Abstract
The pancreatic zymogen granule membrane protein (GP2) is expressed by pancreatic acinar cells and M cells of the ileum. GP2 is the closest related homologue of the urine resident Tamm–Horsfall protein (THP). Recently, it was shown that THP is a ligand of various scavenger receptors (SRs). Therefore, we were interested, if GP2 has similar properties.
cDNA of different SRs was stably transfected into a murine thymoma cell line. GP2 was recombinantly expressed, purified and biotinylated. Binding or uptake of GP2 by transfected cells or monocyte-derived dendritic cells (moDCs) was analyzed by flow-cytometry.
GP2 is a binding partner of the scavenger receptor expressed on endothelial cells I (SREC-I) but not of SR-AI and SR-BI. The dissociation constant (Kd) of GP2 binding to SREC-I is 41.3 nM. SREC transfected cells are able to internalize GP2. moDCs express SREC-I and also bind and internalize GP2. Inhibition of SREC-I on moDCs with anti-SREC-I antibodies does not result in a decreased GP2 binding.
Interaction of GP2 with SREC-I and uptake might have profound effects in antigen clearance and mediation of the immune response. In addition to SREC-I other presently unknown receptors for GP2 on DCs might be involved in this process.
Keywords: SREC-I, GP2, Dendritic cell, THP, Internalization, acLDL
1. Introduction
The pancreatic zymogen granule membrane protein 2 (GP2) is the most abundant membrane glycoprotein in the zymogen granules of the pancreatic acinar cells [1,2], and has been discussed to play a role in the formation and the secretion of these granules [3–5]. In addition, GP2 is expressed on the apical surface of intestinal M cells and appears to be overexpressed in colon biopsies of Crohn’s disease patients [19]. It was demonstrated that membrane resident GP2 is capable of binding pathogenic Escherichia coli. Bacterial binding to GP2 mediated the transcytosis of the pathogens through M cells. This resulted in the preferential formation of IgA antibodies and the establishment of a mucosal immunity [6].
GP2 is the closest related homologue of the Tamm–Horsfall Protein (THP), which is exclusively expressed in the urogenital tract. Both proteins show 52% identity and 85% similarity in their amino acid sequence and might have evolved from gene duplication [7,8]. Like THP, GP2 has a zona pelucida domain and is linked to the apical epithelium via a glycosyl phosphatidyl inositol (GPI) anchor [9,10]. Since both proteins exist also in soluble form, it is supposed that a phenylalanine specific protease mediates the cleavage from the membrane [11]. GP2 and THP have the ability to attach to the FimH adhesin of E. coli or Salmonella typhimurium. Binding to E. coli is dependent on the glycosylation status, and in particular on the presence of mannose residues [12,13]. Whereas soluble THP is considered to be a decoy receptor in the urogenital tract that inhibits the adherence of uropathogenic bacteria, the functional role of soluble GP2 is still unclear.
Scavenger receptors represent a highly heterogenic group of membrane receptors. These molecules are described to have functions in the binding of modified low density lipoproteins (LDL) as well as the recognition and uptake of pathogens [14]. The scavenger receptor expressed on endothelial cells I (SREC-I) is expressed by various cell types, as for example dendritic cells (DCs) [15]. SREC-I mediates the binding and internalization of several proteins, as for example acetylated LDL (acLDL) and heat shock protein (HSP) 90 [16]. Recently, it has been demonstrated that SREC-I is involved in processes that contribute to cross presentation. Murshid et al. showed that HSP90 coupled ovalbumin peptides were taken up by SREC-I and efficiently presented to a peptide specific CD8(+) T-cell hybridoma cell line [17]. Furthermore, it has been demonstrated that this receptor functions as a high affinity receptor for THP, a urinary protein which is considered to play a role in several immunological processes [18].
In the present study we show for the first time that GP2 is a high affinity interaction partner of SREC-I. Moreover, we demonstrate that GP2 can be internalized by SREC-I expressing cell lines. Furthermore, DCs are capable to bind and to take up GP2.
2. Material and methods
2.1. Reagents
All chemicals were of American Chemical Society reagent grade and were purchased from SigmaAldrich (Deisenhofen, Germany) unless stated otherwise. Phycoerythrin (PE) conjugated SR-AI antibodies and biotinylated goat anti-human SREC-I antibodies were purchased from R&D Systems (Minneapolis, MN). Unbiotinylated mouse anti-human SREC-I monoclonal antibodies (AK 503) were a kind gift of Prof. Otto Majdic (Institute of Immunology, Medical University of Vienna, Austria). Mouse anti-human SR-BI antibodies were obtained from BD Biosciences (San Jose, CA). Alexa Fluor 488 acLDL (acLDL-488) was purchased from Invitrogen (Carlsbad, CA). Lymphoprep was obtained from Axis Shield (Oslo, Norway). Paramagnetic CD14 beads were purchased from Miltenyi (Bergisch Gladbach, Germany). IL-4 and GM-CSF were obtained from PeproTech (Rocky Hill, NJ). Streptavidin-PE (SA-PE) and goat anti-mouse (GaM) IgG-PE were purchased from Jackson ImmunoResearch (West Grove, PA). RPMI 1640 supplemented with 10% fetal calf serum (FCS), glutamine and penicillin/streptomycin was used as cell culture medium (all from PAA, Pasching, Austria).
2.2. Expression and purification of GP2
Expression of GP2 has been described in detail elsewhere [19]. In brief, cDNA of the GP2 isoform BAA88166 (pancreatic zymogen granule membrane associated protein GP2 alpha form) was inserted into a pVL1393-vector. 2.5 μg BaculoGold (BD) and 2.5 μg DNA of the insert containing vector were mixed with H2O and Polyfect® transfection reagent (Qiagen, Hilden, Germany). Prepared Sf9 cells were incubated for 5 days with the solution. For infection, Sf9 cells were incubated with the supernatant of the previous infected culture in a 1:10 ratio for 3 days. GP2 producing Sf9 were harvested and lysed in non denaturating lysis buffer supplemented with a protease inhibitor cocktail (Roche, Mannheim, Germany). GP2 was isolated employing an equilibrated Ni-chelate column, followed by anion exchange chromatography (both types of columns were purchased from GE Healthcare, Buckinghamshire, GB). GP2 was biotinylated (GP2-bio) using standard procedures.
2.3. Generation of SR expressing Bw cells
The coding sequences of human SREC-I, SR-AI, SR-BI were PCR-amplified from a cDNA expression library generated from human DC using appropriate primers containing restriction endonuclease recognition sites. The PCR products were cloned into the retroviral expression vector pBMN [20]. The integrity of the resulting retroviral expression constructs was confirmed by DNA sequencing. The murine thymoma cell line Bw5147 (referred to as Bw cells throughout this work) was then transduced retrovirally with pBMN plasmids encoding either SREC-I, SR-AI or SR-BI. For control purposes, a vector containing the lacZ gene was used (pBMN-Z) [21]. Bw cells were cultured in medium supplemented with plasmocin and amphotericin B (PAA).
2.4. Generation of monocyte-derived DCs (moDCs)
Buffy coats from healthy blood donors were obtained from the Austrian Red Cross, where donors are adequately informed and the procedure follows the ethical guidelines according to the declaration of Helsinki. Blood was diluted 1:2 with phosphate buffered saline (PBS; PAA). PBMCs were isolated by density gradient centrifugation. Monocytes were isolated by magnetic cell sorting. moDCs were generated by incubating monocytes in culture medium supplemented with 10 ng/mL IL-4 and 50 ng/mL GM-CSF for 6 days.
2.5. Binding studies and internalization assays
Binding experiments using GP2-bio were performed in PBS supplemented with 0.5% bovine serum albumin (PBS-B). GP2-bio was detected using SA-PE, diluted 1:200 in PBS-B. For affinity measurements Bw-SREC-I cells were incubated with defined concentrations of GP2-bio dissolved in PBS-B for 40 min at 4 °C, washed twice and then stained with SA-PE. For uptake experiments, Bw-SREC-I were incubated with 10 μL GP2-bio at a concentration of 10 μg/mL in PBS-B for 20 min at 4 °C. Cells were washed twice with PBS-B and incubated with SA-PE (group 1) or PBS-B (group 2). Subsequently, cells were washed in culture medium and incubated at 37 °C for 60 min. Cells were washed with ice-cold PBS-B and then incubated with PBS-B (group 1) or stained with SA-PE (group 2). Flow cytometric analysis was performed using a BD FacsCalibur (BD Biosciences).
3. Results
3.1. GP2 binds to SREC-I
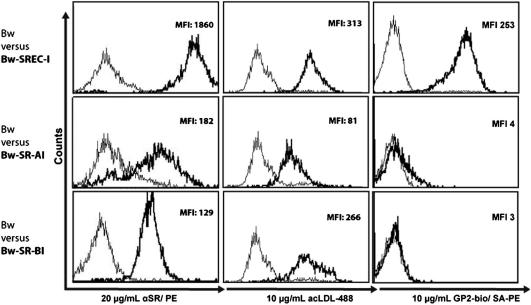
Retrovirally transduced Bw cells were evaluated for the expression of SRs by flow cytometry using SR specific antibodies. SR-AI, SR-BI and SREC-I were expressed on the surface of the respective Bw-clones at high levels (Fig. 1). To address whether SRs can bind their genuine ligand, SR expressing Bw clones were incubated with acLDL-488. All SR expressing clones but not mock transfected Bw cells strongly bound acLDL-488 (Fig. 1) and were therefore considered to be functional. Subsequently, SR expressing Bw clones were incubated with GP2-bio, which strongly bound to clones expressing SREC-I. No binding was observed with SR-AI or SR-BI or mock transfected Bw cells (Fig. 1). These results clearly identify SREC-I as a cellular receptor for GP2.
Fig. 1.
SR expression and binding to acLDL-488 and GP2-bio by transduced Bw cells. SR expressing Bw cells were stained with PE-labeled antibodies (Bw-SR-AI), biotinylated antibodies (Bw-SREC-I) or unlabeled antibodies (Bw-SR-BI). Binding of primary antibodies was detected with appropriate PE-labeled secondary reagents (left panel, bold line). acLDL-488 was tested for its binding to SR expressing clones (middle panel, bold line). GP2-bio was incubated with SR expressing Bw clones and stained with SA-PE (right panel, bold line). Mock transfected Bw cells were used as control in all binding experiments (thin lines). The depicted results are representative for three independent experiments. MFI = mean fluorescence intensity.
3.2. Determination of equilibrium Kd of the SREC-I - GP2 Interaction
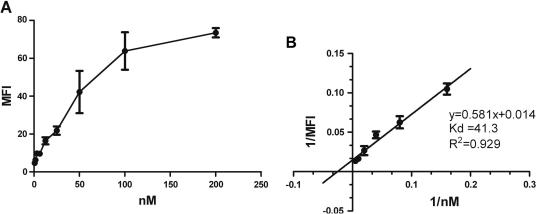
Since GP2 clearly bound to cells expressing SREC-I, it was of interest to assess the affinity of this interaction. Therefore, SREC-I expressing cells were incubated with increasing amounts of GP2, resulting in an increased binding of the ligand (Fig. 2A).
Fig. 2.
Determination of the binding affinity of GP2 for SREC-I (A) Bw cells expressing SREC were incubated with indicated concentrations of GP2-bio and stained with SA-PE. (B) Inverted values of MFI and concentrations were used to create a Lineweaver–Burk diagram (right panel). Kd = −1/X(y0). MFI = Mean fluorescence intensity. Error bars indicate the standard error of the mean (n = 3).
By blotting the inverted values of the resulting MFI against the concentration of GP2, a Lineweaver–Burk diagram was generated, which delivered a regression line of y = 0.581x + 0.014 and an intercept of the x-axis at −0.025 ± 0.0029 1/nM (Fig. 2B). This allowed the calculation of the equilibrium Kd, which is 41.3 ± 5.06 nM. The data sets exhibited a strong correlation (R2 = 0.929).
3.3. Internalization of GP2 by Bw-SREC-I cells and moDCs
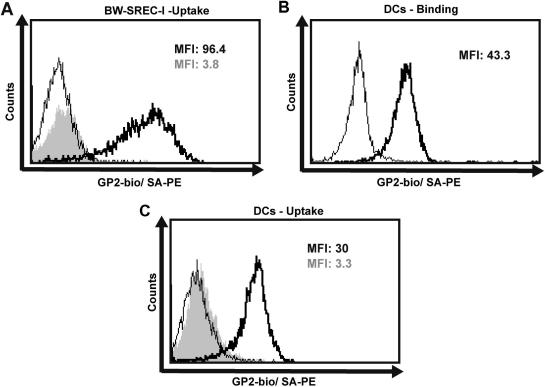
Since SREC-I has been shown to be a receptor with endocytic capacity [22], we were interested whether GP2 is taken up after ligation. Therefore Bw-SREC-I cells were incubated with GP2-bio at 4 °C and washed. SA-PE staining of the cells was either performed after the washing step or following a one hour incubation step at 37 °C. After the one hour incubation step at 37 °C, GP2-bio was no longer accessible for binding of SA-PE, whereas immediate application of the secondary reagent and subsequent incubation for one hour at 37 °C still resulted in a strong fluorescence signal (Fig. 3A). These results clearly demonstrate efficient endocytic uptake of GP2 by the Bw-SREC-I cells. moDCs have been shown to bind and internalize the GP2 homologue THP [23]. Therefore, we were also interested to test the interaction of GP2 with this cell type. moDCs were able to bind (Fig. 3B) and to internalize GP2-bio (Fig. 3C). Using the same approach as described before, we found that moDCs stained with SA-PE before the one hour incubation step retained surface staining with GP2-bio. In contrast, staining after incubation at 37 °C for one hour resulted in a complete loss of SA-PE binding, indicating uptake of GP2.
Fig. 3.
Endocytosis of GP2-bio after binding to Bw-SREC or moDCs (A) SREC-I expressing Bw cells were incubated with 5 μg/mL GP2-bio. Cells were stained with SA-PE before (bold line) or after a one-hour incubation step (grey histogram). For control, untransfected Bw cells were incubated with GP2-bio and stained with SA-PE before the one hour incubation step (thin line). (B) moDCs were stained with 5 μg/mL GP2-bio and stained with SA-PE (bold line) or secondary reagent only (thin line). (C) To assess the internalization of GP2, moDCs were incubated with GP2-bio. Cells were stained with SA-PE before (bold line) or after a one hour incubation step at 37 °C (grey histogram). For control, moDCs were stained with SA-PE (thin line). MFI = Mean fluorescence intensity. One representative result of four independently performed experiments is shown.
3.4. Redundant GP2 binding structures on moDCs
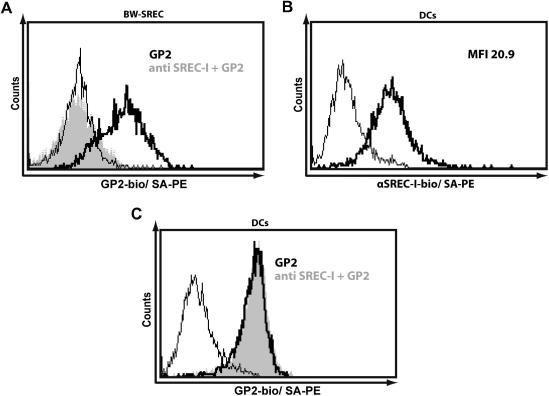
In order to evaluate moDCs for the presence of additional GP2 binding receptors, we investigated the contribution of SREC-I on the total GP2 binding. Therefore, we used anti-SREC-I antibodies to block the interaction of moDCs with GP2-bio. Ligation of SREC-I on SREC-I expressing Bw cells by specific antibodies resulted in a strong decrease of the MFI, indicating that the antibodies are suitable competitors of GP2 binding (Fig. 4A). In Fig. 4B expression of SREC-I on the surface of moDCs is demonstrated. By performing the inhibition experiments as shown in Fig. 4A for SREC-I expressing Bw cells, however, no decrease in GP2-bio binding to moDCs could be observed (Fig. 4C). This indicates the presence of additional GP2 binding structures on these cells.
Fig. 4.
GP2-bio binding on moDCs cannot be inhibited by anti-SREC-I antibodies. (A) GP2-bio binding to SREC-I expressing Bw cells was inhibited by coincubating GP2-bio at a concentration of 5 μg/mL with anti-SREC-I antibody at a concentration of 20 μg/mL (grey histogram). (B) Expression of SREC-I on moDCs cells was evaluated by staining cells with biotinylated SREC-I antibody at a concentration of 20 μg/mL (bold line). (C) Inhibition of GP2-bio binding to moDCs by SREC-I antibodies was investigated by coincubating moDCs with anti-SREC-I antibodies at a concentration of 20 μg/mL and GP2-bio at a concentration of 5 μg/mL (grey histogram). One representative result of three independently performed experiments is shown. MFI = Mean fluorescence intensity.
4. Discussion
GP2 and THP are highly homologous proteins that share several important features. They have a high sequence homology and can attach to the FimH adhesin of E. coli or S. typhimurium. Furthermore, both molecules can be secreted from their producer cells after cleavage of their GPI anchor and are then found in the excretions of the gastrointestinal and urogenital tract, respectively [9]. Because of these profound similarities, we were interested, whether membrane-detached GP2 and THP utilize common receptors and thus analyzed the interaction of GP2 with SRs.
We here demonstrate that GP2 binds to SREC-I with an affinity of 41.3 nM. This resembles the dissociation constant of THP for SREC-I, which was 16.8 nM [23]. Whereas THP was also shown to bind SR-AI and SR-BI, specific interaction of these SRs with GP2 was not observed. Several reasons might be responsible for the differences in the binding behavior. Despite the high sequence homology with THP, GP2 lacks the epidermal growth factor like domains [24], which could explain the lack of binding to SR-AI and SR-BI. Furthermore, GP2 used in the present study was expressed by Sf9 insect cells. Therefore also differences in glycosylation might cause the absence of binding to these receptors [25].
SRs have an important function in the binding and uptake of proteins. SREC-I, for example, has been described to bind and internalize acLDL as well as members of the heat shock protein (HSP) family [22,26]. Recently, it has been revealed that also the urinary glycoprotein THP is taken up by SREC-I [23]. Therefore, we were interested whether SREC-I retrovirally expressed on the Bw cell line also can mediate the internalization of GP2. By employing internalization assays we demonstrated that bound GP2 disappeared from the surface of SREC-I expressing cells. We could also show efficient uptake of GP in moDCs. Although SREC-I is expressed on the surface of moDCs, we found that GP2 binding to moDCs cannot be blocked by anti-SREC-I antibodies. These findings indicate the presence of additional GP2 binding structures on DCs. Thus, screening of receptors represented in a DC cDNA library and expressed in special cell lines with GP2 as ligand might help to discover additional GP2 binding structures.
Recently, membrane resident GP2 was shown to mediate pathogen binding of enteropathogenic E. coli [12]. Binding of enteropathogenic E. coli by GP2 expressed on intestinal M-cells of the ileum resulted in transcytosis of bacteria and the induction of a mucosal immune response [6]. However, a physiological role of soluble GP2 in host defense has not been reported to date. Based on our findings, it can be speculated that binding and ingestion of soluble GP2 bound to proteins or pathogens might also be an important determinant in various immunological processes. A model for the internalization of molecules by DCs via SREC-I was recently provided by Murshid et al. The authors observed that SREC-I, expressed on DCs bound and internalized complexes existing of HSP90 and ovalbumin (OVA) [17]. OVA peptides that did not require processing were directly loaded on MHC class I and mediated efficient cross presentation by the antigen presenting cells. It is well conceivable that GP2 also could act as a chaperone that enables the uptake of proteins or peptides via SREC-I. The identification of proteins or peptides that bind to GP2 will help to clarify, whether GP2 might play a role in immunological processes, as for example cross presentation.
Interestingly, after secretion of pancreatic acinar cells, GP2 is not digested by zymogens. Therefore, it could also function as a decoy receptor that inhibits the adhesion of potential pathogenic bacteria to the intestinal epithelium. This function has already been shown for THP, which inhibits bacterial linkage to urothelial uroplakin receptors by covering uropathogenic E. coli [13]. Furthermore, GP2 could act as a bridge that mediates microbial binding to SR expressing immune cells. Opsonization of microbes with soluble GP2 would thus facilitate their uptake by phagocytic cells of the immune system. Such interaction of local substances with resident cells of the immune system should be suitable to draw the attention of the immune system to local invaders so that it becomes well prepared in case these microbes indeed get access to local tissues.
In conclusion, the present study reveals GP2 as a novel high affinity interaction partner of SREC-I. Further investigations will be necessary to unravel the possible implications of this receptor/ligand interaction on physiological processes, as for example infection and inflammation.
5. Disclosures
GA Generic Assays GmbH (D Ro) is a manufacturer and distributor of in vitro diagnostics. All remaining authors have no competing financial interests.
Acknowledgments
The project was funded by the Austrian Science Foundation (FWF) grant P20508-B11. We thank Claus Wenhardt and Margarethe Merio for the excellent technical assistance. We thank Prof. Otto Majdic for providing us with SREC-I monoclonal antibodies.
References
- 1.Fukuoka S. Analysis of ZAPs, zymogen granule membrane associated proteins, in the regulated exocytosis of the pancreas. Biosci. Biotechnol. Biochem. 1994;58:1282–1285. doi: 10.1271/bbb.58.1282. [DOI] [PubMed] [Google Scholar]
- 2.Ronzio R.A., Kronquist K.E., Lewis D.S., MacDonald R.J., Mohrlok S.H., O’Donnell J.J., Jr. Glycoprotein synthesis in the adult rat pancreas. IV. Subcellular distribution of membrane glycoproteins. Biochim. Biophys. Acta. 1978;508:65–84. doi: 10.1016/0005-2736(78)90189-x. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt K., Dartsch H., Linder D., Kern H.F., Kleene R. A submembranous matrix of proteoglycans on zymogen granule membranes is involved in granule formation in rat pancreatic acinar cells. J. Cell Sci. 2000;113(Pt12):2233–2242. doi: 10.1242/jcs.113.12.2233. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt K., Schrader M., Kern H.F., Kleene R. Regulated apical secretion of zymogens in rat pancreas. Involvement of the glycosylphosphatidylinositol-anchored glycoprotein GP-2, the lectin ZG16p, and cholesterol-glycosphingolipid-enriched microdomains. J. Biol. Chem. 2001;276:14315–14323. doi: 10.1074/jbc.M006221200. [DOI] [PubMed] [Google Scholar]
- 5.Yu S., Michie S.A., Lowe A.W. Absence of the major zymogen granule membrane protein, GP2, does not affect pancreatic morphology or secretion. J. Biol. Chem. 2004;279:50274–50279. doi: 10.1074/jbc.M410599200. [DOI] [PubMed] [Google Scholar]
- 6.Hase K., Kawano K., Nochi T., Pontes G.S., Fukuda S., Ebisawa M., Kadokura K., Tobe T., Fujimura Y., Kawano S., Yabashi A., Waguri S., Nakato G., Kimura S., Murakami T., Iimura M., Hamura K., Fukuoka S., Lowe A.W., Itoh K., Kiyono H., Ohno H. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K., Yanagihara K., Ishiguro K., Fukuoka S. GP2/THP gene family of self-binding, GPI-anchored proteins forms a cluster at chromosome 7F1 region in mouse genome. Biochem. Biophys. Res. Commun. 2004;322:659–664. doi: 10.1016/j.bbrc.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 8.Hoops T.C., Rindler M.J. Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. Homology to uromodulin/Tamm–Horsfall protein. J. Biol. Chem. 1991;266:4257–4263. [PubMed] [Google Scholar]
- 9.Serafini-Cessi F., Malagolini N., Cavallone D. Tamm–Horsfall glycoprotein: Biology and clinical relevance. Am. J. Kidney Dis. 2003;42:658–676. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 10.Rindler M.J., Naik S.S., Li N., Hoops T.C., Peraldi M.N. Uromodulin (Tamm–Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J. Biol. Chem. 1990;265:20784–20789. [PubMed] [Google Scholar]
- 11.Fukuoka S., Kobayashi K. Analysis of the C-terminal structure of urinary Tamm–Horsfall protein reveals that the release of the glycosyl phosphatidylinositol-anchored counterpart from the kidney occurs by phenylalanine-specific proteolysis. Biochem. Biophys. Res. Commun. 2001;289:1044–1048. doi: 10.1006/bbrc.2001.6112. [DOI] [PubMed] [Google Scholar]
- 12.Yu S., Lowe A.W. The pancreatic zymogen granule membrane protein, GP2, binds Escherichia coli Type 1 fimbriae. BMC Gastroenterol. 2009;9:58. doi: 10.1186/1471-230X-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pak J., Pu Y., Zhang Z.T., Hasty D.L., Wu X.R. Tamm–Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. Coli from binding to uroplakin Ia and Ib receptors. J. Biol. Chem. 2001;276:9924–9930. doi: 10.1074/jbc.M008610200. [DOI] [PubMed] [Google Scholar]
- 14.Areschoug T., Gordon S. Pattern recognition receptors and their role in innate immunity: Focus on microbial protein ligands. Contrib. Microbiol. 2008;15:45–60. doi: 10.1159/000135685. [DOI] [PubMed] [Google Scholar]
- 15.Beauvillain C., Meloni F., Sirard J.C., Blanchard S., Jarry U., Scotet M., Magistrelli G., Delneste Y., Barnaba V., Jeannin P. The scavenger receptors SRA-1 and SREC-I cooperate with TLR2 in the recognition of the hepatitis C virus non-structural protein 3 by dendritic cells. J. Hepatol. 2010;52:644–651. doi: 10.1016/j.jhep.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Adachi H., Tsujimoto M., Arai H., Inoue K. Expression cloning of a novel scavenger receptor from human endothelial cells. J. Biol. Chem. 1997;272:31217–31220. doi: 10.1074/jbc.272.50.31217. [DOI] [PubMed] [Google Scholar]
- 17.Murshid A., Gong J., Calderwood S.K. Heat shock protein 90 mediates efficient antigen cross presentation through the scavenger receptor expressed by endothelial cells-I. J. Immunol. 2010;195:2903–2917. doi: 10.4049/jimmunol.0903635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holzl M.A., Hofer J., Steinberger P., Pfistershammer K., Zlabinger G.J. Host antimicrobial proteins as endogenous immunomodulators. Immunol. Lett. 2008;119:4–11. doi: 10.1016/j.imlet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Roggenbuck D., Hausdorf G., Martinez-Gamboa L., Reinhold D., Buttner T., Jungblut P.R., Porstmann T., Laass M.W., Henker J., Buning C., Feist E., Conrad K. Identification of GP2, the major zymogen granule membrane glycoprotein, as the autoantigen of pancreatic antibodies in Crohn’s disease. Gut. 2009;58:1620–1628. doi: 10.1136/gut.2008.162495. [DOI] [PubMed] [Google Scholar]
- 20.Hitoshi Y., Lorens J., Kitada S.I., Fisher J., LaBarge M., Ring H.Z., Francke U., Reed J.C., Kinoshita S., Nolan G.P. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 21.Steinberger P., Majdic O., Derdak S.V., Pfistershammer K., Kirchberger S., Klauser C., Zlabinger G., Pickl W.F., Stockl J., Knapp W. Molecular characterization of human 4Ig-B7–H3, a member of the B7 family with four Ig-like domains. J. Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 22.Tamura Y., Osuga J., Adachi H., Tozawa R., Takanezawa Y., Ohashi K., Yahagi N., Sekiya M., Okazaki H., Tomita S., Iizuka Y., Koizumi H., Inaba T., Yagyu H., Kamada N., Suzuki H., Shimano H., Kadowaki T., Tsujimoto M., Arai H., Yamada N., Ishibashi S. Scavenger receptor expressed by endothelial cells I (SREC-I) mediates the uptake of acetylated low density lipoproteins by macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 2004;279:30938–30944. doi: 10.1074/jbc.M313088200. [DOI] [PubMed] [Google Scholar]
- 23.Pfistershammer K., Klauser C., Leitner J., Stockl J., Majdic O., Weichhart T., Sobanov Y., Bochkov V., Saemann M., Zlabinger G., Steinberger P. Identification of the scavenger receptors SREC-I, Cla-1 (SR-BI), and SR-AI as cellular receptors for Tamm–Horsfall protein. J. Leukoc. Biol. 2008;83:131–138. doi: 10.1189/jlb.0407231. [DOI] [PubMed] [Google Scholar]
- 24.Wong S.M., Lowe A.W. Sequence of the cDNA encoding human GP-2, the major membrane protein in the secretory granule of the exocrine pancreas. Gene. 1996;171:311–312. doi: 10.1016/0378-1119(96)00065-0. [DOI] [PubMed] [Google Scholar]
- 25.Altmann F., Staudacher E., Wilson I.B., Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj. J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- 26.Facciponte J.G., Wang X.Y., Subjeck J.R. Hsp110 and Grp170, members of the Hsp70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. Eur. J. Immunol. 2007;37:2268–2279. doi: 10.1002/eji.200737127. [DOI] [PubMed] [Google Scholar]