Abstract
Background
The probiotic Escherichia coli strain Nissle 1917 (EcN) has been shown to interfere in a human in vitro model with the invasion of several bacterial pathogens into epithelial cells, but the underlying molecular mechanisms are not known.
Methodology/Principal Findings
In this study, we investigated the inhibitory effects of EcN on Salmonella Typhimurium invasion of porcine intestinal epithelial cells, focusing on EcN effects on the various stages of Salmonella infection including intracellular and extracellular Salmonella growth rates, virulence gene regulation, and adhesion. We show that EcN affects the initial Salmonella invasion steps by modulating Salmonella virulence gene regulation and Salmonella SiiE-mediated adhesion, but not extra- and intracellular Salmonella growth. However, the inhibitory activity of EcN against Salmonella invasion always correlated with EcN adhesion capacities. EcN mutants defective in the expression of F1C fimbriae and flagellae were less adherent and less inhibitory toward Salmonella invasion. Another E. coli strain expressing F1C fimbriae was also adherent to IPEC-J2 cells, and was similarly inhibitory against Salmonella invasion like EcN.
Conclusions
We propose that EcN affects Salmonella adhesion through secretory components. This mechanism appears to be common to many E. coli strains, with strong adherence being a prerequisite for an effective reduction of SiiE-mediated Salmonella adhesion.
Introduction
E. coli Nissle 1917 (EcN; Mutaflor) is a widely employed probiotic strain and several in vivo studies demonstrated its promising probiotic activity in humans and animals [1], [2], [3], [4], [5], [6]. Proposed probiotic actions of EcN include effects on pathogens, host epithelial cells, host smooth muscle cell activity and the host immune system [7], [8], [9], [10], [11], [12], [13]. In vitro, EcN has been shown to prevent invasion of host cells by several pathogens, including Salmonella, Yersinia, Shigella, Legionella, Listeria and adherent-invasive E. coli [14], [15], [16]. These studies demonstrated that EcN inhibited invasion in a dose-dependent manner. Interestingly, EcN supernatants were also effective in inhibiting invasion. However, the underlying molecular mechanisms involved in this process are poorly understood to date.
Successful probiotic action of a bacterial strain is often associated with its colonization of the intestine. The colonization of hosts by EcN can be very successful, but is likely specific for each individual [15], [17]. EcN has been shown to express type 1 fimbriae and F1C fimbriae (which have usually been associated with uropathogenic E. coli), but not P and S fimbriae [18]. Recently, it was demonstrated that EcN F1C fimbriae or cellulose production play an important role in EcN biofilm formation, adherence to intestinal epithelial cells in vitro, and intestinal colonization of mice [19], [20].
The aim of the present study was to characterize the effects of EcN on Salmonella invasion of the porcine intestinal epithelial cell line IPEC-J2 [21], with a focus on EcN effects on single Salmonella infection steps. In addition, EcN adhesion capacities were tested as possible requisites for an EcN specific probiotic activity. We show that adhesion rates always correlate with inhibitory effects against Salmonella invasion, and the initial Salmonella invasion process, especially adhesion, is likely affected.
Materials and Methods
Bacterial strains used in this study
E. coli Nissle 1917 DSM6601 (O6:H1:K5, EcN) was kindly provided by G. Breves (Hannover, Germany). E. coli MG1655 was kindly provided by C.A. Gross (San Francisco, USA). Salmonella enterica serovar Typhimurium (Salmonella Typhimurium) strain SL1344 was kindly provided by F. Norel (Paris, France). SL1344::kan was generated by introduction of a kanamycin resistance cassette into the downstream, non-coding region between the avrA and sitD genes of Salmonella typhimurium LT2 as previously described [22]. Following PCR screening for correct insertion and orientation, the kanamycin resistance cassette was introduced into strain SL1344 by bacteriophage P22 transduction using standard protocols. The non-invasive Salmonella Typhimurium strain VV341 (SL1344 hilA-339::kan) was kindly provided by C.A. Lee (Boston, USA). The non-invasive Salmonella Typhimurium strain SB161 (SL1344 invG) was kindly provided by M. Hensel (Erlangen, Germany) and was transformed with the pEGFP plasmid. β-Galactosidase assays were performed with strains CL87 (SL1344 hilA(iagB)::lacZY), EE635 (SL1344 hilC9::Tn5lacZY), and RL696 (SL1344 hilD696::lacZY) provided by C.A. Lee. Strain KT4166 harboring a lacZ fusion to the SPI4-encoded siiE gene (SL1344 icgA1(siiE)::MudJ(kan)) was constructed by P22 transduction of the siiE::MudJ(kan) fusion into strain SL1344 with kanamycin selection using lysates prepared on strain JS296 (J.M. Slauch, Urbana, USA).
E. coli 140815 (IMT13962) was isolated from feces of a clinically healthy pig and found by PCR to be negative for the virulence genes eae, stx2e, faeG, fanA, fasA, fedA, fimF41a, est-1a, eltB-Ip [15]. A Δfim mutant of EcN was provided by T. Ölschläger [14]. Additional ΔfocA and ΔfliA mutants of EcN were generated in this study using the protocol of Datsenko and Wanner [22]. Both mutants were complemented with the plasmid pACYC177 harboring sequences encoding focA or fliA of E. coli CFT073. This was necessary as there were no gene sequences from EcN for focA and fliA available, but the genome of EcN shows high homology to uropathogenic E. coli CFT073 [18]. E. coli WS15C1, WS30C1 and WS46C1 were isolated from intestinal contents of wild boars ([23] and this study).
In vitro assays
IPEC-J2 cell culture conditions
The porcine intestinal epithelial cell line IPEC-J2 [21] was grown in Dulbecco's modified Eagle Medium (DMEM) HAM'S/F-12 (1∶1) (Biochrom, Germany), supplemented with 5% fetal calf serum, and maintained in an atmosphere of 5% CO2 at 37°C. Cells reached confluence after 3–4 days and were used consistently within 8 days from seeding. Cell cultures were tested routinely and found to be free from mycoplasma contamination.
In vitro Salmonella invasion assays
Invasion assays were performed essentially as previously described [24]. E. coli were grown in LB medium to an optical density at 600 nm (OD600) of approximately 1, washed by centrifugation, re-suspended in cell culture medium and adjusted by dilution to provide a multiplicity of infection (MOI) of 100∶1 or 10∶1 E. coli to host cells in culture wells of a 24-well plate using a conversion of approximately 3×108 bacteria/ml/OD600. Confluent monolayers of IPEC-J2 cells were first incubated with the respective E. coli strain for 2 or 6 hours at 37°C. Cells were washed three times with PBS to remove non-adherent E. coli. S. Typhimurium was grown in LB medium to an OD600 of approximately 2, washed by centrifugation, re-suspended in cell culture medium and adjusted by dilution to provide a MOI of 100∶1 or 1∶1 Salmonella to host cells using a conversion of approximately 3×108 bacteria/ml/OD600. Confluent monolayers were infected after E. coli pre-incubation for one hour, followed by an additional hour of incubation in media containing 50 µg/ml gentamicin to kill extracellular bacteria. Infected cells were washed twice with PBS and lysed with 0.1% Triton X-100 in deionized, distilled water. Dilutions of the resulting cell lysates were plated on LB agar plates for determination of intracellular bacterial counts.
For kinetics of intracellular Salmonella growth, IPEC-J2 cells were infected with Salmonella Typhimurium at a MOI of 1∶1 for one hour, with an additional incubation of one hour in media containing 50 µg/ml gentamicin to kill extracellular bacteria (time point “2 hours”). IPEC-J2 cells were washed three times with media and incubated with the respective E. coli strain for 2 hours with a MOI of 100∶1 E. coli to host cells, followed by incubation in media containing 50 µg/ml gentamicin for one hour to kill extracellular bacteria (time point “5 hours”). Finally, cells were incubated over 19 hours in media containing 10 µg/ml gentamicin (end time point “24 hours”).
To determine the effects of EcN in mixed E. coli cultures, EcN was mixed with other E. coli in a ratio of 1∶1 (mixture with one other E. coli strain) or in a ratio of 1∶1∶1 (mixture with two other E. coli strains) with a final MOI in the mixture of 100∶1 E. coli to host cells.
To determine the effects of E. coli supernatants, E. coli were grown in DMEM HAM'S/F-12 (1∶1) supplemented with 5% fetal calf serum at 37°C to an OD600 of 1. Bacteria were centrifuged at 800×g for 5 min. Supernatants were sterile-filtered (pore size 0.22 µm) and used in the cell culture assays.
To determine the effects of E. coli supernatants on Salmonella growth rates, Salmonella counts were determined in the culture supernatants during the respective invasion assays. After a one-hour incubation step with Salmonella, the supernatant of each well was removed and plated in serial dilutions on LB agar plates. Invasion and adhesion assays were performed in duplicate.
In vitro Salmonella adhesion assays
Salmonella adhesion assays with SL1344 hilA-339::kan were performed similarly to the invasion assays. E. coli were grown in LB medium to an OD600 of approximately 1, washed by centrifugation, re-suspended in cell culture medium and adjusted by dilution to provide a MOI of 100∶1 E. coli to host cells in culture wells of a 24-well plate. Confluent monolayers of IPEC-J2 cells were first incubated with the respective E. coli strain for 2 or 6 hours at 37°C. Cells were washed three times with PBS to remove non-adherent E. coli. SL1344 hilA-339::kan was grown in LB medium to an OD600 of approximately 2, washed by centrifugation, re-suspended in cell culture medium and adjusted by dilution to provide a MOI of 100∶1 Salmonella to host cells. Confluent monolayers were infected after E. coli pre-incubation for one hour. After the one hour incubation of IPEC-J2 cells with SL1344 hilA-339::kan, cells were washed 3 times with PBS to remove non-adherent Salmonella. IPEC-J2 cells were lysed and lysates were plated on to LB agar plates containing 50 µg/ml kanamycin for determination of adherent Salmonella counts.
Salmonella adhesion assays with SL1344 pEGFP invG-339::kan were performed similarly to the Salmonella adhesion assays with SL1344 hilA-339::kan until the washing step after the 1 hour incubation with Salmonella. After washing IPEC-J2 cells 3 times with PBS to remove non-adherent Salmonella, cell culture plates were analyzed by the Aklides apparatus (GA Generic Assays GmbH, Germany). This apparatus automatically recognizes fluorescent Salmonella cells, photographs cell monolayers with adherent fluorescent bacteria, and analyses pictures by counting fluorescent cell numbers. IPEC-J2 cell lysis, dilutions of lysates, plating of dilutions and counting of bacterial colonies are omitted.
In vitro E. coli adhesion assays
E. coli were grown in LB medium to an OD600 of approximately 1, washed by centrifugation, re-suspended in cell culture medium and adjusted by dilution to provide a MOI of 100∶1 E. coli to host cells in culture wells of a 24-well plate. Confluent monolayers of IPEC-J2 cells were incubated with the respective E. coli strain (EcN, EcN mutants, E. coli WS15C1, E. coli WS30C1 and E. coli WS46C1) for 2 hours at 37°C. Cells were washed three times with PBS to remove non-adherent E. coli. IPEC-J2 cells were lysed and lysates were plated on to LB agar plates for determination of adherent E. coli counts.
β-Galactosidase assays
β-Galactosidase assays were performed as previously described [25], [26] with Salmonella strains grown aerobically to late-log/early stationary phase (OD600 ∼2), unless otherwise noted.
Statistical analysis
P values were calculated using the Student's t test implemented in the Statistical Package for the Social Sciences (SPSS Statistics; version 17.0).
Results
E. coli Nissle 1917 as well as E. coli supernatants inhibit Salmonella invasion into IPEC-J2 cells
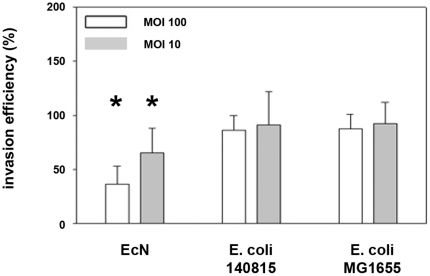
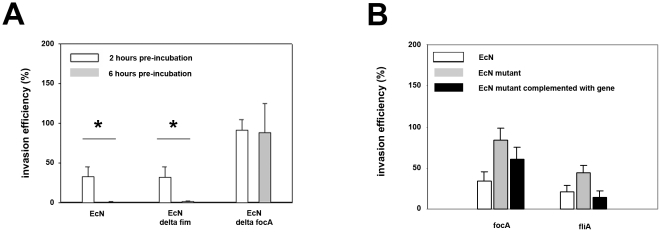
Initially, we verified a probiotic effect of E. coli Nissle 1917 (EcN) on Salmonella invasion of porcine intestinal epithelial cells (IPEC-J2). We compared the effects of EcN with those of two control E. coli strains (E. coli 140815 and E. coli MG1655), and the effects of EcN in a mix with these two strains, and included two multiplicities of infections (MOI). In general, employing the gentamicin protection assay the invasion efficiency of Salmonella Typhimurium strain SL1344 into the porcine intestinal epithelial cell line IPEC-J2 was 27%. A two-hour pre-incubation of IPEC-J2 cells with EcN resulted in a decrease in Salmonella invasion efficiency, while pre-incubation with E. coli 140815 or E. coli MG1655 did not. This effect was stronger using an MOI of 100∶1 (E. coli:epithelial cells) compared to 10∶1 (Figure 1). The inhibitory effect of EcN was markedly increased by a pre-incubation period of six hours compared to two hours (Figure 2A).
Figure 1. Invasion efficiency of Salmonella Typhimurium into IPEC-J2 cells after pre-incubation with E. coli Nissle 1917.
Confluent monolayers of IPEC-J2 cells were pre-incubated with E. coli Nissle 1917 (EcN), E. coli 140815 or E. coli MG1655 at an MOI of 100∶1 or 10∶1 bacteria to host cells. After two hours, cells were washed and infected with Salmonella Typhimurium using an MOI of 100∶1 Salmonella to host cells. Invasion levels in percent (%) are expressed as invasion of Salmonella relative to invasion without pre-incubation with E. coli (Salmonella mono-infection). The data are the mean ± S.E.M. of at least three separate experiments in duplicate wells. * = p<0.01 compared to Salmonella mono-infection.
Figure 2. Inhibitory effects of E. coli Nissle 1917 on Salmonella Typhimurium invasion is dependent on adhesion.
Confluent monolayers of IPEC-J2 cells were pre-incubated with E. coli Nissle 1917 (EcN), EcN ΔfocA, EcN Δfim or EcN ΔfliA using an MOI of 100∶1 E. coli to host cells. After two or six hours, cells were washed and infected with Salmonella Typhimurium using an MOI of 100∶1 Salmonella to host cells. Invasion levels in percent (%) are expressed as invasion of Salmonella relative to invasion without pre-incubation with E. coli (Salmonella mono-infection). The data are the mean ± S.E.M. of at least three separate experiments in duplicate wells. * = p<0.01 compared to Salmonella mono-infection. A) Effects of EcN ΔfocA and EcN Δfim mutants on Salmonella invasion after a 2 or 6 hours pre-incubation period. B) Effects of EcN ΔfocA and EcN ΔfliA mutants and their respective strains complemented with the plasmid pACYC 177 containing the relevant gene on Salmonella invasion after 2 hours pre-incubation.
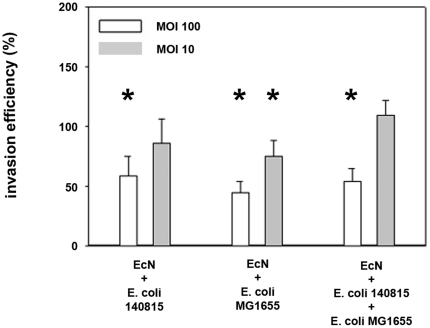
Inhibition of Salmonella invasion by EcN was also observed in mixed E. coli cultures although the effects on invasion were less effective in mixed E. coli cultures compared to EcN in the monoculture model. Using a mixture of EcN:E. coli 140815:E. coli MG1655 (1∶1∶1), at a total MOI of 10∶1 the inhibitory effects of EcN were abolished (Figure 3).
Figure 3. Invasion efficiency of Salmonella Typhimurium into IPEC-J2 cells after pre-incubation with E. coli mixed cultures.
Confluent monolayers of IPEC-J2 cells were pre-incubated with E. coli Nissle 1917 (EcN) monocultures or mixed cultures using an MOI of 100∶1 or 10∶1 E. coli to host cells. After two hours, cells were washed and infected with Salmonella Typhimurium using an MOI of 100∶1 Salmonella to host cells. Invasion levels in percent (%) are expressed as invasion of Salmonella relative to invasion without pre-incubation with E. coli (Salmonella mono-infection). The data are the mean ± S.E.M. of at least three separate experiments in duplicate wells. * = p<0.01 compared to Salmonella mono-infection.
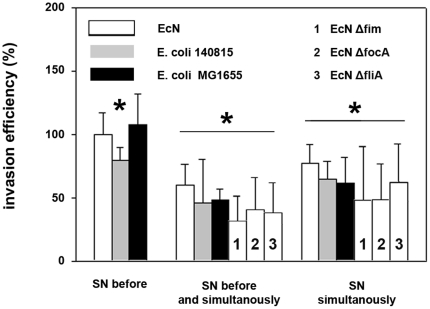
We also tested the effects of cell-free EcN supernatants compared to supernatants of the two control strains. To create a scenario relevant to the initial experiments with bacteria (for which EcN was present in the pre- and co-incubation period), we included pre- and pre-/co-incubation experiments using E. coli culture supernatants. As shown in Figure 4, pre-incubation with E. coli supernatants (E. coli 140815) showed little or no effects on Salmonella invasion efficiency, while pre-/co-incubation noticeably inhibited Salmonella invasion. We tested the hypothesis that co-incubation of E. coli supernatants with Salmonella was predominantly responsible for the inhibitory effect in subsequent co-incubation experiments. However, co-incubation of supernatants of all three tested E. coli strains similarly inhibited Salmonella invasion (Figure 4) as was the case with E. coli pre-incubations. Also supernatants of EcN mutants ΔfocA (F1C fimbriae), ΔfimA (type 1 fimbriae) and ΔfliA inhibited Salmonella invasion comparable to EcN wildtype (Figure 4).
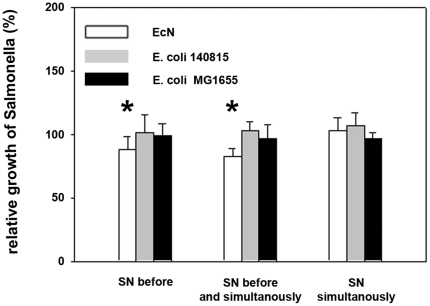
Figure 4. Invasion efficiency of Salmonella Typhimurium into IPEC-J2 cells after incubation with E. coli culture supernatants.
Confluent monolayers of IPEC-J2 cells were pre-incubated (SN before) and/or co-incubated (SN simultaneously) with E. coli supernatants (SN). Cells were infected with Salmonella Typhimurium using an MOI of 100∶1 Salmonella to host cells. Invasion levels in percent (%) are expressed as invasion of Salmonella relative to invasion without pre- and/or co-incubation with E. coli SN. The data are the mean ± S.E.M. of at least three separate experiments in duplicate wells. * = p<0.05 compared to Salmonella infection without influence of E. coli SN. EcN: E. coli Nissle 1917.
Effects of E. coli supernatants on extracellular growth
The inhibitory effect of EcN against Salmonella invasion might have been due to the inhibition of Salmonella growth by E. coli supernatants, which inevitably affects invasion efficiencies. To exclude such effects, Salmonella numbers were determined in cell culture supernatants in parallel to the invasion assays. Supernatants of all three E. coli did not, or only slightly (EcN), affect extracellular growth of Salmonella within the one hour Salmonella incubation time (Figure 5).
Figure 5. Effects of E. coli supernatants on growth of Salmonella Typhimurium.
Confluent monolayers of IPEC-J2 cells were pre- and/or co-incubated with E. coli supernatants (SN). Cells were infected with Salmonella Typhimurium using an MOI of 100∶1 Salmonella to host cells for one hour. Thereafter, numbers of extracellular, non-adherent Salmonella were determined. Growth rates are expressed as growth in percent (%) relative to Salmonella growth in cell culture medium (Salmonella mono-infection). The data are the mean ± S.E.M. of at least three separate experiments in duplicate wells. * = p<0.01 compared to Salmonella mono-infection. EcN: E. coli Nissle 1917.
Effects of E. coli on adhesion of Salmonella
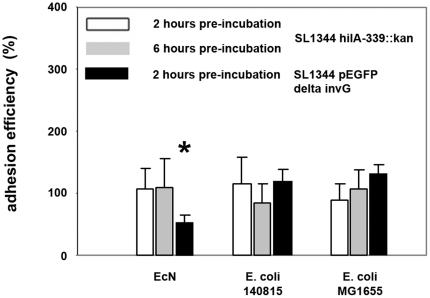
We determined whether EcN inhibited Salmonella adhesion, which is also a prerequisite for Salmonella invasion. Salmonella adhesion assays were performed with non-invasive S. Typhimurium SL1344 hilA-339::kan. All three E. coli strains showed no effects on Salmonella adhesion to IPEC-J2 cells in pre-incubation experiments (Figure 6). Even after a six-hour pre-incubation period with E. coli, adhesion of S. Typhimurium SL1344 hilA-339::kan was not affected (Figure 6).
Figure 6. Adhesion efficiency of Salmonella Typhimurium to IPEC-J2 cells after pre-incubation with E. coli.
Confluent monolayers of IPEC-J2 cells were pre-incubated with E. coli Nissle 1917 (EcN), E. coli 140815 or E. coli MG1655 using an MOI of 100∶1 E. coli to host cells. After two or six hours, cells were washed and infected with non-invasive Salmonella Typhimurium SL1344 hilA-339::kan or SL1344 pEGFP invG-339::kan using an MOI of 100∶1 Salmonella to host cells. Adhesion levels in percent (%) are expressed as adhesion of Salmonella relative to adhesion without pre-incubation with E. coli (Salmonella mono-infection). The data are the mean ± S.E.M. of at least three separate experiments in duplicate wells. * = p<0.01 compared to Salmonella mono-infection.
Additional Salmonella adhesion tests were performed with non-invasive S. Typhimurium SL1344 pEGFP invG-339::kan. This mutant adhered twice to IPEC-J2 cells than non-invasive S. Typhimurium SL1344 hilA-339::kan. EcN inhibited SL1344 pEGFP invG-339::kan adhesion by 40%. In contrast, E. coli strain MG1655 and 140815 enhanced Salmonella adhesion to IPEC-J2 cells in pre-incubation experiments (Figure 6). The kanamycin resistance of non-invasive Salmonella mutants does not contribute to this effect since strain SL1344::kan harboring a chromosomal kanamycin resistance cassette in a non-coding, intergenic region not affecting the expression of any genes was similarly invasive and had a similar probiotic effect against Salmonella invasion (reduction of invasion by 55.3%) as the wild type strain itself.
E. coli Nissle 1917 does not inhibit intracellular growth of Salmonella
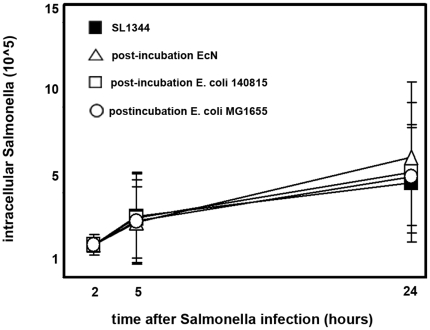
To test whether EcN might have been able to affect Salmonella post-invasion, EcN and the two control strains were incubated with Salmonella in post-incubation experiments. Here, IPEC-J2 cells were initially infected with Salmonella for one hour, and extracellular Salmonella were inactivated by incubation with gentamicin for another hour. After removal of gentamicin IPEC-J2 cells with intracellular Salmonella were incubated with E. coli for 2 hours and then incubated for up to 20 hours with medium containing gentamicin to kill extracellular bacteria. Intracellular Salmonella were determined at 2, 5 and 24 hours after the initial Salmonella incubation period. As shown in Figure 7, all three E. coli had no effect on intracellular Salmonella growth at 2, 5 as well as 24 hours after the initial Salmonella incubation.
Figure 7. Intracellular growth of Salmonella Typhimurium in IPEC-J2 cells after post-incubation with E. coli Nissle 1917.
Confluent monolayers of IPEC-J2 cells were infected with Salmonella Typhimurium for one hour using an MOI of 1∶1 Salmonella to host cells. After one additional hour of incubation in media containing gentamicin, IPEC-J2 cells were incubated with E. coli Nissle 1917 (EcN), E. coli 140815 or E. coli MG1655 for two hours using an MOI of 100∶1 E. coli to host cells, followed by incubation in media with gentamicin. Intracellular Salmonella numbers are presented per well of a 24-well plate. The data are the mean ± S.E.M. of at least three separate experiments in duplicate wells.
F1C fimbriae mediates inhibitory effects of EcN on Salmonella invasion
Adhesion might be a prerequisite for the inhibitory effect of EcN. To test this, we used EcN ΔfocA (F1C fimbriae), ΔfimA (type 1 fimbriae) and ΔfliA (flagellae) mutants. Adhesion by strains EcN ΔfocA (reduction by 92.7%) and EcN ΔfliA (reduction by 47.9%) on IPEC-J2 cells was reduced compared to EcN wild type strain. After complementation with the pACYC 177 plasmids, respectively containing the focA or fliA gene, adhesion was enhanced to 53.4% (focA) and 122.1% (fliA), compared to the EcN wild type strain. Adhesion by strain EcN ΔfimA on IPEC-J2 cells was not reduced compared to EcN wild type strain. A decrease or increase in adhesion correlated with a decrease or increase of the inhibitory effect on Salmonella invasion, respectively. Thus EcN ΔfocA did not inhibit Salmonella invasion compared to EcN wildtype strain, whereas EcN ΔfliA inhibited Salmonella invasion by 50%. EcN ΔfimA inhibited Salmonella invasion at levels similar to those of EcN wildtype strain (Figure 2). The results were more prominent using a 6-hour EcN pre-incubation period.
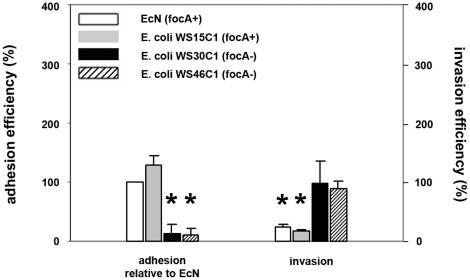
To test whether adhesion via F1C fimbriae is essential for a subsequent inhibitory effect of EcN and the specificity of EcN inhibition, we compared adhesion rates as well as inhibition between EcN and another focA gene-positive (WS15C1), and two other focA gene-negative (WS30C1 and WS46C1) E. coli strains. These bacteria all carry the type 1 fimbriae and flagellae and were isolated from the intestine of clinically healthy wild boars [23]. As shown in Figure 8, adhesion rates of the focA gene-positive strain WS15C1 were higher compared to EcN, but focA gene-negative strains WS30C1 and WS46C1 adhered much less compared to EcN. Additionally, these high adhesion rates correlated with high inhibitory effects of EcN and WS15C1 on Salmonella invasion (Figure 8).
Figure 8. Inhibitory effects of focA-positive and focA-negative E. coli isolates on Salmonella Typhimurium invasion.
Confluent monolayers of IPEC-J2 cells were pre-incubated with E. coli Nissle 1917 (EcN, focA-positive strain), E. coli WS15C1 (focA-positive strain), E. coli WS30C1 (focA-negative strain) and E. coli WS46C1 (focA-negative strain) using an MOI of 100∶1 E. coli to host cells. After two hours, cells were washed and adhesion efficiencies of E. coli isolates were determined (left side); alternatively, cells were washed after two hours and infected with Salmonella Typhimurium using an MOI of 100∶1 Salmonella to host cells (right side). Adhesion levels in percent (%) were expressed relative to adhesion of EcN. Invasion levels in percent (%) were expressed relative to Salmonella invasion without pre-incubation with bacteria (Salmonella mono-infection). The data are the mean ± S.E.M. of at least three separate experiments in duplicate wells. * = p<0.01 compared to EcN adhesion (left side) or Salmonella mono-infection (right side).
Effects of EcN on expression of Salmonella invasion genes
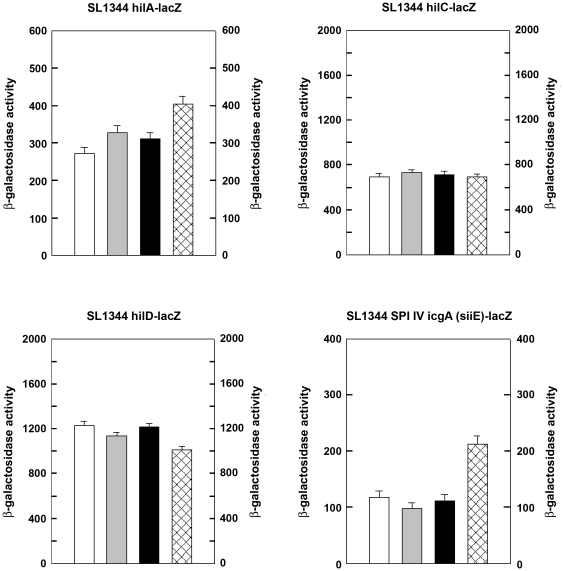
As the previous results indicated that Salmonella adhesion - but not extracellular or intracellular Salmonella growth - was affected by EcN, we tested the effects of EcN supernatants on the expression of Salmonella invasion gene regulatory and dependent genes. Salmonella strains harboring lacZ fusions to the invasion locus regulatory genes hilC, hilD, hilA, and the SPI4-encoded siiE gene were incubated with supernatants of EcN, E. coli 140815 or E. coli MG1655. As shown in Figure 9, expression of HilC was not affected by any of the three E. coli supernatants. HilD expression was slightly enhanced by all three E. coli supernatants and HilA and SiiE expressions were inhibited by all three E. coli supernatants.
Figure 9. Salmonella invasion gene regulation by E. coli supernatants.
E. coli were cultivated in cell culture medium (DMEM HAM'S/F-12) until an OD600nm = 1. Supernatants were collected by centrifugation with subsequent sterile filtration. Subsequently, SL1344 fusion strains (SL1344 hilC-lacZ, SL1344 hilD-lacZ, SL1344 hilA-lacZ, SL1344 icgA(siiE)-lacZ) were cultivated in supernatants of EcN, E. coli 140815 or E. coli MG1655. B-Galactosidase activity was measured as previously described [25], [26]. The results shown are representative of at least two independent experiments. White bar: Salmonella grown in EcN supernatant, gray bar: Salmonella grown in E. coli 140815 supernatant, black bar: Salmonella grown in E. coli MG1655 supernatant, patterned bar: Salmonella grown in pure cell culture medium.
Discussion
Probiotic E. coli Nissle 1917 (EcN) is being successfully used for the prevention and treatment of various intestinal diseases of humans and animals. However, the basis of its mode of action remains largely unanswered. EcN has been shown in in vitro studies to protect human embryonic intestinal epithelial cells (INT407 cells) against infection by different enteropathogens, including enteroinvasive bacteria, but the underlying mechanisms were not clarified [14], [16]. In the present study, we initially defined possible probiotic effects of EcN against Salmonella invasion of porcine intestinal epithelial cells (IPEC-J2) and subsequently verified such probiotic effects against single stages of the Salmonella invasion process.
EcN successfully inhibited Salmonella infection of IPEC-J2 cells. The specificity of this effect was demonstrated in that two control E. coli strains, 140815 and MG1655, showed no such inhibitory effects. Inhibition of Salmonella infection could have been due to effects on several infection steps including inhibition of intracellular and extracellular Salmonella growth, inhibition of adhesion, or other factors. The probiotic effect of EcN was found to be highly dependent upon its adherence to IPEC-J2 cells, preferentially through F1C fimbriae (Figure 2). Salmonella adhesion and Salmonella adhesion gene expressions were affected by EcN and/or EcN supernatants respectively, but not Salmonella extracellular or intracellular growth, supporting a role of adhesion genes in the probiotic effects.
We propose two mechanisms for the probiotic effect of EcN. First, EcN supernatants could be responsible for this effect. This might be mediated through interactions with the Salmonella SiiE-dependent adhesion mechanism, as well as down-regulation of the expression of the adhesion factor SiiE. We suggest that Salmonella adhesion was reduced via SiiE-mediated adhesion by EcN since adhesion of a non-invasive invG Salmonella mutant with a functional SiiE mediation system was affected, whereas adhesion of a non-invasive Salmonella mutant defective in hilA with an SiiE-minus genotype was not, however; E. coli supernatants suppressed both SiiE and HilA expression, as well as suppression of both genes contributed to the probiotic effect. HilA is a transcriptional regulator of the OmpR/ToxR family that is encoded on Salmonella pathogenicity island 1 and plays a key role in the regulation of invasiveness of Salmonella Typhimurium. HilA has been shown to be required for the optimal expression of both invasion genes as well as the adhesin SiiE [27], [28]. While HilA-dependent regulation has been intensively studied, other HilA-dependent adhesion genes have not been reported so far. HilA expression is also regulated by the regulators HilC and HilD [29]. In our study, HilC expression was not influenced by E. coli supernatants, while HilD expression was slightly enhanced. Thus the regulation of HilA by HilD, which would have up-regulated HilA and SiiE expression, was weaker than the direct effects of E. coli supernatants on HilA. SiiE is secreted from Salmonella by a type I secretion system into the cell culture supernatant and can function as an adhesin when in contact with polarized epithelial cells. The IPEC-J2 cell line has also been shown to form a polarized cell monolayer [21], [30]. This mechanism might not be EcN-specific since supernatants from other E. coli also similarly inhibited Salmonella invasion, and similarly regulated Salmonella virulence gene expression.
A second mechanism is suggested by the strong adherence of EcN on IPEC-J2 cells and the subsequent inhibition of Salmonella invasion which may be a major contributing factor in the probiotic effect of EcN. This strong adhesion was EcN-specific compared to the control strains 140815 and MG1655 and was predominantly mediated by F1C fimbriae. However, other F1C fimbriae expressing E. coli strains could also strongly adhere to IPEC-J2 cells and reduce Salmonella invasion, as was the case with E. coli strain WS15C1.
In summary, in the presence of E. coli, components present in culture supernatants appear to reduce Salmonella SiiE-mediated adhesion by down-regulation of SiiE production. E. coli supernatant compounds might also block SiiE-mediated adhesion by binding to either the SiiE protein itself or to SiiE receptors on the host cell surface. Such effects on the adhesion mediated by the SiiE protein are a subject for future research. In the presence of high concentrations of E. coli supernatants components might bind to the host cell surface. After washing host cells, followed by incubation in fresh cell culture medium (pre-incubation experiments), these bound components might detach and be diluted by added fresh cell culture medium to concentrations that become ineffective in inhibiting Salmonella adhesion. If cells are however incubated at high concentrations of supernatants after washing (pre- plus post-incubation with supernatants), components of supernatants will still bind to the host cell surface in high concentrations and affect Salmonella adhesion. This might explain why pre- and co-incubation with E. coli supernatants resulted in a higher reduction of Salmonella invasion than pre-incubation or post-incubation only. Attachment to the cell surface through F1C fimbriae may support intimate EcN contact at the host cell surface and increase the local concentrations of supernatant compounds which decrease Salmonella adhesion and invasion.
A supernatant-dependent mechanism seems to be common for different E. coli strains (this study, [14]) as well as other bacterial species e.g. Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus plantarum, Pediococcus pentosaceus and Leuconostoc mesenteroides [31], [32], [33]. In these latter studies, acidification of the medium followed by a bacteriocidal effect, the production of microcins, and other undefined bacteriocidal effects or inhibition of Salmonella growth were found to be responsible for probiotic supernatant-dependent mechanisms. However, in this study, Salmonella growth rates were similar in all different E. coli culture supernatants indicating that E. coli culture supernatants had no bacteriocidal effects against Salmonella. Such a finding does not rule out the possibility of other unknown supernatant factors binding to, and inhibiting SiiE or other molecules. Other authors reported that L. casei supernatant inhibited Salmonella invasion, but did not modify the viability of Salmonella. These authors supposed that a hypothetical substance of L. casei supernatant directly modified the ability of Salmonella to invade enterocyte-like cells in vitro in an acidic environment [33].
The adhesion of EcN to IPEC-J2 cells was a prerequisite for its probiotic effects. As shown in our study, F1C fimbriae as well as flagellae contributed to the adherence but not type 1 fimbriae. This is in accordance with recent observations that F1C fimbriae, not type 1 fimbriae, contributed to the adherence of EcN to the human larynx epithelial cell line Hep-2, and to their persistence in infant mouse colonization and biofilm formation [19]. EcN is therefore able to adhere via F1C fimbriae to different types of cells. The contribution of adherence to the probiotic effect was clearly indicated. For example, the adhesion of the EcN ΔfliA mutant complemented with a plasmid containing the fliA gene was enhanced to 122.1% compared to the EcN wild type strain, while Salmonella invasion decreased to 14.9% in the presence of the EcN ΔfliA/pACYC177 fliA construct, compared to 35% in the presence of the EcN wild type strain.
The conclusion of this study is three-fold. First, EcN suppressed Salmonella invasion by suppressing Salmonella adhesion. Second, effects of bacterial supernatants might be also common for other E. coli. And third, strong adherence is a prerequisite for the probiotic effect of EcN.
Acknowledgments
We thank Matthias Filter (Bundesinstitut für Risikobewertung, Berlin, Germany) for helping with the statistical analysis and Bryon Nicholson (Department of Veterinary Microbiology and Preventive Medicine, Ames, USA) for helpful comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the German Research Foundation (DFG) through the Collaborative Research Centre 852 (grant no. SFB852/1), by grant FOR 438/1-1 from the DFG and by InnoProfile IP 03 IP 611 funded by the Bundesministerium für Bildung und Forschung (BMBF, Germany). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz M, Strauch UG, Linde HJ, Watzl S, Obermeier F, et al. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol. 2004;11:372–378. doi: 10.1128/CDLI.11.2.372-378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder B, Duncker S, Barth S, Bauerfeind R, Gruber AD, et al. Preventive effects of the probiotic Escherichia coli strain Nissle 1917 on acute secretory diarrhea in a pig model of intestinal infection. Dig Dis Sci. 2006;51:724–731. doi: 10.1007/s10620-006-3198-8. [DOI] [PubMed] [Google Scholar]
- 4.Henker J, Laass MW, Blokhin BM, Maydannik VG, Bolbot YK, et al. Probiotic Escherichia coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. Pediatr Infect Dis J. 2008;27:494–499. doi: 10.1097/INF.0b013e318169034c. [DOI] [PubMed] [Google Scholar]
- 5.von Buenau R, Jaekel L, Schubotz E, Schwarz S, Stroff T, et al. Escherichia coli strain Nissle 1917: significant reduction of neonatal calf diarrhea. J Dairy Sci. 2005;88:317–323. doi: 10.3168/jds.S0022-0302(05)72690-4. [DOI] [PubMed] [Google Scholar]
- 6.Krammer HJ, Kamper H, von Bunau R, Zieseniss E, Stange C, et al. [Probiotic drug therapy with E. coli strain Nissle 1917 (EcN): results of a prospective study of the records of 3,807 patients]. Z Gastroenterol. 2006;44:651–656. doi: 10.1055/s-2006-926909. [DOI] [PubMed] [Google Scholar]
- 7.Reissbrodt R, Hammes WP, dal Bello F, Prager R, Fruth A, et al. Inhibition of growth of Shiga toxin-producing Escherichia coli by nonpathogenic Escherichia coli. FEMS Microbiol Lett. 2009;290:62–69. doi: 10.1111/j.1574-6968.2008.01405.x. [DOI] [PubMed] [Google Scholar]
- 8.Bar F, Von Koschitzky H, Roblick U, Bruch HP, Schulze L, et al. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil. 2009;21:559–566, e516-557. doi: 10.1111/j.1365-2982.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- 9.Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamada N, Maeda K, Inoue N, Hisamatsu T, Okamoto S, et al. Nonpathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect Immun. 2008;76:214–220. doi: 10.1128/IAI.01193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bickert T, Trujillo-Vargas CM, Duechs M, Wohlleben G, Polte T, et al. Probiotic Escherichia coli Nissle 1917 suppresses allergen-induced Th2 responses in the airways. Int Arch Allergy Immunol. 2009;149:219–230. doi: 10.1159/000199717. [DOI] [PubMed] [Google Scholar]
- 12.Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, et al. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9:804–816. doi: 10.1111/j.1462-5822.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 13.Helwig U, Lammers KM, Rizzello F, Brigidi P, Rohleder V, et al. Lactobacilli, bifidobacteria and E. coli nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol. 2006;12:5978–5986. doi: 10.3748/wjg.v12.i37.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altenhoefer A, Oswald S, Sonnenborn U, Enders C, Schulze J, et al. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol Med Microbiol. 2004;40:223–229. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- 15.Kleta S, Steinruck H, Breves G, Duncker S, Laturnus C, et al. Detection and distribution of probiotic Escherichia coli Nissle 1917 clones in swine herds in Germany. J Appl Microbiol. 2006;101:1357–1366. doi: 10.1111/j.1365-2672.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 16.Boudeau J, Glasser AL, Julien S, Colombel JF, Darfeuille-Michaud A. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn's disease. Aliment Pharmacol Ther. 2003;18:45–56. doi: 10.1046/j.1365-2036.2003.01638.x. [DOI] [PubMed] [Google Scholar]
- 17.Prilassnig M, Wenisch C, Daxboeck F, Feierl G. Are probiotics detectable in human feces after oral uptake by healthy volunteers? Wien Klin Wochenschr. 2007;119:456–462. doi: 10.1007/s00508-007-0808-1. [DOI] [PubMed] [Google Scholar]
- 18.Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, et al. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol. 2004;186:5432–5441. doi: 10.1128/JB.186.16.5432-5441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasaro MA, Salinger N, Zhang J, Wang Y, Zhong Z, et al. F1C fimbriae play an important role in biofilm formation and intestinal colonization by the Escherichia coli commensal strain Nissle 1917. Appl Environ Microbiol. 2009;75:246–251. doi: 10.1128/AEM.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteiro C, Saxena I, Wang X, Kader A, Bokranz W, et al. Characterization of cellulose production in Escherichia coli Nissle 1917 and its biological consequences. Environ Microbiol. 2009;11:1105–1116. doi: 10.1111/j.1462-2920.2008.01840.x. [DOI] [PubMed] [Google Scholar]
- 21.Schierack P, Nordhoff M, Pollmann M, Weyrauch KD, Amasheh S, et al. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol. 2006;125:293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- 22.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schierack P, Romer A, Jores J, Kaspar H, Guenther S, et al. Isolation and characterization of intestinal Escherichia coli clones from wild boars in Germany. Appl Environ Microbiol. 2009;75:695–702. doi: 10.1128/AEM.01650-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CA, Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson A, Rolfe MD, Lucchini S, Schwerk P, Hinton JC, et al. The bacterial signal molecule, ppGpp, mediates the environmental regulation of both the invasion and intracellular virulence gene programs of Salmonella. J Biol Chem. 2006;281:30112–30121. doi: 10.1074/jbc.M605616200. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez VJ, Bremer H. Guanosine tetraphosphate (ppGpp) dependence of the growth rate control of rrnB P1 promoter activity in Escherichia coli. J Biol Chem. 1990;265:11605–11614. [PubMed] [Google Scholar]
- 27.Bajaj V, Hwang C, Lee CA. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 28.Main-Hester KL, Colpitts KM, Thomas GA, Fang FC, Libby SJ. Coordinate regulation of Salmonella pathogenicity island 1 (SPI1) and SPI4 in Salmonella enterica serovar Typhimurium. Infect Immun. 2008;76:1024–1035. doi: 10.1128/IAI.01224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellermeier CD, Ellermeier JR, Slauch JM. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2005;57:691–705. doi: 10.1111/j.1365-2958.2005.04737.x. [DOI] [PubMed] [Google Scholar]
- 30.Gerlach RG, Jackel D, Geymeier N, Hensel M. Salmonella pathogenicity island 4-mediated adhesion is coregulated with invasion genes in Salmonella enterica. Infect Immun. 2007;75:4697–4709. doi: 10.1128/IAI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernet-Camard MF, Lievin V, Brassart D, Neeser JR, Servin AL, et al. The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu HH, Tsai CC, Hsih HY, Tsen HY. Screening from pickled vegetables the potential probiotic strains of lactic acid bacteria able to inhibit the Salmonella invasion in mice. J Appl Microbiol. 2008;104:605–612. doi: 10.1111/j.1365-2672.2007.03573.x. [DOI] [PubMed] [Google Scholar]
- 33.Hudault S, Lievin V, Bernet-Camard MF, Servin AL. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol. 1997;63:513–518. doi: 10.1128/aem.63.2.513-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]