Abstract
The RAD51 protein has been shown to participate in homologous recombination by promoting ATP-dependent homologous pairing and strand transfer reactions. In the present study, we have investigated the possible involvement of RAD51 in non-homologous recombination. We demonstrate that overexpression of CgRAD51 enhances the frequency of spontaneous non-homologous recombination in the hprt gene of Chinese hamster cells. However, the rate of non-homologous recombination induced by the topoisomerase inhibitors campothecin and etoposide was not altered by overexpression of RAD51. These results indicate that the RAD51 protein may perform a function in connection with spontaneous non-homologous recombination that is not essential to or not rate-limiting for non-homologous recombination induced by camptothecin or etoposide. We discuss the possibility that the role played by RAD51 in non-homologous recombination observed here may not be linked to non-homologous end-joining.
INTRODUCTION
The RAD51 protein is the mammalian homologue of the RecA protein in Escherichia coli and promotes ATP-dependent homologous pairing and strand transfer reactions in vitro (1), in addition to supporting homologous recombination in mammalian cells (2–4). The RAD51 gene has been identified in many higher eukaryotes and the amino acid sequence of the protein shown to be highly conserved (3,5–7). The RAD51 protein performs essential functions in higher eukaryotes, in contrast to bacteria and yeast, since RAD51 knockout mice die at an early stage of embryogenesis (8,9) and cells lacking the RAD51 protein exhibit chromosome breaks and cell-cycle arrest (10).
Compelling evidence suggests that the RAD51 protein participates in the repair of double-strand breaks in mammalian cells (for reviews see 11,12). Cells that overexpress RAD51 demonstrate increased resistance towards X-rays (3,13). Furthermore, foci containing RAD51 are observed in the nucleus after exposure of somatic cells to methylmethanesulfonate (MMS), UV irradiation or γ-irradiation (14).
There are several pathways of non-homologous recombination in mammalian cells, all probably employing the Ku70, Ku80 and DNA Protein Kinase catalytic subunit (DNA-PKcs) proteins (15–20). The pathway of non-homologous recombination used in DNA double-strand break repair is referred to as non-homologous end-joining (for reviews see 19,20). Two other pathways of non-homologous recombination are required in the production of the mature heavy-chain immunoglobulin (Ig) gene, i.e., V(D)J-recombination and class switch recombination. An elevated level of RAD51 is associated with stimulation of Ig heavy-chain class switch recombination by lipopolysaccharide and RAD51 foci are also observed within the nuclei of such switching B cells (21).
The RAD51-containing nuclear foci associated with class switch recombination in B cells are distinct from the corresponding foci induced by the DNA-damaging agent MMS (22). This observation suggests that the RAD51 protein performs another function in connection with class switch recombination, possibly related to non-homologous recombination.
In the present study, we have investigated the possible involvement of the RAD51 protein in non-homologous recombination in mammalian cells. The system employed for these studies was the unique Sp5 cell line obtained while selecting for spontaneous mutations in the endogenous hprt gene of V79 Chinese hamster cells with 6-thioguanine (23). The hprt gene in Sp5 cells contains a displaced partial duplication of exon 2 together with flanking intron regions, giving rise to a non-functional truncated protein (24,25) (Fig. 1A). This situation renders Sp5 cells unable to grow in medium containing HAsT (hypoxanthine, l-azaserine and thymidine), because of the blockage of de novo purine biosynthesis by l-azaserine. HAsT-containing medium thus selects for Sp5 cells that have reverted to a functional hprt gene and are able to metabolise guanine into GMP, a reaction catalysed by the HGPRT protein. In revertants from Sp5, the duplication of exon 2 has been excised by non-homologous recombination to restore the wild-type hprt gene (24,25).
Figure 1.
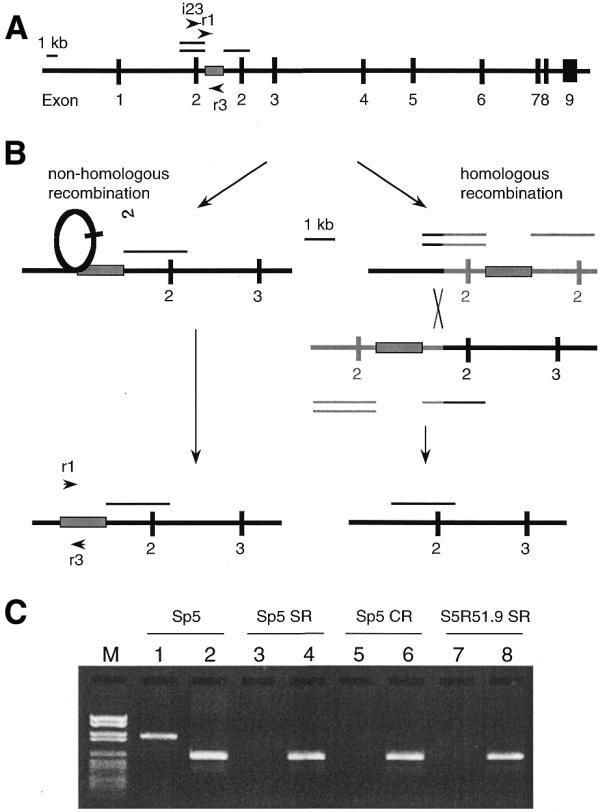
Molecular mechanisms for reversion of the hprt gene in Sp5 cells to wild-type. (A) Duplication of exon 2 in the hprt gene of the Sp5 cell line results in a non-functional gene, which can be selected for on the basis of its [HPRT]– phenotype. The single line designates the parental region, while the double lines indicate the duplicated copy of exon 2 together with flanking intron regions (24,25). The box represents the sequence between the duplicated regions. Arrowheads indicate the primers employed. (B) Recombination pathways for reversion to a [HPRT]+ phenotype: if the reversion from a [HPRT]– to a [HPRT]+ phenotype involves non-homologous recombination, the region represented by the box will be retained in the revertants. If homologous recombination is involved in this reversion event, this region will invariably be lost. (C) Lanes 1, 3, 5 and 7 contain the PCR products obtained employing the primers i23 and r3 together with DNA from Sp5 cells or from spontaneous (Sp5 SR), CPT-induced (Sp5 CR) Sp5 revertants or from spontaneous S5R51.9 revertants (S5R51.9 SR), respectively. This gel demonstrates that the duplicated exon 2 has been lost in all of these revertants. Lanes 2, 4, 6 and 8 contain the PCR products obtained employing the primers r1 and r3 together with DNA from Sp5 cells or from Sp5 SR, Sp5 CR or S5R51.9 SR, respectively, and demonstrate that the DNA region (boxed) is present in all of these revertants, i.e., that non-homologous recombination was involved in restoring the functional hprt gene. Identical results were obtained for all of the 32 revertants analysed. Marker VI (Boehringer Mannheim) was used as a size indicator. Thus, these data demonstrate that, in all revertants, the original duplication of exon 2 is lost, whereas the DNA region originally located between the duplicated regions (indicated by the box) is retained.
Sp5 cells were transfected with a constitutive mammalian expression vector containing the RAD51 cDNA and both spontaneous and induced recombination were investigated. The results suggest that RAD51 is involved in spontaneous non-homologous recombination but not in non-homologous recombination induced by the topoisomerase inhibitors camptothecin (CPT) and etoposide.
MATERIALS AND METHODS
Cell lines
The Sp5 cell line and revertants thereof were cultured under the conditions described previously (24).
Sequencing the p53 gene in Sp5 cells
Genomic DNA was extracted from Sp5 cells and PCR amplifications covering an internal part of the p53 gene were performed under conditions described previously (4). The PCR products were subsequently separated by agarose gel electrophoresis [1% (w/v) agarose in 1× TBE and stained with ethidium bromide], purified and sequenced as described elsewhere (26).
Transfection and fixation of transformants
The pZeoRAD51 [containing the Cricetulus griseus RAD51 (CgRAD51) cDNA] and pZeoSV1 (empty) constitutive expression vectors (3,4) were extracted from E.coli and subsequently transfected into 7.5 × 106 Sp5 cells by electroporation (voltage of 2.5 kV/cm, capacitance setting of 25 µF). Individual clones were isolated after culturing on Petri dishes in medium containing zeocin (500 µg/ml) and 6TG (5 µg/ml) and subsequently subcultured for 1 month.
Plasmid integration
Genomic DNA was extracted from Sp5 cells and from four transfected clones designated: S5S1.1, S5S1.5, S5R51.1 and S5R51.9. PCR amplifications designed to detect the pZeoSV1 and pZeoRAD51 vectors as well as to characterise the endogenous RAD51 gene in these cell lines were performed as described earlier (4).
Quantitation of the level of overexpression
Overexpression of RAD51 was quantitated using RT–PCR on 5 ng total RNA isolated from the Sp5, S5S1.1, S5S1.5, S5R51.1 or S5R51.9 cell lines, with the level of β-actin mRNA serving as a control, under the conditions described previously (4). To assure quantitation of RNA levels using RT–PCR, serial dilutions of total RNA were amplified by RT–PCR using the primers R51f1–R51r1 (4). All RT–PCR products were separated by agarose gel electrophoresis [2% (w/v) agarose in 1× TBE and stained with ethidium bromide].
The level of RAD51 protein was quantitated by western blotting. In this case, sub-confluent cells were trypsinised and counted; 1 × 107 cells were lysed in phosphate-buffered saline (PBS) containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and 0.1 mM phenylmethylsulfonyl fluoride on ice for 30 min and insoluble debris removed by centrifuging at 13 000 g for 20 min. An equal amount of protein (as determined by the Bio-Rad assay procedure) from each sample was mixed with SDS–gel loading buffer, boiled for 2 min and electrophoresed on a 10% SDS–polyacrylamide gel. The gel was then blotted onto a nitrocellulose membrane (Hybond ECL, Amersham Life Sciences) and blocked overnight at 4°C with 5% milk in PBS-T (PBS with 0.1% Tween-20). CgRAD51 protein was detected by incubation with rabbit polyclonal antibodies (kindly provided by Dr Stephen West) at a dilution of 1:1000 in PBS-T for 1 h. Actin was detected using rabbit antibodies raised towards a synthetic actin N-terminal peptide (A5060, Sigma), at a dilution of 1:5000 in PBS-T with an incubation time of 1 h. Incubation with secondary goat anti-rabbit antibodies conjugated to peroxidase (A9169, Sigma) at a dilution of 1:50 000 in PBS-T was then performed for 1 h in the case of both CgRAD51 and actin. Detection was achieved employing the ECLTM system (Amersham Life Sciences). Quantitation of CgRAD51 and actin was subsequently performed using the NIH Image 1.61 program. The level of overexpression of CgRAD51 was defined as the ratio between CgRAD51 and actin for each cell line.
Recombination assay
The recombination assay involved inoculation of 1.5 × 106 cells into each flask (75 cm2) and growth in medium without 6-thioguanine for 3 days. The 24 h treatment with CPT or etoposide was initiated 4 h after inoculation. After this treatment, the cells were rinsed three times with HBSS++ and subsequently allowed to recover for 48 h, after which they were removed from the flasks by trypsinisation and seeded onto Petri dishes (500 cells per dish; two dishes per dose) for cloning. In addition, 3 × 105 cells were seeded onto Petri dishes (three dishes per dose) in medium containing HAsT (50 mM hypoxanthine, 10 mM l-azaserine and 5 mM thymidine) for selection. The plates employed for cloning and selection were fixed after 7 and 10 days, respectively, using methylene blue in methanol (4 g/l) and the colonies observed then counted.
Characterisation of the reversion events
Prior to staining of the plates employed for selection in the recombination assay, individual revertant clones originating from the Sp5, CPT-treated Sp5, S5R51.1 and S5R51.9 cell lines were isolated and cultured in medium containing HAsT for 14 days. Thereafter, their genomic DNA was extracted and PCR amplification performed employing the primers i23 (5′-CCATC CCTAA TGAGA TCTGG TGCCC-3′), r1 (5′-GCTCT TTGTG GAAGC AGCCA TTCT-3′) and r3 (5′-GGCAG TCACC AACAG GAAAA TACC-3′) under the following conditions: 2.5 µg template DNA was mixed with 10 pmol of each primer, 12.5 nmol dNTPs, 5 µl 10× PCR Buffer 1, 0.875 U DNA Polymerase Mix Enzyme (Expand™ Long Template PCR System; Boehringer Mannheim) and 0.1% diethyl pyrocarbonate-treated distilled H2O to obtain a final volume of 50 µl. The optimal PCR cycle was found to be: 94°C for 1 min; (94°C for 15 s, 60°C for 30 s, 72°C for 1 min)35; 72°C for 10 min; 4°C. The PCR products thus obtained were separated by agarose gel electrophoresis (1% agarose in 1× TBE) and stained with ethidium bromide.
RESULTS
Characterisation of the p53 gene in the Sp5 cell line
The p53 gene in the Sp5 cell line was found to contain two point mutations in the sequence coding for the DNA-binding domain of the p53 protein (data not shown). These two point mutations (L136Q and C138W) have previously been suggested to inactivate the p53 protein (27).
Establishment of stably transfected cells overexpressing RAD51
Sp5 cells were transfected with the control pZeoSV1 (empty) or pZeoRAD51 (containing RAD51 cDNA) expression vectors employed previously (3,4). These plasmids carry the Sh ble gene, which encodes a protein conferring resistance to zeocin. pZeoRAD51 (constructed as described previously; 3) resulted in a constitutive and stable overexpression of RAD51 in transfected cells. Two pZeoSV1-transfected (designated S5S1.1 and S5S1.5) and two pZeoRAD51-transfected (S5R51.1 and S5R51.9) cell lines, all selected randomly, were isolated and integration of the plasmid into the genomic DNA verified as described previously (4; data not shown). The growth of these transfected cell lines was similar to that of the parent cell line (data not shown). Overexpression of RAD51 in such transfected cell lines has been verified earlier employing western blotting and RT–PCR (3,4). In the present study, the level of RAD51 in the S5R51.1 and S5R51.9 clones was found to be ∼2–3-fold higher than in Sp5, S5S1.1 and S5S1.5 cells (controls), again as determined by RT–PCR (Fig. 2A) and western blotting (Fig. 2B). This increase is similar to that obtained previously upon transfection with the pZeoRAD51 vector (3,4).
Figure 2.
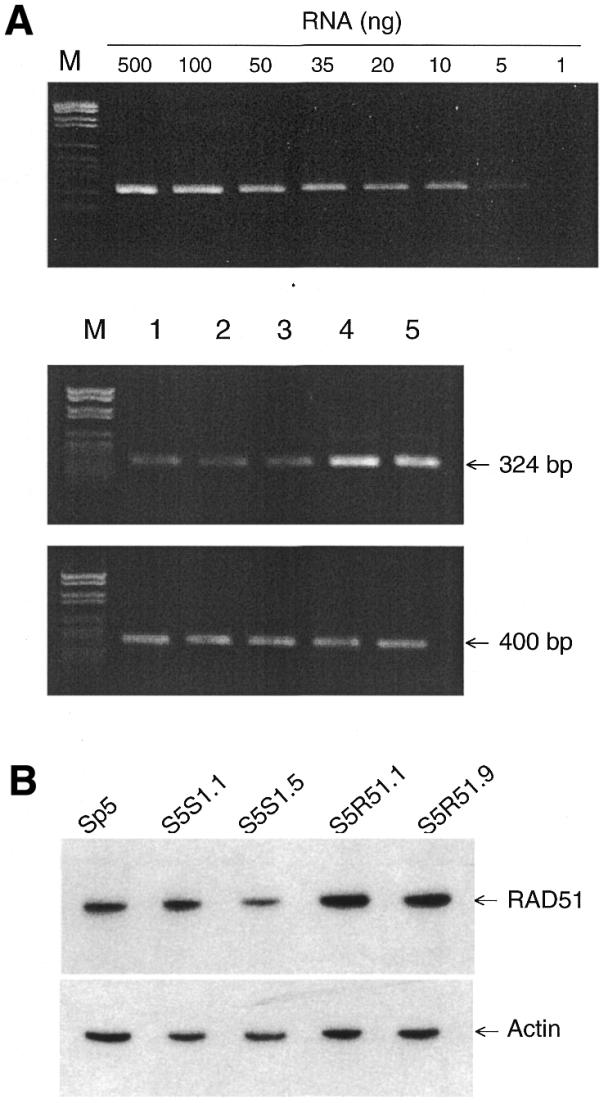
Overexpression of RAD51 in cell lines stably transfected with pZeoRAD51. Sp5 cells were stably transfected and plasmid integration into the genomic DNA verified employing methods described earlier (4). (A) A series of dilutions of total RNA were amplified by RT–PCR using the primers R51f1–R51r1 to ensure that the RT–PCR was quantitative. Overexpression of RAD51 was examined by RT–PCR using 5 ng of the total RNA isolated from Sp5, S5S1.1, S5S1.5, S5R51.1 and S5R51.9 cells (lanes 1–5, respectively), with the level of β-actin mRNA serving as a control, under conditions described previously (4). Marker VI (Boehringer Mannheim) was used as a size indicator. (B) Levels of RAD51 and actin proteins were quantitated by western blotting of extracts from Sp5, S5S1.1, S5S1.5, S5R51.1 and S5R51.9 cells. The overexpression level of CgRAD51 was determined as the ratio between CgRAD51 and actin in each cell line.
Non-homologous recombination in the Sp5 cell line
We employed the Sp5 cell line, which has previously been used as a tool to study non-homologous recombination (28). The duplication of exon 2 together with flanking intron regions in the Sp5 cell line is displaced; i.e., there is a short sequence between the duplicated segments (Fig. 1A). Theoretically, the reversion from [HPRT]– to [HPRT]+ phenotype can be the result of either homologous or non-homologous recombination (Fig. 1B). If the sequence located between the duplicated regions is retained, reversion involves non-homologous recombination, while the loss of this region implies that the reversion event occurred by homologous recombination. By means of PCR amplification (Fig. 1C), the duplicated region was shown to be absent from all the revertants, demonstrating that the restoration of the [HPRT]+ phenotype is invariably associated with loss of the extra copy of exon 2. Furthermore, the sequence located between the duplicated regions in the Sp5 cell line was present in all revertants examined, i.e., in 16 spontaneous and eight CPT-induced revertants as well as in eight revertants overexpressing RAD51. The retention of this sequence indicates that the reversion process can only involve non-homologous recombination (Fig. 1B). No sequence homologies, such as those mediating recombination in transposons or between Alu repeats, were present at the ends of the duplicated segment. Nonetheless, the high frequency of spontaneous reversion in this region suggests that it might be a ‘hotspot’ for recombination of a non-homologous nature.
Effects of the overexpression of RAD51 on spontaneous and induced non-homologous recombination
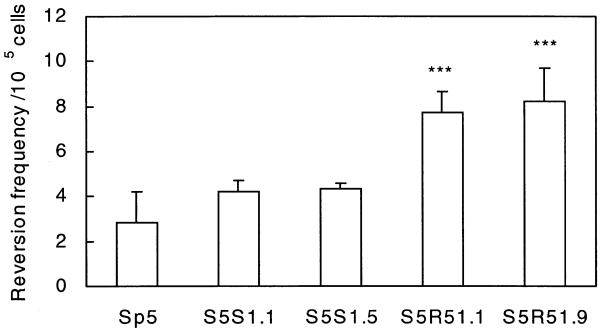
To investigate the potential role of RAD51 in non-homologous recombination, the reversion frequency to a [HPRT]+ phenotype was compared between the parental Sp5 cell line, RAD51 overexpressing cells and control cells. In comparison to the parental Sp5 cell line, the frequency of spontaneous non-homologous recombination was increased ∼2-fold in S5R51.1 and S5R51.9 cells (P < 0.001) and was unaltered in S5S1.1 and S5S1.5 control cells (Fig. 3). These data indicate that the RAD51 protein supports spontaneous non-homologous recombination of the type found in Sp5 cells.
Figure 3.
Increased frequency of spontaneous non-homologous recombination in cell lines overexpressing RAD51. The frequency of spontaneous non-homologous recombination in these cell lines was measured as the reversion to the [HPRT]+ phenotype. The S5R51.1 and S5R51.9 cell lines, which overexpress RAD51, demonstrated a statistically significant increase in the frequency of spontaneous non-homologous recombination compared to the control cell lines (***, P < 0.001 in both cases). The error bars indicate the standard deviations of values from four independent experiments.
In contrast, when Sp5 and S5R51.9 cells were treated with CPT or etoposide, the same dose–response relationship was observed and the increased frequency of non-homologous recombination was additive to that caused by overexpression of RAD51 (Fig. 4). This indicates that RAD51 is either not involved in or not rate-limiting for induced non-homologous recombination in these cell lines.
Figure 4.
Lack of influence of overexpression of RAD51 on the frequency of induced non-homologous recombination. The level of non-homologous recombination in cells treated over 24 h with (A) CPT or (B) etoposide. The reversion frequencies reflect the frequencies of recombinational events. The error bars indicate the standard errors of values from two independent experiments.
DISCUSSION
There are several pathways of non-homologous recombination in mammalian cells. All of these pathways probably employ the Ku70, Ku80 and DNA-PKcs proteins (15–20). However, only class switch recombination has been implicated to involve RAD51 (21). Also, this is the only pathway that has been suggested to involve homologous pairing between RNA/DNA hybrids (29,30).
In the present study, the frequency of spontaneous reversion of [HPRT]– Sp5 cells to the [HPRT]+ phenotype was shown to be increased by overexpression of RAD51. Such reversion involves non-homologous recombination and was found to be of the same nature as spontaneous reversion (Fig. 1). Therefore, we propose that RAD51 may play a role in spontaneous non-homologous recombination in mammalian cells.
We report that non-homologous recombination is always involved in the reversion event in the hprt gene of Sp5 cells. Moreover, the high frequency of spontaneous reversion suggests that the region examined might be a ‘hotspot’ for recombination of a non-homologous nature. Theoretically, the reversion event could involve homologous recombination between the two copies of exon 2 (Fig. 1), which would invariably involve loss of a large portion of intron 1. However, no viable revertants from Sp5 cells were identified lacking this portion of intron 1 and one explanation could be that this part of intron 1 may contain the branch site used in the splicing process.
The reversion frequency of Sp5 cells can be elevated in a dose-dependent fashion by treatment with various agents (31). CPT (an inhibitor of topoisomerase I) has previously been reported to induce recombination in this cell line; whereas etoposide (an inhibitor of topoisomerase II) is without effect (31).
CPT induces DNA double-strand breaks associated with replication forks (32), that in turn can recruit the Ku proteins which activate the DNA-PKcs, known to be involved in non-homologous end-joining (33). Furthermore, cells deficient in non-homologous end-joining exhibit increased sensitivity to CPT (C.Arnaudeau, C.Lundin and T.Helleday, submitted for publication), which altogether suggests that CPT-induced DNA double-strand breaks are, to some extent, repaired by non-homologous end-joining. Here, an additive effect on the CPT-induced reversion frequency was seen in Sp5 cells overexpressing RAD51 (Fig. 4A). This indicates that the level of RAD51 does not influence induction of non-homologous recombination in Sp5 cells. Also, this might indicate that RAD51 is not involved in or rate limiting in non-homologous end-joining induced by CPT.
Although etoposide does not induce non-homologous recombination in Sp5 cells, this inhibitor has been shown to induce homologous recombination in mammalian cells in culture (34). Furthermore, nuclear foci containing RAD51 have been found to accumulate at single-stranded DNA regions after etoposide treatment (35,36). These observations make it probable that RAD51 is involved in the repair of etoposide-induced DNA damage by homologous recombination. At the same time, Ku70- and Ku80-deficient cell lines are hypersensitive to DNA damage caused by etoposide (37), indicating that at least some components of non-homologous end-joining are also involved in repairing such DNA damage. However, the lack of response of the S5R51.9 cell line to etoposide suggests that the pathway for non-homologous end-joining used to repair etoposide-induced DNA damage differs from the non-homologous recombination investigated here and, thus, the former might not involve the RAD51 protein.
Nuclear RAD51-containing foci associated with B cell activation differ from the corresponding foci induced by the DNA-damaging agent MMS (22), suggesting that the RAD51 protein might have several different functions. MMS has also been shown to induce homologous, but not non-homologous recombination (28), in agreement with these observations.
Previously, we have reported that RAD51 is involved in homologous recombination in mammalian cells (3,4). The present findings suggest that RAD51 might also be involved in non-homologous recombination. Although RAD51 mediates end-to-end ligation in vitro (1), we believe that this might not necessarily be the molecular basis for the involvement of RAD51 in non-homologous recombination, since RAD51 does not play an essential role in non-homologous end-joining (38). Additional support for this view comes from the fact that RAD51 appears not to change the level of non-homologous recombination after treatment with CPT or etoposide (Fig. 4).
Considered together, these data suggest that the non-homologous recombination involving RAD51 studied here is not related to non-homologous end-joining and the repair of double-strand breaks.
RAD51 is expressed at high levels in the thymus and spleen (7) and enrichment of RAD51 in nuclear foci has been observed after induction of class switch recombination by lipopolysaccharide (21). In fibroblasts, the level of RAD51 expression is normally low and overexpression of RAD51 in this cell type might provide a valuable model for the normal situation in the thymus and spleen. The results presented here demonstrate that, in the case of fibroblasts, an increased level of RAD51 is associated with an increased frequency of non-homologous recombination, which might possibly reflect the biological function of RAD51 in the thymus and spleen.
Class switch recombination is a process executed in the thymus and spleen and both the Ku70 and Ku80 proteins also play essential roles in connection with this recombination process, implying the involvement of non-homologous recombination (17,18). RNA/DNA hybrids, possibly involving pairing of homologous sequences, have been suggested to be intermediates in class switch recombination (29,30), which is, as in the case in Sp5, a pathway of non-homologous recombination that to some extent differs from non-homologous end-joining.
We speculate that a possible function of the RAD51 protein in non-homologous recombination could be related to the homologous pairing required for the formation of RNA/DNA hybrids. Furthermore, the putative role of RAD51 to form RNA/DNA hybrids is supported in that its bacterial homologue RecA has been shown to mediate homologous pairing between RNA/DNA hybrids (39,40). However, the putative role of RAD51 in this context remains to be investigated.
In conclusion, we suggest that the mechanism underlying spontaneous non-homologous recombination in the Sp5 cell line involves the RAD51 protein. Furthermore, we speculate that this finding might be related to the function of this protein in relation to homologous pairing of RNA/DNA hybrids that has been suggested in class switch recombination in B cells (29,30). In addition, we report that the RAD51 protein seems not to be involved in induced non-homologous recombination by CPT or etoposide and, therefore, probably not involved in the repair of double-strand breaks via such a mechanism (i.e., non-homologous end-joining).
Acknowledgments
ACKNOWLEDGEMENTS
We wish to thank Drs Stephen West and Fiona Benson for their generous gift of the anti-RAD51 antibody and Dr Mark Meuth and John Hinz for critically reading this manuscript. The Lawski Foundation, the Swedish Cancer Society, the Swedish Foundation for Strategic Environmental Research (MISTRA), EU 5th frame work program contract no. QLK4-1999-01142 and ARC grant no. 9238 supported this work.
References
- 1.Baumann P., Benson,F.E. and West,S.C. (1996) Human RAD51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell, 87, 757–766. [DOI] [PubMed] [Google Scholar]
- 2.Xia S.J., Shammas,M.A. and Reis,R.J. (1997) Elevated recombination in immortal human cells is mediated by HsRAD51 recombinase. Mol. Cell. Biol., 17, 7151–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vispe S., Cazaux,C., Lesca,C. and Defais,M. (1998) Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res., 26, 2859–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnaudeau C., Helleday,T. and Jenssen,D. (1999) The RAD51 protein supports homologous recombination by an exchange mechanism in mammalian cells. J. Mol. Biol., 289, 1231–1238. [DOI] [PubMed] [Google Scholar]
- 5.Shinohara A., Ogawa,H., Matsuda,Y., Ushio,N., Ikeo,K. and Ogawa,T. (1993) Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nature Genet., 4, 239–243. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura Y., Morita,T., Yamamoto,A. and Matsushiro,A. (1993) Cloning and sequence of the human RecA-like gene cDNA. Nucleic Acids Res., 21, 1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morita T., Yoshimura,Y., Yamamoto,A., Murata,K., Mori,M., Yamamoto,H. and Matsushiro,A. (1993) A mouse homologue of the Escherichia coli RecA and Saccharomyces cerevisiae RAD51 genes. Proc. Natl Acad. Sci. USA, 90, 6577–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim D.S. and Hasty,P. (1996) A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol., 16, 7133–7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuzuki T., Fujii,Y., Sakumi,K., Tominaga,Y., Nakao,K., Sekiguchi,M., Matsushiro,A., Yoshimura,Y. and Morita,T. (1996) Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc. Natl Acad. Sci. USA, 93, 6236–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonoda E., Sasaki,M.S., Buerstedde,J.M., Bezzubova,O., Shinohara,A., Ogawa,H., Takata,M., Yamaguchi-Iwai,Y. and Takeda,S. (1998) Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J., 17, 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumann P. and West,S.C. (1998) Role of the human RAD51 protein in homologous recombination and double-stranded-break repair. Trends Biochem. Sci., 23, 247–251. [DOI] [PubMed] [Google Scholar]
- 12.Thompson L.H. and Schild,D. (1999) The contribution of homologous recombination in preserving genome integrity in mammalian cells. Biochimie, 81, 87–105. [DOI] [PubMed] [Google Scholar]
- 13.Yanez R.J. and Porter,A.C. (1999) Gene targeting is enhanced in human cells overexpressing hRAD51. Gene Ther., 6, 1282–1290. [DOI] [PubMed] [Google Scholar]
- 14.Haaf T., Golub,E.I., Reddy,G., Radding,C.M. and Ward,D.C. (1995) Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc. Natl Acad. Sci. USA, 92, 2298–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taccioli G.E., Gottlieb,T.M., Blunt,T., Priestley,A., Demengeot,J., Mizuta,R., Lehmann,A.R., Alt,F.W., Jackson,S.P. and Jeggo,P.A. (1994) Ku80: product of the XRCC5 gene and its role in DNA repair and V(D)J recombination. Science, 265, 1442–1445. [DOI] [PubMed] [Google Scholar]
- 16.Blunt T., Finnie,N.J., Taccioli,G.E., Smith,G.C., Demengeot,J., Gottlieb,T.M., Mizuta,R., Varghese,A.J., Alt,F.W. and Jeggo,P.A. (1995) Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell, 80, 813–823. [DOI] [PubMed] [Google Scholar]
- 17.Casellas R., Nussenzweig,A., Wuerffel,R., Pelanda,R., Reichlin,A., Suh,H., Qin,X.F., Besmer,E., Kenter,A., Rajewsky,K. and Nussenzweig,M.C. (1998) Ku80 is required for immunoglobulin isotype switching. EMBO J., 17, 2404–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manis J.P., Gu,Y., Lansford,R., Sonoda,E., Ferrini,R., Davidson,L., Rajewsky,K. and Alt,F.W. (1998) Ku70 is required for late B cell development and immunoglobulin heavy chain class switching. J. Exp. Med., 187, 2081–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karran P. (2000) DNA double strand break repair in mammalian cells. Curr. Opin. Genet. Dev., 10, 144–150. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer P., Goedecke,W. and Obe,G. (2000) Mechanisms of DNA double-strand break repair and their potential to induce chromosomal aberrations. Mutagenesis, 15, 289–302. [DOI] [PubMed] [Google Scholar]
- 21.Li M.J., Peakman,M.C., Golub,E.I., Reddy,G., Ward,D.C., Radding,C.M. and Maizels,N. (1996) Rad51 expression and localization in B cells carrying out class switch recombination. Proc. Natl Acad. Sci. USA, 93, 10222–10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M.J. and Maizels,N. (1997) Nuclear Rad51 foci induced by DNA damage are distinct from Rad51 foci associated with B cell activation and recombination. Exp. Cell Res., 237, 93–100. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L.H., Vrieling,H., van Zeeland,A.A. and Jenssen,D. (1992) Spectrum of spontaneously occurring mutations in the hprt gene of V79 Chinese hamster cells. J. Mol. Biol., 223, 627–635. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L.H. and Jenssen,D. (1992) Reversion of the hprt mutant clone SP5 by intrachromosomal recombination. Carcinogenesis, 13, 609–615. [DOI] [PubMed] [Google Scholar]
- 25.Dare E., Zhang,L.H. and Jenssen,D. (1996) Characterization of mutants involving partial exon duplications in the hprt gene of Chinese hamster V79 cells. Somat. Cell Mol. Genet., 22, 201–210. [DOI] [PubMed] [Google Scholar]
- 26.Helleday T., Arnaudeau,C. and Jenssen,D. (1998) A partial hprt gene duplication generated by non-homologous recombination in V79 Chinese hamster cells is eliminated by homologous recombination. J. Mol. Biol., 279, 687–694. [DOI] [PubMed] [Google Scholar]
- 27.Chaung W., Mi,L.J. and Boorstein,R.J. (1997) The p53 status of Chinese hamster V79 cells frequently used for studies on DNA damage and DNA repair. Nucleic Acids Res., 25, 992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helleday T., Arnaudeau,C. and Jenssen,D. (1998) Effects of carcinogenic agents upon different mechanisms for intragenic recombination in mammalian cells. Carcinogenesis, 19, 973–978. [DOI] [PubMed] [Google Scholar]
- 29.Reaban M.E. and Griffin,J.A. (1990) Induction of RNA-stabilized DNA conformers by transcription of an immunoglobulin switch region. Nature, 348, 342–344. [DOI] [PubMed] [Google Scholar]
- 30.Muller J.R., Giese,T., Henry,D.L., Mushinski,J.F. and Marcu,K.B. (1998) Generation of switch hybrid DNA between Ig heavy chain-mu and downstream switch regions in B lymphocytes. J. Immunol ., 161, 1354–1362. [PubMed] [Google Scholar]
- 31.Zhang L.H. and Jenssen,D. (1994) Studies on intrachromosomal recombination in SP5/V79 Chinese hamster cells upon exposure to different agents related to carcinogenesis. Carcinogenesis, 15, 2303–2310. [DOI] [PubMed] [Google Scholar]
- 32.Strumberg D., Pilon,A.A., Smith,M., Hickey,R., Malkas,L. and Pommier,Y. (2000) Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5′-phosphorylated DNA double-strand breaks by replication runoff. Mol. Cell. Biol., 20, 3977–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao R.G., Cao,C.X., Zhang,H., Kohn,K.W., Wold,M.S. and Pommier,Y. (1999) Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J., 18, 1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnaudeau C., Tenorio Miranda,E., Jenssen,D. and Helleday,T. (2000) Inhibition of DNA synthesis is a potent mechanism by which cytostatic drugs induce homologous recombination in mammalian cells. Mutat. Res., 461, 221–228. [DOI] [PubMed] [Google Scholar]
- 35.Haaf T., Raderschall,E., Reddy,G., Ward,D.C., Radding,C.M. and Golub,E.I. (1999) Sequestration of mammalian Rad51-recombination protein into micronuclei. J. Cell Biol., 144, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raderschall E., Golub,E.I. and Haaf,T. (1999) Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc. Natl Acad. Sci. USA, 96, 1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin S., Inoue,S. and Weaver,D.T. (1998) Differential etoposide sensitivity of cells deficient in the Ku and DNA-PKcs components of the DNA-dependent protein kinase. Carcinogenesis, 19, 965–971. [DOI] [PubMed] [Google Scholar]
- 38.Hanakahi L.A., Bartlet-Jones,M., Chappell,C., Pappin,D. and West,S.C. (2000) Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair. Cell, 102, 721–729. [DOI] [PubMed] [Google Scholar]
- 39.Kirkpatrick D.P., Rao,B.J. and Radding,C.M. (1992) RNA-DNA hybridization promoted by E.coli RecA protein. Nucleic Acids Res., 20, 4339–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkpatrick D.P. and Radding,C.M. (1992) RecA protein promotes rapid RNA-DNA hybridization in heterogeneous RNA mixtures. Nucleic Acids Res., 20, 4347–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]