3 cm proximal to the anal verge with screenshots and measurements taken at each centimeter distally at four locations (12, 3, 6 and 9 o'clock). Any defects in either sphincter were noted. All images were performed by the senior author who was blinded to continence status but not age.
Statistical Analysis
All statistical analyses were completed using SPSS software version 14.0 (Chicago, IL). Using the Wexner scores of the participants at screening, women were divided into groups: young continent (YC), older continent (OC), and older incontinent (OI). Bivariate relationships were explored between the YC, OC and OI groups and demographic data, questionnaire scores, POP-Q points, anorectal manometry measures and ultrasound measures with ANOVA tests. Additional pair-wise comparisons with Student's t-tests were made when a significant difference between the groups was detected with the ANOVA. An alpha of .05 was used for significance in all tests.
Results
The demographics of the three groups are shown in Table 1. The OI group was significantly older than the YC group and also had more vaginal deliveries, forceps-assisted deliveries and cesarean sections. The OC group was also older and had more vaginal deliveries than the YC group. No differences were observed between the OI and OC groups.
Table 1. Demographics among three cohorts (mean ± S.E.).
| YC (N=9) |
OC (N=9) |
OI (N=8) |
ANOVA P |
|
|---|---|---|---|---|
| Age (Yrs) | 28.7 ± 2.4*† | 71.6 ± 2.6 † | 71.6 ± 2.6 * | <0.001 |
| BMI (kg/m2) | 27.2 ± 2.5 | 25.4 ± 1.4 | 27.6 ± 1.5 | 0.682 |
| NSVD | 0.2 ± 0.1*† | 2.7 ± 0.6 † | 2.6 ± 0.4 * | <0.001 |
| FAVD | 0* | 0.6 ± 0.3 | 1.0 ± 0.4* | 0.068 |
| C/S | 0.1 ± 0.1 | 0 | 0.1 ± 0.1 | 0.592 |
Pairwise comparison YC vs. OI, p<0.001
Pairwise comparison YC vs. OC, p<0.001
All three groups were similar with respect to number of bowel movements per week (YC= 8.3 ± 1.3, OC= 7.6 ± 1.2, OI= 9.9 ± 2.3, p=0.34). The OI group scores differed from the other two groups on all fecal incontinence and quality of life scales. The OI group scored higher on the Wexner scale and significantly lower on the FIQOL in all scales. FISI scores were higher among the OI group as well (Table 2).
Table 2. Instrument scores among three cohorts (mean ± S.E.).
| YC | OC | OI | YC vs. OI P value |
YC vs. OC P value |
OC vs. OI P value |
|
|---|---|---|---|---|---|---|
| Wexner Score | 0.6 ± 0.7 | 1.3 ± 1.7 | 13.9 ± 2.8 | <0.001 | 0.22 | <0.001 |
| FIQOL Score | ||||||
| Lifestyle | 4.0 ± 0.1 | 3.7 ± 0.6 | 2.5 ± 1.1 | <0.001 | 0.17 | 0.02 |
| Coping | 4.0 ± 0.1 | 3.5 ± 0.8 | 2.0 ± 1.0 | <0.001 | 0.13 | 0.004 |
| Depression | 4.3 ± 0.1 | 3.6 ± 1.2 | 2.8 ± 0.8 | <0.001 | 0.16 | 0.12 |
| Embarrassment | 4.0 ± 0.1 | 3.4 ± 1.1 | 1.8± 0.6 | <0.001 | 0.17 | 0.003 |
| FISI | ||||||
| Patient Scale | 8.1 ± 11.2 | 8.2 ± 6.6 | 34.8 ± 13.3 | 0.001 | 0.98 | <0.001 |
| Surgeon Scale | 7.3 ± 11.9 | 7.0 ± 6.5 | 34.4 ± 12.1 | <0.001 | 0.94 | <0.001 |
Differences in pelvic organ support among the three groups varied. The YC group had better anterior vaginal wall support than the OC and OI groups at point Aa (YC= -2.4 ± 0.2 vs. OC= -1.4 ± 0.4 and OI= -0.1± 0.7, p<0.01) and Ba (YC= -2.4 ± 0.2 vs. OC= -1.6 ± 0.4 and OI= 0.2 ± 0.9). Apical support (point D) was also statistically higher in the YC group than the OC group (YC: -9.6 ± 0.4 vs. OC: -7.8 ± 0.4, p<0.01). There was a minimal difference in apical support between the YC and OI groups as well (YC= -9.6 ± 0.4 vs. OI= -5.7 ± 1.6, p<0.05). No other differences in POP-Q points were observed.
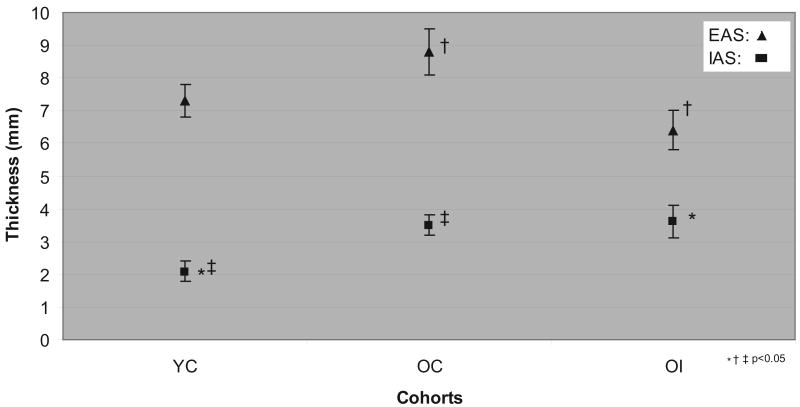
At two centimeters proximal to the anal verge, mean external anal sphincter (EAS) measurements in the OI group were significantly thinner than those in the OC group. On the other hand, both the OI and OC groups overall had thicker internal anal sphincter measures at that location than the YC group (Figure 1). Two women in the OI group had a focal defect in the anterior external anal sphincter which was detected on ultrasound.
Figure 1. Sphincter thickness among cohorts at 2 cm proximal to anal verge (mean ± S.E.).
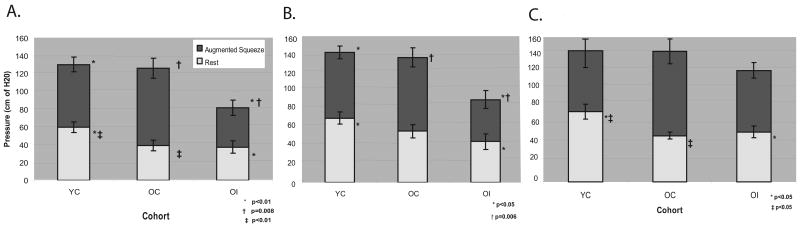
At 1 and 3 centimeters proximal to the anal verge, the YC group had higher mean resting pressures (about 60 cm H2O) than both the OC and OI groups; at 2 centimeters this was also true, however, the difference between the YC and OC group did not reach statistical significance. At all three levels (1, 2 and 3 cm proximal to the anal verge), no differences were seen in resting pressures between the OC and OI groups (Figure 2A-C).
Figure 2.
(A) Resting and squeeze pressures at 1cm proximal to anal verge; (B) Resting and squeeze pressures at 2cm proximal to anal verge (C) Resting and squeeze pressures at 3cm proximal to anal verge (mean ± S.E.)
Both the YC and OC groups demonstrated significantly higher augmented squeeze pressures than the OI group at 1 and 2 centimeters (Figure 2A-B). This trend was also seen at 3 centimeters but, again, did not reach statistical significance (Figure 2C). No differences in augmented squeeze pressures were detected between the YC and OC groups at all levels.
During the balloon distention phase of testing, the OI group felt the sense to defecate at a much lower volume than the YC group and also could tolerate much less volume overall than the OC group; the volume difference in tolerable volume between the OI and the YC group approached significance as well (Table 3).
Table 3. Balloon anorectal manometry volumes.
| Group | Mean Volume (cubic cm of air)* | YC vs. OI P value |
OC vs. OI P value |
YC vs. OC P value |
|
|---|---|---|---|---|---|
| Defecation Sensation Volume | YC | 60 ± 6.0 | 0.04 | 0.2 | 0.9 |
| OC | 58.9 ± 11.5 | ||||
| OI | 42.5 ± 4.5 | ||||
| Max Tolerable Volume | YC | 177.8 ± 26.4 | 0.07 | 0.02 | 1.0 |
| OC | 176.7 ± 13.8 | ||||
| OI | 113.8 ± 18.3 |
Values reported as mean ± standard error
Comment
In this study, aging (irrespective of continence status) was associated with a thickening of the IAS smooth muscle and decrease in resting tone. FI alone (not aging) was associated with thinning of the striated EAS muscle and a corresponding decrease in augmented squeeze pressures. Similarly, FI was also associated with rectal hypersensitivity, but not aging.
In our study, older continent women were able to augment their resting rectal pressures similarly to younger women. Old incontinent women, on the other hand, were unable to do so. This is similar to other published studies. 11 Our findings that reduced squeeze pressures did not correlate with aging, however, are similar to some studies in asymptomatic volunteers 12-14 and contrary to others.15 For example, Fox et al. found that in 61 asymptomatic women, aging was associated with reduced anal squeeze pressures and reduced rectal compliance and sensation. 16 The discrepancy in these findings could be attributed to differences in the patient populations; only 6 women in Fox's study were over the age of 70.
Both older continent and older incontinent groups showed a thickening of the IAS on ultrasound and decreased resting pressures compared to the young continent women. These findings are consistent with our previous work evaluating the IAS on MRI images in old versus young asymptomatic women. 17 This decrease is resting pressures would confirm our hypothesis that the thickening seen is not secondary to active muscle fibers, but probable fibrosis that (while increasing the appearance of muscle bulk), generates less resting pressure. This finding was associated with age and did not differ with continence status. Our manometry results complement these ultrasound findings and are consistent with the literature showing decreased resting pressures with aging in asymptomatic and symptomatic women. 15,18,19
Rectal hypersensitivity (i.e. lower volume and pressure thresholds for the desire to defecate) is common in patients with FI and is associated with the symptom of urge.3, 20-23 This was true in our study as well. Older incontinent women felt the urge to defecate and had a maximum tolerance at a much lower volume than younger and older continent subjects. Interestingly, while many studies have shown that aging alone is also associated with rectal hypersensitivity16, the young continent and old continent women in this study did not differ in that respect. Wald describes three broad categories of fecal incontinence among the elderly: (1) overflow incontinence; (2) reservoir incontinence; and (3) rectosphincteric incontinence.24 Our older incontinent women had normal bowel function and no constipation, and therefore none had overflow incontinence. Reservoir incontinence, as demonstrated by rectal hypersensitivity and decreased tolerable rectal volumes on manometry was seen in the majority of the OI women. Sphincteric incontinence was also seen in these women, with two patients having an EAS defect and significantly lower squeeze augmentation. Limitations of this study include the small sample size. A larger study will be needed to further validate our results. Also, the majority of our older incontinence women were Caucasian, and a more diverse cohort would be more representative of community-dwelling women. The median parity of the older continent group was higher than the younger continent group; it is difficult, however, to control for this, as most older women have had vaginal deliveries. Differences in pelvic support (reflecting the function of the puborectalis muscle) could confound our results, however, the clinical relevance of the minimal differences in POP-Q findings in this study is debatable.
A variety of therapeutic interventions can be used to help elderly women with FI. These include dietary, behavioral, pharmacological and surgical modalities, chosen on the basis of accurate diagnosis and understanding of the primary pathophysiology. Our findings support treatment modalities, such as pelvic floor exercises and biofeedback with rectal balloon therapy, targeting the striated EAS muscle and rectal hypersensitivity. With an understanding of the underlying diseases processes and the anatomical and physiological changes that occur with aging, we can design preventive therapies. With this knowledge, significant improvements in quality of life can be achieved in most elderly persons with fecal incontinence.
Acknowledgments
We gratefully acknowledge research support from the Claude A. Pepper Foundation Grant P30AG024824 for this project and investigator support from the Office for Research on Women's Health SCOR on Sex and Gender Factors Affecting Women's Health and the National Institute of Child Health and Human Development 1 P50 HD044406.
Footnotes
To be presented at the 29th Annual Scientific Meeting of the American Urogynecologic Society, Chicago, IL, September 4-6, 2008.
Reprints will not be available.
There are no disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perry S, Shaw C, McGrother C, Matthews RJ, Assassa RP, Dallosso H, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50(4):480–4. doi: 10.1136/gut.50.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson RL. Epidemiology of fecal incontinence. Gastroenterology. 2004;126(1 Suppl 1):S3–7. doi: 10.1053/j.gastro.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE, Zinsmeister AR, Locke GR, Seide BM, McKeon K, Schleck CD, et al. Prevalence and burden of fecal incontinence: A population-based study in women. Gastroenterology. 2005;129(1):42–9. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Specht JK. 9 myths of incontinence in older adults: Both clinicians and the over-65 set need to know more. Am J Nurs. 2005;105(6):58–68. doi: 10.1097/00000446-200506000-00029. quiz 69. [DOI] [PubMed] [Google Scholar]
- 5.Boreham MK, Richter HE, Kenton KS, Nager CW, Gregory WT, Aronson MP, et al. Anal incontinence in women presenting for gynecologic care: Prevalence, risk factors, and impact upon quality of life. Am J Obstet Gynecol. 2005;192(5):1637–42. doi: 10.1016/j.ajog.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in US women: A population-based study. Am J Obstet Gynecol. 2005;193(6):2071–6. doi: 10.1016/j.ajog.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Pretlove SJ, Radley S, Toozs-Hobson PM, Thompson PJ, Coomarasamy A, Khan KS. Prevalence of anal incontinence according to age and gender: A systematic review and meta-regression analysis. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(4):407–17. doi: 10.1007/s00192-005-0014-5. [DOI] [PubMed] [Google Scholar]
- 8.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: The fecal incontinence severity index. Dis Colon Rectum. 1999;42(12):1525–32. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, Thorson AG, et al. Fecal incontinence quality of life scale: Quality of life instrument for patients with fecal incontinence. Dis Colon Rectum. 2000;43(1):9–16. doi: 10.1007/BF02237236. discussion 16-7. [DOI] [PubMed] [Google Scholar]
- 10.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 11.Salvioli B, Bharucha AE, Rath-Harvey D, Pemberton JH, Phillips SF. Rectal compliance, capacity, and rectoanal sensation in fecal incontinence. Am J Gastroenterol. 2001;96(7):2158–68. doi: 10.1111/j.1572-0241.2001.03954.x. [DOI] [PubMed] [Google Scholar]
- 12.Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94(3):773–83. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 13.Lagier E, Delvaux M, Vellas B, Fioramonti J, Bueno L, Albarede JL, et al. Influence of age on rectal tone and sensitivity to distension in healthy subjects. Neurogastroenterol Motil. 1999;11(2):101–7. doi: 10.1046/j.1365-2982.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- 14.Sloots CE, Felt-Bersma RJ, Cuesta MA, Meuwissen SG. Rectal visceral sensitivity in healthy volunteers: Influences of gender, age and methods. Neurogastroenterol Motil. 2000;12(4):361–8. doi: 10.1046/j.1365-2982.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- 15.Bannister JJ, Abouzekry L, Read NW. Effect of aging on anorectal function. Gut. 1987;28(3):353–7. doi: 10.1136/gut.28.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum. 2006;49(11):1726–35. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 17.Huebner M, Margulies RU, Fenner DE, Ashton-Miller JA, Bitar KN, DeLancey JO. Age effects on internal anal sphincter thickness and diameter in nulliparous females. Dis Colon Rectum. 2007;50(9):1405–11. doi: 10.1007/s10350-006-0877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McHugh SM, Diamant NE. Effect of age, gender, and parity on anal canal pressures. contribution of impaired anal sphincter function to fecal incontinence. Dig Dis Sci. 1987;32(7):726–36. doi: 10.1007/BF01296139. [DOI] [PubMed] [Google Scholar]
- 19.Jameson JS, Chia YW, Kamm MA, Speakman CT, Chye YH, Henry MM. Effect of age, sex and parity on anorectal function. Br J Surg. 1994;81(11):1689–92. doi: 10.1002/bjs.1800811143. [DOI] [PubMed] [Google Scholar]
- 20.Andrews C, Bharucha AE, Seide B, Zinsmeister AR. Rectal sensorimotor dysfunction in women with fecal incontinence. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G282–9. doi: 10.1152/ajpgi.00176.2006. [DOI] [PubMed] [Google Scholar]
- 21.Chan CL, Scott SM, Williams NS, Lunniss PJ. Rectal hypersensitivity worsens stool frequency, urgency, and lifestyle in patients with urge fecal incontinence. Dis Colon Rectum. 2005;48(1):134–40. doi: 10.1007/s10350-004-0774-x. [DOI] [PubMed] [Google Scholar]
- 22.Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of ‘idiopathic’ faecal incontinence. Gut. 1992;33(6):807–13. doi: 10.1136/gut.33.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharucha AE, Fletcher JG, Harper CM, Hough D, Daube JR, Stevens C, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic faecal incontinence. Gut. 2005;54(4):546–55. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wald A. Faecal incontinence in the elderly : Epidemiology and management. Drugs Aging. 2005;22(2):131–9. doi: 10.2165/00002512-200522020-00004. [DOI] [PubMed] [Google Scholar]