Abstract
Sex-linked factors may alter risk for neurodegenerative diseases. Definitive diagnoses are not established until autopsy, so neuropathological studies are critical. There have not been reported gender-related differences in neocortical Lewy bodies (LBs) using large multi-center autopsy series. We evaluated the associations between gender and pathologically characterized neurodegenerative diseases. Cases with Alzheimer's disease (AD), neocortical LBs, AD + neocortical LBs, or neither pathology were evaluated as separate groups. Results were corrected for possible confounders including age at death, smoking history, and education. The settings were the University of Kentucky Alzheimer's Disease Center and the National Alzheimer's Coordinating Center (NACC) Registry autopsy series; 3,830 subjects met inclusion criteria. Patients with neocortical (“diffuse”) or intermediate (“limbic”) LB pathologies tended to be male (male:female odds ratios ∼2.9 with 95% CI 2.02–4.18). The preponderance of males dying with neocortical LB pathology was seen consistently across age groups and was not due to the potential confounders evaluated. By contrast, individuals dying with AD pathology were more likely to be female if dying over 80 (male:female odds ratio 0.66, 95% CI 0.50–0.88), but that tendency was not seen in individuals dying with AD pathology prior to age 80. Increased understanding of the male predominance in neocortical LB pathology may help guide clinicians, because males are more likely to be “undercalled” for neocortical LBs clinically, and females are more likely to be “overcalled” (P < 0.05 for both). Males are far more likely than females to die with neocortical LB pathology. This phenomenon may help guide medical practice including clinical trial study design.
Keywords: DLB, Synuclein, Human, Dementia, Neuropathology
Introduction
Correlations between gender and neurodegenerative disease (ND) risks are not clearly established. At a population level, men and women differ in their genetic repertoires and environmental exposures. Specific sex chromosome-linked genes, sex hormones, and sexually disparate co-morbidities are hypothesized to affect ND pathogenesis [4, 12, 21].
Testing the association between gender and ND risk requires human studies because NDs are essentially human-specific. However, the application of epidemiological methods must overcome multiple challenges. Potential confounders include the ∼5 year average longevity difference between males and females, non-identical exposures, and distinctive behavior patterns including different rates of volunteerism for clinical studies. Furthermore, definitive ND diagnoses require autopsy confirmation. Neuropathological diagnoses are especially important for cases with mixed pathology, such as Alzheimer's disease (AD) with concomitant dementia with Lewy bodies (DLB), where precise clinical diagnostic criteria are lacking.
Perhaps due to these challenges, there have been relatively few studies providing assessment of gender-related risk for neuropathologically proven DLB. DLB is characterized clinically by fluctuating cognition, well-formed hallucinations, parkinsonism, and other supportive features [15]. Definitive diagnosis of DLB requires pathological confirmation, which rests on α-synuclein-immunoreactive cortical Lewy bodies [15]. Although there has been recent progress in DLB diagnoses, the positive predictive value of the clinical diagnosis remains rather low [13, 14, 16, 17]. In contrast to DLB, prior studies in AD and Parkinson's disease (PD) have found firmer evidence of links between gender and risk for diagnosis or mortality [2, 4, 21].
There have been prior autopsy series that have included pathologically-verified DLB cases and most, but not all, have shown a trend for more males than females to die with this pathology [3, 7, 8, 10, 20, 25–27]. However, there has not been a study that has evaluated the phenomenon systematically, nor has there been a relevant well-powered, systematic study using a multi-center autopsy series. There also has never been an evaluation in a large dataset as to whether the clinical-pathological correlation differs between the genders. We hypothesize that if the gender link in DLB is underappreciated in the clinical community, men and women would have different rates of agreement between clinical and pathological diagnoses. It may be helpful to understand these correlations for better diagnostic and therapeutic decision-making in the future.
In the present study, associations were tested between gender and risks for AD, neocortical LB, and mixed (AD + neocortical LB) pathologies in some large autopsy cohorts derived from multiple academic centers. We also tested whether men and women tend to be misdiagnosed before death at different rates in terms of the clinical prediction of cortical LB pathology. Data were analyzed from the National Alzheimer's Coordinating Center (NACC) [5] Registry with separate subanalyses from the University of Kentucky Alzheimer's disease Center (UK ADC) autopsy series.
Methods
Databases
The NACC Registry represents data obtained from 25 different ADCs [5]. The study was performed with approval by local institutional review boards. Inclusion criteria and numbers excluded are described in detail in Supplemental file 1. The following groups were excluded from the NACC data (total N = 2311): death prior to 1998; no data about education level or neuropathology; and, clinical history of prion disease, triplet repeat diseases, brain cancer, or frontotemporal dementia that might explain a dementia syndrome with early death. PD and Parkinson's disease with dementia (PDD) cases were excluded due to lack of statistical power. There were no inclusion or exclusion criteria with regard to cognitive decline during life, because neuropathological diagnoses were used as the gold standard for the disease process. Using these criteria, 3,172 cases were included from the NACC Registry (excluding UK ADC cases) and an additional 658 from the UK ADC [18] autopsy series from which no autopsied cases were excluded from initial analyses. Mean number of included subjects per ADC was 129, median 125, and range 16–340.
Pathological criteria
Neocortical LBs were determined by indication of “diffuse” or “intermediate” cortical LB pathology using the NACC registry “NPLEWY” variable, based on consensus diagnostic features [15]. The “brainstem-predominant” variant was not included initially because this pathology has a low probability of correlating with a DLB syndrome clinically [9]. AD-type pathology was determined using a modified version of the National Institute on Aging-Reagan Institute criteria [1] that incorporated both CERAD stages of neuritic plaque densities and Braak stages for neurofibrillary pathology. AD was indicated by Braak stages V or VI with CERAD “moderate” or “frequent”. If a sufficient number of cortical LBs were present to merit the designation of DLB by pathology according to the NPLEWY code in the NACC Registry, then, AD was deemed also present (i.e. AD + DLB) with Braak neurofibrillary stages IV, V, or VI. Braak stage IV cases were included for AD + DLB because AD pathology in the presence of cortical LBs tends to be lower despite commensurate clinical disease [18].
Statistical methods
For the NACC data, a logistic regression model was used to control for age at death and years of education. This model was also run on an age-stratified sample, where 80 years of age was the cut-point. This age was selected because it is the approximate mean age at death for this research cohort (Table 1). As additional variables of interest were available in the UK ADC data, a separate logistic regression model predicting male gender included age at death, years of education, ND pathology type, smoking history (reported smoker vs. not), and apolipoprotein E (ApoE) alleles. Simple odds ratio tabulations, confidence intervals, and all other analyses were conducted using PC-SAS 9.1.3®. Odds ratios were used rather than relative risk statistics because the populations reported were convenience samples rather than random cohorts. Gender-specific rates of overcall and undercall of clinical diagnoses, using the pathological diagnoses as the gold standard, were compared using the Chi-square statistic.
Table 1. Patient characteristics by gender including neuropathology and demographic data from NACC Registry and UK ADC autopsy series.
| Research subjects grouped by neuropathology | Total | |||||
|---|---|---|---|---|---|---|
| AD only | DLB Only | AD + DLB | Neither AD nor DLB | |||
| Patients | Male | 734 | 109 | 240 | 451 | 1,534 |
| NACC | Female | 883 | 42 | 230 | 483 | 1,638 |
| Total | 1,617 | 151 | 470 | 934 | 3,172 | |
| OR, M:F (95% CI) | 0.78 (0.68–0.90) | 2.91 (2.02–4.18) | 1.14 (0.93–1.38) | 1.00 (0.85–1.16) | ||
| Patients | Male | 77 | 22 | 35 | 141 | 275 |
| UK ADC | Female | 147 | 11 | 61 | 164 | 383 |
| Total | 224 | 33 | 96 | 305 | 658 | |
| OR, M:F (95% CI) | 0.62 (0.45–0.87) | 2.94 (1.40–6.17) | 0.77 (0.49–1.20) | 1.41 (1.03–1.92) | ||
| Mean | Male | 76.7 (75.9–77.4) | 78.9 (77.3–80.4) | 78.0 (76.9–79.1) | 83.6 (82.8–84.4) | 79.1 (78.6–79.6) |
| Age | Female | 80.9 (80.2–81.7) | 81.9 (78.8–84.9) | 80.4 (79.1–81.7) | 86.6 (85.7–87.4) | 82.5 (82.0–83.1) |
| NACC | Total | 79.0 (78.5–79.5) | 79.7 (78.3–81.1) | 79.2 (78.3–80.0) | 85.1 (84.5–85.7) | 80.9 (80.5–81.2) |
| Mean | Male | 14.9 (14.6–15.1) | 15.7 (15.1–16.4) | 15.0 (14.5–15.4) | 15.5 (15.1–15.8) | 15.1 (14.9–15.3) |
| Education | Female | 13.4 (13.2–13.6) | 14.0 (12.9–15.0) | 13.3 (12.9–13.7) | 14.6 (14.3–14.9) | 13.8 (13.6–13.9) |
| NACC | Total | 14.1 (13.9–14.3) | 15.2 (14.7–15.8) | 14.1 (13.8–14.5) | 15.0 (14.8–15.2) | 14.4 (14.3–14.5) |
Results
Although the dataset is skewed toward individuals with cognitive decline, the full gamut of cognitive impairment severity was observed, with 23% of patients in the NACC Registry data having final MMSE scores above 25. Neuropathological findings were stratified by gender (Table 1); mean age and education level are provided to convey additional information about the various subgroups. UK ADC data is provided separately from the NACC Registry data because the latter was a validation dataset used to evaluate the original finding in the UK ADC data. Persons who died with pathologically verified DLB—unlike AD, AD + DLB, or controls—tended to be males in both the UK ADC autopsy series (P < 0.003) and in the larger NACC Registry excluding UK ADC patients (P < 0.0001). Note that the odds ratio for pure neocortical LB pathology in males is almost identical (∼2.8) in the two cohorts. Although 25 different ADCs provided cases for the current study, DLB cases in the NACC Registry clustered from 17 ADCs which contributed more than two DLB cases each. From each of these 17 different ADCs, the number of males with DLB pathology exceeded the number of females.
Results of the logistic regression analysis indicated that male preponderance in DLB diagnoses is not due to possible confounders including age of death, years of education, in either the NACC registry or UK ADC cohorts, nor due to the additional predictors of apolipoprotein E alleles or smoking history in the UK ADC cohort (P < 0.01 comparing “DLB Only” to “Neither AD nor DLB”). The interaction between age and diagnosis was assessed in a separate logistic model—in the NACC Registry data—and found not to be significant.
The NACC Registry data was used to test the importance of age at death and the LB pathology subtype. Figure 1a (NACC registry data) shows that the relatively high male:female ratio of neocortical LB pathology is found at autopsy almost uniformly across different age groups. As shown in Table 2, the 95% confidence intervals for relative risk for ND pathology in males indicate that individuals dying both before or after 80 years tend to be males if dying with DLB pathology. By contrast, those that die after age 80 with AD pathology tend to be females, but this gender-related risk effect is not seen in persons dying before 80 years of age. When the results are stratified by pathological subtype of DLB pathology [15] (Fig. 1b), male predominance is greatest in the neocortical “diffuse” subtype of DLB, less so in the “intermediate”/limbic subtype, and absent in the brainstem predominant subtype. If Braak stage IV cases were included in the “DLB Only” group, and excluded from the “AD + DLB” group, then the relative risk for males having “DLB Only” pathology was still 2.39 (181M:84F in the 47.3% male NACC Registry cohort; P < 0.0001).
Fig. 1.
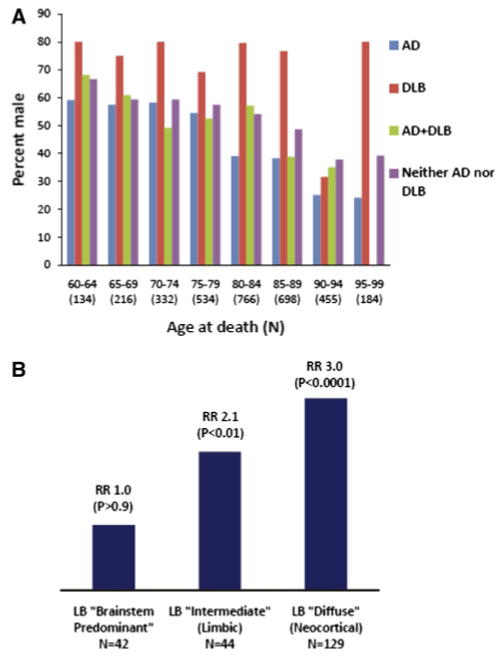
NACC Registry data was analyzed to test the effects of age and neocortical Lewy body (LB) pathology subtype. a Percent male by age at death cohort: AD, DLB, AD + DLB or neither pathology. The association between gender and pathologically-verified neurodegenerative disease diagnoses across different cohorts stratified by age at death. In each cohort, males are far more likely to die with neocortical LBs. By contrast, Alzheimer's disease is not skewed to women when death occurs before age 80. Shown are NACC Registry data with autopsy-verified neurodegenerative disease diagnoses (N = 2,718 in these age ranges). b Male:female risk ratio by subgroup of cases with Lewy body (LB) pathology. The more the pathology is concentrated in the neocortex instead of the brainstem, the higher is the odds ratio that the patient is male, indicating neuropathological subtype of LB disease is important in male preponderence
Table 2. Male:female odds ratio, controlling for age at death and years of education.
| Younger than 80 (total N = 1,329) 95% CI |
80 and over (total N = 2,165) 95% CI |
|
|---|---|---|
| AD versus neither | 1.02 (0.74–1.39) | 0.65 (0.53–0.80) |
| AD versus DLB | 0.50 (0.29–0.88) | 0.26 (0.16–0.42) |
| AD versus AD + DLB | 1.11 (0.82–1.51) | 0.66 (0.50–0.88) |
| DLB versus neither | 2.03 (1.11–3.71) | 2.50 (1.56–4.00) |
| DLB versus AD + DLB | 2.21 (1.21–4.04) | 2.55 (1.53–4.23) |
| AD + DLB versus neither | 0.92 (0.63–1.34) | 0.98 (0.73–1.31) |
NACC Registry data for AD (N = 1,749), DLB (173), AD + DLB (525) or neither (1,047)
Having shown that males are more likely than females to die with pure neocortical LB pathology, we sought to test the hypothesis that this phenomenon could be important in a clinical context. More specifically, we wanted to ascertain whether the phenomenon was under-appreciated in the academic environment of ADCs in such a way as to promote clinical (antemortem) DLB misdiagnoses. To test this, we evaluated cases where the clinical (N = 99) and/or pathological (N = 162) diagnoses of pure DLB were documented (Fig. 2). In cases with clinical DLB diagnoses, an individual who was not found to have DLB at autopsy was said to be an “overcall” (false positive). By contrast, in cases with pathological DLB diagnoses, an individual whose DLB had been clinically unsuspected was said to be an “undercall” (false negative). The data indicate that men tend to be “undercalled”, and women tend to be “overcalled” for DLB clinically (P < 0.05 in both cases), consistent with the hypothesis that there is suboptimal appreciation of the phenomenon of neocortical LB pathology being over-represented in males in ADCs.
Fig. 2.
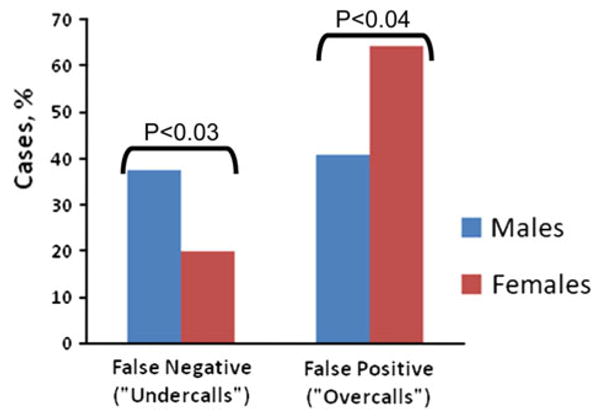
Clinical misdiagnoses in DLB: males versus females. Gender differences in neocortical Lewy body (LB) pathology is directly relevant to clinical diagnoses in neurodegenerative diseases and appears to be under-appreciated by clinicians. The presence of LB pathology in males is relatively likely to be missed by clinicians, whereas in females the diagnosis tends to be “overcalled”, according to NACC Registry data tabulated since 2000 (total N = 2,862). These data involve only cases where the clinical (N = 99) and/or pathological (N = 162) diagnoses of pure dementia with Lewy bodies (DLB) were documented. For individuals where cortical LB pathology was observed upon autopsy (left bars), a false-negative “undercall” was indicated if the diagnosis of DLB was not made during life. In the case of patients where DLB was suspected clinically (right bars), a false-positive “overcall” meant that cortical LB pathology was not judged to neuropathologically contribute to the dementia. Results were compared using the Chi-square statistic. These data indicate that an appreciation of the relative tendency for males to have cortical LB pathology may help prevent clinical diagnostic misdiagnoses
Discussion
Data from large autopsy series indicate that there is a positive association between male gender and risk for dying with relatively “pure” cortical DLB pathology. This effect does not appear to be related to potential confounders such as patients' age of death, education level, smoking status, or ApoE alleles. These data may provide an important new link in understanding DLB pathogenesis. Prior American, European, and Japanese autopsy series have found disproportionate numbers of males in pathologically-confirmed DLB cohorts [3, 7, 8, 10, 20, 25, 26]. However, the question has not been systematically analyzed using large multi-center cohorts. Other previous studies have also relied on clinical, rather than pathological, criteria for DLB diagnoses, which are far less dependable.
In the present study, cases with AD, DLB, and AD + DLB pathology were considered separately. This approach was chosen partly because DLB and AD + DLB tend to have distinct clinical manifestations [10, 19, 24]. In addition, the fundamental biology of AD + DLB may be unique, a point highlighted by the finding of specific genetic mutations associated with AD + DLB pathology [11, 22]. Thus, we found it preferable a priori to separate AD, DLB, and AD + DLB cases by pathology, whether they might be considered individual diseases or a distinguishable combination of diseases.
Using neuropathological diagnoses as the gold standard, the associations between gender risk for AD or AD + DLB were relatively weak. Prior studies have noted that AD mortality and prevalence are higher in women than in men [4]. However, in the NACC (N = 3,494 including UK ADC) database, correcting for age at death caused the AD/gender association to lessen. Further work is required to better understand the associations between gender and AD risk.
The present study has limitations. The cases derived from a large, multi-center dataset that constitutes a cross-sectional convenience sample. There are no doubt a number of relevant ascertainment, selection, and sampling biases referent to this study design. However, the overall gender composition in the NACC Registry is nearly one-half men (see Table 1). Since we included groups with pathology other than DLB, the study could compare the features most specific for patients that died with DLB. Unlike cortical LB pathology, there was a trend for more women to die with AD pathology. We see no reason why there should be differential ascertainment, selection, and sampling biases for the disease subtypes since the neuropathological diagnoses were not known during life. Moreover, any study that includes autopsy data will have some such bias because autopsy rates and study volunteer rates are never perfect. For all its imperfections, the NACC Registry is designed to represent the state of the art in this regard. Another consideration related to the use of neuropathology as “gold standard” is that there currently are no consensus criteria for cutoffs to delineate “AD + DLB” versus “DLB only”. It is hence near-certain that some experts would disagree with our working criteria as we explore the ill-defined interface between AD and DLB.
Another note of caution pertinent to the study design is the axiom that one should not “fish for small P values in a large dataset”. Strictly speaking, the clinical-pathological correlation was used to provide a proxy for determining “odds ratios” for this pathological process in males and females. However, since the phenomenon of male predominance in DLB has been observed previously in other, smaller studies, and here shows such strong statistical significance it is likely that this phenomenon could be clinically and biologically important in a wider sense. Further, the implications are direct and substantial: the fact that physicians may benefit from incorporating these data into their clinical practice is perhaps best demonstrated in Fig. 2. Research studies, including clinical trials, should likewise incorporate this expectation.
It is important to note that, in contrast to AD, PD has been shown to have a male predominance [21]. Perhaps surprisingly, we found a lack of association between gender and “brainstem-predominant” Lewy body-type pathology. Nonetheless, these data may underscore a key pathogenetic link between PD/PDD and DLB. In addition to PD/PDD cases, we also excluded from the NACC Registry analyses of thousands of cases with other strong pathologies including frontotemporal diseases, Huntington's disease, and brain cancer. These exclusions may have introduced additional confounders related to gender and NDs. However, we did not apply such exclusions to the UK ADC cohort and the results were very similar with regard to the male predominance of neocortical LB pathology.
Why would males have a higher risk for neocortical Lewy bodies? Sex hormones have been suggested to confer altered risk to NDs [4, 12, 21]. PD has been linked to environmental exposures, and environmental factors may also influence neocortical Lewy bodies in DLB [23]. Alternatively, in patients with DLB, women may have a relatively greater tendency to develop concomitant AD pathology, so these patients would be shifted into the AD + DLB category. This latter hypothesis is credible because synucleinopathy and AD-type pathology can be synergistic in humans and in animal models [6]. However, many clinical and biological issues related to the gender, AD, and DLB remain unresolved.
Supplementary Material
Acknowledgments
We are deeply grateful to all of the study participants. We thank Ela Patel, Ann Tudor, Paula Thomason, Dr. Huaichen Liu and Sonya Anderson for technical support, Greg Cooper, MD, Nancy Stiles, MD and Allison Caban-Holt, PhD for the clinical evaluations, and Daron Davis MD for pathological evaluations. We also thank Leslie Phillips, MS for help with NACC data. Corresponding Author Dr. Nelson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study was supported by NIH grants R01 NS061933, K08 NS050110, P30 AG028383, and U01 AG016976.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00415-010-5630-4) contains supplementary material, which is available to authorized users.
Contributor Information
Peter T. Nelson, Email: pnels2@email.uky.edu, Division of Neuropathology, Department of Pathology, Sanders-Brown Center on Aging, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA.
Frederick A. Schmitt, Division of Neuropathology, Department of Pathology, Sanders-Brown Center on Aging, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA; Department of Neurology, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536, USA
Gregory A. Jicha, Division of Neuropathology, Department of Pathology, Sanders-Brown Center on Aging, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA; Department of Neurology, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536, USA
Richard J. Kryscio, Division of Neuropathology, Department of Pathology, Sanders-Brown Center on Aging, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA; Department of Statistics, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536, USA
Erin L. Abner, Division of Neuropathology, Department of Pathology, Sanders-Brown Center on Aging, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA
Charles D. Smith, Division of Neuropathology, Department of Pathology, Sanders-Brown Center on Aging, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA; Department of Neurology, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536, USA
Linda J. Van Eldik, Division of Neuropathology, Department of Pathology, Sanders-Brown Center on Aging, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA; Department of Anatomy and Neurobiology, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536, USA
William R. Markesbery, Division of Neuropathology, Department of Pathology, Sanders-Brown Center on Aging, University of Kentucky, Rm 311, Sanders-Brown Building, 800 S. Limestone, Lexington, KY 40536-0230, USA; Department of Neurology, Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536, USA
References
- 1.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease The National Institute on aging, and Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer's disease. Neurobiol Aging. 1997;18(4):S1–S2. [PubMed] [Google Scholar]
- 2.Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: the EURODEM studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- 3.Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Barrett AM. Probable Alzheimer's disease: gender-related issues. J Gend Specif Med. 1999;2:55–60. [PubMed] [Google Scholar]
- 5.Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA. The National Alzheimer's Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–277. [PubMed] [Google Scholar]
- 6.Crews L, Tsigelny I, Hashimoto M, Masliah E. Role of synucleins in Alzheimer's disease. Neurotox Res. 2009;16(3):306–317. doi: 10.1007/s12640-009-9073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, Wszolek ZK, Knopman DS, Petersen RC, Parisi JE, Dickson DW. Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol. 2008;67:649–656. doi: 10.1097/NEN.0b013e31817d7a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hishikawa N, Hashizume Y, Yoshida M, Sobue G. Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol. 2003;105:341–350. doi: 10.1007/s00401-002-0651-4. [DOI] [PubMed] [Google Scholar]
- 9.Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta. 2008;1792:730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, Bowen JD, Kukull WA, Leverenz JB, Cherrier MM. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, Schmidt ML, Nee LE, O'Connell B, Pollen DA, St George-Hyslop P, Ghetti B, Nochlin D, Bird TD, Cairns NJ, Lee VM, Iwatsubo T, Trojanowski JQ. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manly JJ, Merchant CA, Jacobs DM, Small SA, Bell K, Ferin M, Mayeux R. Endogenous estrogen levels and Alzheimer's disease among postmenopausal women. Neurology. 2000;54:833–837. doi: 10.1212/wnl.54.4.833. [DOI] [PubMed] [Google Scholar]
- 13.McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, Gauthier S, Halliday G, Lawlor B, Lippa C, Lopez OL, Carlos Machado J, O'Brien J, Playfer J, Reid W. Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- 14.McKeith IG, Ballard CG, Perry RH, Ince PG, O'Brien JT, Neill D, Lowery K, Jaros E, Barber R, Thompson P, Swann A, Fairbairn AF, Perry EK. Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology. 2000;54:1050–1058. doi: 10.1212/wnl.54.5.1050. [DOI] [PubMed] [Google Scholar]
- 15.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 16.McKeith IG, Rowan E, Askew K, Naidu A, Allan L, Barnett N, Lett D, Mosimann UP, Burn D, O'Brien JT. More severe functional impairment in dementia with lewy bodies than Alzheimer disease is related to extrapyramidal motor dysfunction. Am J Geriatr Psychiatry. 2006;14:582–588. doi: 10.1097/01.JGP.0000216177.08010.f4. [DOI] [PubMed] [Google Scholar]
- 17.Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, Xu LO, Smith CD, Markesbery WR. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2009;257(3):359–366. doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olichney JM, Galasko D, Salmon DP, Hofstetter CR, Hansen LA, Katzman R, Thal LJ. Cognitive decline is faster in Lewy body variant than in Alzheimer's disease. Neurology. 1998;51:351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- 21.Shulman LM. Gender differences in Parkinson's disease. Gend Med. 2007;4:8–18. doi: 10.1016/s1550-8579(07)80003-9. [DOI] [PubMed] [Google Scholar]
- 22.Trembath Y, Rosenberg C, Ervin JF, Schmechel DE, Gaskell P, Pericak-Vance M, Vance J, Hulette CM. Lewy body pathology is a frequent co-pathology in familial Alzheimer's disease. Acta Neuropathol. 2003;105:484–488. doi: 10.1007/s00401-003-0670-9. [DOI] [PubMed] [Google Scholar]
- 23.Tsuang D, Larson EB, Li G, Shofer JB, Montine KS, Thompson ML, Sonnen JA, Crane PK, Leverenz JB, Montine TJ. Association between lifetime cigarette smoking and lewy body accumulation. Brain Pathol. 2009;20(2):412–418. doi: 10.1111/j.1750-3639.2009.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner MF, Hynan LS, Parikh B, Zaki N, White CL, III, Bigio EH, Lipton AM, Martin-Cook K, Svetlik DA, Cullum CM, Vobach S, Rosenberg RN. Can alzheimer's disease and dementias with Lewy bodies be distinguished clinically? J Geriatr Psychiatry Neurol. 2003;16:245–250. doi: 10.1177/0891988703258671. [DOI] [PubMed] [Google Scholar]
- 25.Weiner MF, Risser RC, Cullum CM, Honig L, White C, III, Speciale S, Rosenberg RN. Alzheimer's disease and its Lewy body variant: a clinical analysis of postmortem verified cases. Am J Psychiatry. 1996;153:1269–1273. doi: 10.1176/ajp.153.10.1269. [DOI] [PubMed] [Google Scholar]
- 26.Williams MM, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67:1935–1941. doi: 10.1212/01.wnl.0000247041.63081.98. [DOI] [PubMed] [Google Scholar]
- 27.Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34:561–566. doi: 10.1093/ageing/afi190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


