Abstract
Long-distance migrations of wildlife have been identified as important biological phenomena, but their conservation remains a major challenge. The Mongolian Gobi is one of the last refuges for the Asiatic wild ass (Equus hemionus) and other threatened migratory mammals. Using historic and current distribution ranges, population genetics, and telemetry data we assessed the connectivity of the wild ass population in the context of natural and anthropogenic landscape features and the existing network of protected areas. In the Mongolian Gobi mean biomass production is highly correlated with human and livestock density and seems to predict wild ass occurrence at the upper level. The current wild ass distribution range largely falls into areas below the 250 gC/m2/year productivity isoline, suggesting that under the present land use more productive areas have become unavailable for wild asses. Population genetics results identified two subpopulations and delineated a genetic boundary between the Dzungarian and Transaltai Gobi for which the most likely explanation are the mountain ranges separating the two areas. Home ranges and locations of 19 radiomarked wild asses support the assumed restricting effects of more productive habitats and mountain ranges and additionally point towards a barrier effect of fences. Furthermore, telemetry data shows that in the Dzungarian and Transaltai Gobi individual wild ass rarely ventured outside of the protected areas, whereas in the southeast Gobi asses only spend a small fraction of their time within the protected area network. Conserving the continuity of the wild ass population will need a landscape level approach, also including multi-use landscapes outside of protected areas, particularly in the southeast Gobi. In the southwest Gobi, allowing for openings in the border fence to China and managing the border area as an ecological corridor would connect three large protected areas together covering over 70,000 km2 of wild ass habitat.
Keywords: Asiatic wild ass, Barriers, China, Equus hemionus, Fragmentation, Landscape genetics, Mongolia, Protected areas
1. Introduction
Habitat loss and fragmentation have been identified as key threats to biodiversity conservation worldwide. Busy transportation routes and fences are significant mortality factors (Harrington and Conover, 2006; Lovari et al., 2007), impede movement of wildlife by creating access barriers to important resources (Frair et al., 2008), stop or slow population expansion (Kramer-Schadt et al., 2004), or subdivide once-continuous populations into more or less isolated subpopulations (Lankester et al., 1991). Large-bodied, far-ranging mammals like large carnivores and large herbivores are particularly sensitive to fragmentation because they need access to large tracts of continuous habitat. Seasonal changes in habitat conditions can force large herbivore populations to migrate between distinct seasonal ranges (Wolanski et al., 1999; Ferguson and Elkie, 2004), whereas unpredictable changes in habitat conditions can force them to resume nomadic movements (Mueller et al., 2008). The fragmentation of habitat into small and often non-contiguous patches decreases their capacity to escape locally poor habitat conditions and may result in dramatic population declines (Berger, 2004; Bolger et al., 2007). Furthermore, small and fragmented subpopulations become vulnerable to chance events like demographic, genetic, and environmental stochasticity (van Noordwijk, 1994; Frankham, 2005). The smaller the subpopulation and the more unpredictable the habitat, the higher the risk of local extinctions becomes.
Landscape genetics has become a powerful tool for addressing population fragmentation on the landscape level (Holderegger and Wagner, 2008). Several studies have revealed clear associations between habitat fragmentation and the genetic structure of wide-ranging, long-lived, and large-bodied mammal species (McRae et al., 2005), and identified barriers (Riley et al., 2006) as well as corridors (Dixon et al., 2006). A recent approach applied landscape genetics to optimize dispersal and corridor models (Epps et al., 2007); however, the application of genetic tools for conservation is still largely method and theory driven, rather than focused on real data sets with relevance to conservation problems (Vernesi et al., 2008).
Although long-distance migrations and nomadic movements over extensive areas have been identified as important biological phenomena (Convention on Migratory Species (CMS), 2002), their conservation remains a major challenge of the 21st century (Berger, 2004; Thirgood et al., 2004; Bolger et al., 2007; Wilcove and Wikelski, 2008). The steppes, desert steppes, and deserts of Central Asia are still home to several globally threatened migratory or nomadic large herbivores (Berger, 2004; Bolger et al., 2007). However, a growing human population, changes in land management, exploitation of natural resources, and the development of infrastructure place increasing pressure on these species and their habitats (Reading et al., 1998; Milner-Gulland et al., 2003; Ito et al., 2005; Clark et al., 2006; Qui, 2007; Wingard and Zahler, 2006). Among these species is the Asiatic wild ass, Equus hemionus.
The Mongolian Gobi and adjacent areas in northern China provide the last refuge for the Asiatic wild ass and other threatened wildlife (Clark et al., 2006; Yang, 2007). Anecdotal evidence suggests that the Asiatic wild ass may have lost as much as 70% of its range since the 19th century because of direct persecution and competition with humans and their livestock over water and pasture use (Zevegmid and Dawaa, 1973; Reading et al., 2001). Reliable historic population numbers for wild asses are unavailable (Reading et al., 2001) and recent estimates are either plagued by a high variance of the estimate (Reading et al., 2001; B. Lkhagvasuren and S. Strindberg, unpubl. data) or a lack of statistical rigor in the analysis (Lhagvasuren 2007; Yang, 2007). Most likely the Mongolian population still numbers in the magnitude of 10–20,000 animals (B. Lkhagvasuren and S. Strindberg, unpubl. Data; Kaczensky, unpubl. Data), while adjacent China likely houses another few thousand animals (Yang, 2007; Yang, unpubl. data).
The Asiatic wild ass has been fully protected in Mongolia since the 1950s (Clark et al., 2006), and large portions of its habitat are under formal protection. Nevertheless, little is known about the degree of connectivity and whether or not the current protected area system is adequate to safeguard the wild ass population of the Gobi.
People consider wild asses to compete with their livestock for pasture and water. As a consequence wild asses are actively chased away or illegally killed by people (Kaczensky et al., 2006; Wingard and Zahler, 2006) and the mere presence of people and their livestock at water points can limit or block access for Asiatic wild asses (Denzau and Denzau, 1999; Kaczensky et al., 2006). In recent years, Mongolia has been anticipating the development of a commercialized agricultural sector that could easily cause greater intrusion of human activities into the Gobi areas (World Bank, 2003). Development of other sectors of the Mongolian economy, especially mining and road construction (World Bank, 2006), could further affect the environmental security and habitat needs of the Asiatic wild ass and associated wildlife in the Gobi (Kaczensky et al., 2006).
An evaluation of the connectivity of the still abundant Asiatic wild ass population would yield important information about the integrity of the Gobi ecosystem and identify possible movement barriers. Such barriers are likely to also affect other species that presently have a more restricted distribution range, such as the wild Bactrian camel (Camelus ferus bactrianus), the saiga (Saiga tatarica), or the re-introduced Przewalski’s horse (Equus ferus przewalskii, Clark et al., 2006). Using telemetry, population genetics, and distribution range data, we assessed the connectivity of the wild ass population in the context of natural and anthropogenic landscape features.
2. Study area
The Gobi areas cover roughly 300,000 km2 of desert steppe and desert areas in southern Mongolia (Fig. 1). The climate is strongly continental with long cold winters (January mean, −15 °C to −20 °C) and short hot summers (July mean, 20–25 °C). Average annual precipitation ranges from 50 mm in the Transaltai Gobi, to 100 mm in the Dzungarian Gobi, and up to 200 mm in parts of the southeastern Gobi (von Wehrden and Wesche, 2007). Because the area also shows high levels of inter-annual variation in precipitation, the majority of the Gobi is believed to follow non-equilibrium dynamics (von Wehrden et al., submitted for publication) and thus to have a low risk for degradation caused by grazing.
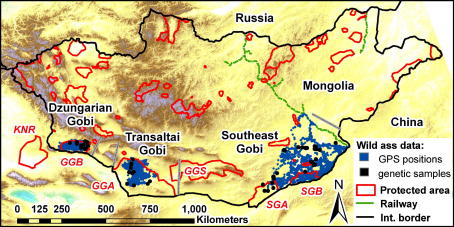
Fig. 1.
GPS locations and ranges of 18 Asiatic wild asses in the Dzungarian, Transaltai, and southeast Gobi of Mongolia 2002–2008. Grey lines delineate the three geo-biographical areas of the Mongolian Gobi. KNR = Kalimalai Nature Reserve, GGA = Great Gobi A strictly protected area, GGB = Great Gobi B strictly protected area, GGS = Gobi Gurvan Saikhan National Park, SGA = Small Gobi A strictly protected area, SGB = Small Gobi B strictly protected area.
Elevations range from 550 to 3750 m. The Dzungarian Gobi is located in a natural basin flanked by the southern tip of the Altai Mountain range to the north and east and a mountain range along the border to China in the south. The Transaltai Gobi is flanked by the Edrene mountain range in the north but also encompasses a medium-sized mountain range in the south–central part. The southeastern Gobi is largely a flat landscape with few distinct topographic features (Fig. 1).
The plant community of the desert areas is widely dominated by Chenopodiaceae, such as saxaul (Haloxylon ammodendron) and Anabasis brevifolia. Asteraceae, such as Artemisia and Ajania, and Poaceae like Stipa and Ptilagrostis, dominate the steppe areas. High productivity riparian vegetation and Nitraria sibirica communities are rare and restricted to larger oases and intermittent river valleys (Hilbig, 1995).
Open water is unevenly distributed, with varying predictability among the areas. In the Dzungarian Gobi, open water is rare, but springs tend to be permanent. In the Transaltai Gobi, open water is extremely rare and except for a few large oases, smaller water points may fall dry during certain seasons or years. Although the southeastern Gobi receives the most precipitation, intra-annual and inter-annual availability of open water is highly variable (Kaczensky et al., 2006).
The Gobi region is at the center of the Cashmere goat industry in Mongolia, and livestock products generate the main income of local herders (World Bank, 2003). Human population density in the 24 Gobi districts (>30% of the area within the wild ass range) is very low, averaging 0.2 inhabitants/km2, and there are only 17 villages, with 500–1500 inhabitants each. Livestock numbers, on the other hand, total 2 million sheep and goats, 614,000 horses, 413,000 domestic camels, and 280,000 cows and yaks (National Statistical Office of Mongolia, 2004 and 2007, unpubl. data). The state owns all grazing land in Mongolia, and the district governments allocate grazing rights based on pasture condition, previous use, and family relationships. In most Gobi areas, herders and their livestock follow a semi-nomadic lifestyle (Fernandez-Gimenez and Batbuyan, 2004). No fences are allowed to delineate grazing plots; the only fences dissecting the Gobi today are along the international border to China and the Ulaanbaatar–Beijing railway (Fig. 1). The Gobi is rich in mineral deposits, and official exploration and mining activities are increasing (World Bank, 2006). In October 2009 Ivanhoe Mines and Rio Tinto signed an Investment Agreement with the Government of Mongolia for the construction and operation of the Oyu Tolgoi copper–gold mining complex in the southeast Gobi (Ivanhoe Mines, 2009). Additionally, illegal mining by so-called “Ninja miners” mechanically destroys large tracts of pastureland and depletes or pollutes local water sources (Grayson 2007).
There are eight protected areas within or intersecting the present-day wild ass distribution range amounting to 29% of the area being under formal protection: Great Gobi B strictly protected area (SPA; 9000 km2), Great Gobi A SPA (44,000 km2), Small Gobi A SPA (11,500 km2), Small Gobi B SPA (6500 km2), the southern parts of Gobi Gurvan Saikhan National Park (5900 km2), Ergeliin Zoo Nature Reserve (620 km2), Zagiin Us Nature Reserve (2500 km2), and the Suikhent Uul National Monument (50 km2; Fig. 1). The closest protected area on the Chinese side, less than 40 km from the border, is the Kalimalai Nature Reserve (17,300 km2). Distances between the four large protected areas in the Mongolian wild ass range are 190 km between Great Gobi A and B, 420 km between Great Gobi A and Small Gobi A, and 80 km between Small Gobi A and B.
The ungulate community of the Mongolian Gobi consists of goitered gazelle (Gazella subgutturosa), Mongolian gazelle (Procapra gutturosa), saiga, Asiatic wild ass, re-introduced Przewalski’s horse, and wild Bactrian camels on the plains, and Siberian ibex (Capra sibirica) and argali sheep (Ovis ammon) in the mountains (Clark et al., 2006). The wild ass population is not evenly distributed over the entire Gobi. The majority of wild asses, likely as many as 70%, are found in the eastern part of the southeast Gobi. The remaining asses largely occur in the Dzungarian Gobi, while the Transaltai Gobi only houses wild asses at very low densities (Reading et al. 2001; Lhagvasuren, 2007; B. Lkhagvasuren and S. Strindberg, unpubl. data; P. Kaczensky, unpubl. data).
3. Methods
3.1. Wild ass distribution range and habitat database
Historic wild ass distribution ranges were digitized from maps provided in Zevegmid and Dawaa (1973) and from information summarized in Denzau and Denzau (1999). To determine the northern border of the current distribution range of Asiatic wild ass in Mongolia, we combined our telemetry data with wild ass observations made during the national surveys in 2003 and 2009 and observations made during multiple trips to the Transaltai and southeast Gobi between 2003 and 2007 (see Supporting Data Appendix S1).
3.2. Mean biomass production as a proxi for human and livestock densities
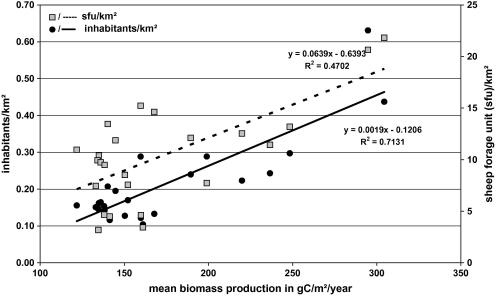
We purchased livestock numbers for all 24 Gobi districts (sums) from the Statistical Office of Mongolia for the year 2004 for the eastern Gobi and for the year 2007 for the western Gobi. We additionally obtained a digital layer with all sum boundaries and human inhabitants dating from 2002 (see Supporting Data Appendix S2). However, the Gobi districts are rather large (range: 7106–27,784 km2) and often expand into more productive mountainous habitat, particularly in the west. In addition, Mongolia does not have a common database with exact locational data on herder camp distribution, movement paths, and associated herd sizes. Furthermore, in the highly variable non-equilibrium Gobi ecosystem pasture quality, and thus human and livestock presence, can vary tremendously on an inter- as well as an intra-annual basis. Nevertheless, on average human population- and livestock densities in the Gobi clearly increase with higher biomass production (Fig. 2). In order to have a measure independent of administrational units we thus chose to use mean biomass production as a proxy for human and livestock densities.
Fig. 2.
Relationship between biomass production (expressed in grams of carbon per square meter and year (gC/m2/years)) and human population- and livestock density (expressed as sheep forage units (sfu*)) in the 24 Gobi districts (sums) of Mongolia. *1 sfu is the amount of dry forage needed to feed an average Mongolian sheep for 1 year, which is approximately 365 kg (Fernandez-Gimenez 1999). The equivalencies for the other species are: 1 camel = 5 sfu, 1 horse = 7 sfu, 1 cow/yak = 6 sfu, 1 goat = 0.9 sfu.
For the overall estimate of biomass production, we used the global layer of biomass production expressed in grams of carbon per square meter and year (gC/m2/years) for 1981–2000. This open-source GIS data set is available on an 8 × 8 km raster basis under http://glcf.umiacs.umd.edu/data/glopem/ with data processing described in Prince and Goward (1995). For our analysis, we used the mean biomass production over all 20 years.
Shuttle Radar Topography Mission tiles for Mongolia and northern China were downloaded from http://glcf.umiacs.umd.edu/ and merged into one file with a spatial resolution of 90 m (Fig. 1). We extracted slope from the digital elevation model and classified slopes ⩽ 5°as “flat”; slopes 5–20° as “mountains”; and slopes >20° as “steep mountains”.
3.3. Genetic sampling and extraction
We collected 80 wild ass samples between 2002 and 2005 that yielded sufficient DNA for analysis: 19 in the Dzungarian Gobi, 18 in the Transaltai Gobi, and 43 in the southeastern Gobi (Fig. 1). We obtained 65 samples from carcass remains and 15 from fecal deposits. For each sample, we recorded the GPS position and the date. The distances between the arithmetic means of the sample coordinates from the Dzungarian and the Transaltai Gobi were 325 km (range for individual samples: 265–504 km), and between the Transaltai and the southeastern Gobi, 930 km (range for individual samples: 640–1205 km). Fresh fecal pellets were stored in 90% ethanol. Old fecal samples, tissue, and bones were preserved dry, packed in plastic bags, and stored at −20 °C prior to DNA extraction.
We used commercially available DNA extraction kits (Qiagen and MACHEREY- NAGEL GmbH and Co. KG, Germany) with some modifications to prepare genomic DNA from deep-frozen fecal and 90% ethanol–preserved fecal pellets as well as from bones and dried tissue samples. We used the SYBR Green detection system in a LightCycler (Roche) for quantitative PCR (qPCR) for quality control (see Supporting Data Appendix S3). For species verification, we used restriction fragment length polymorphism analysis of the mitochondrial cytochrome b gene fragment as described in Kuehn et al. (2006).
We tested 11 equine microsatellites (COR70, SGCV28, ASB23, ASB2, COR58, LEX68, COR18, UM11, COR007, LEX74, and COR71) for successful cross-species amplification and high polymorphism in Asiatic wild ass (see Supporting Data Appendix S4). Annealing temperatures and MgCl2 concentrations were adjusted for stringent amplification conditions. To avoid linkage, we chose microsatellite loci from different chromosomes of the domestic horse for genotyping analyses (genome map of the horse: www.thearkdb.org).
To avoid contamination of PCR products or concentrated genomic DNA and misinterpretations of microsatellite data based on allelic drop-out and false alleles (Taberlet and Luikart, 1999), we (i) included negative controls without sample material in every DNA isolation and amplification experiment to check for contamination; (ii) rejected samples with ⩽100 pg/μl for microsatellite DNA amplification; and (iii) repeated all genotyping analyses at least three times, accepting only genotypes that produced three consistent results.
3.4. Population genetic analyses
We calculated allele frequencies, average allele numbers per locus (A), expected and observed heterozygosities (HE, HO), deviation from Hardy–Weinberg equilibrium, and pairwise genetic differentiation values (FST) using GENEPOP v. 3.4 (Raymond and Rousset, 1995a), assuming three subpopulations for the Mongolian wild ass population. Probability tests were performed applying the Markov Chain algorithm (Raymond and Rousset, 1995b). We additionally calculated the inbreeding coefficient of a group of inbred organisms relative to the subpopulation to which they belong (FIS) and allelic richness (AR) as a standardized measure for the number of alleles corrected by the sample size with the FSTAT v. 2.9.3 program package (Goudet, 2001). Alleles were considered private if they showed a frequency higher than 5% in one population and did not occur in any other population (Geist and Kuehn, 2005).
We used STRUCTURE 2.2 software (Pritchard et al., 2000) to determine the number of genetic clusters (K) and to probabilistically assign individuals to these clusters. We chose the population admixture without sampling information and correlated allele frequency models. We tested K from one to eight with 10 iterations (20,000 burn-in; 200,000 Markov chain Monte Carlo replicates in each run) to assess convergence of ln Pr (X|K). The number of clusters present was then determined from posterior probabilities of K and additionally by an ad hoc statistic ΔK based on the rate of change in the log probability of data (Evanno et al., 2005). For the selected values of K, we assessed the average proportion of membership of the samples to the inferred clusters (PMIs) by combining the 10 replications using CLUMPP (Jakobsson and Rosenberg, 2007), applying the LargeKGreedy algorithm.
Within the framework of landscape genetics, we performed Mantel’s test to evaluate the effect of geographical distance on the level of genetic differentiation using module Mantel in the R software (R Development Core Team 2005). The statistical significance of the relationship was determined by 100,000 randomizations. To identify possible genetic boundaries—zones where genetic differences between pairs of populations are highest—we applied the Monmonier maximum difference algorithm (Monmonier, 1973) using the software BARRIER version 2.2 (Manni, 2004). The robustness of the genetic boundaries was assessed by 100 bootstrap iterations of the pairwise FST-matrices (Weir and Cockerham, 1984).
3.5. Telemetry
Between 2002 and 2007, we captured and radio-collared 19 Asiatic wild asses, seven mares and 12 stallions, in the three assumed subpopulations (Fig. 1, Table 1). For darting, we approached animals using a 4 × 4 vehicle or hid at water points (for details see Walzer et al., 2006). The first four wild asses were collared with Argos (2-D cell Doppler PTT; NorthStar, Baltimore, Maryland, USA) and the remaining 15 with GPS-Argos collars (TWG-3580, Telonics, Mesa, Arizona, USA). While the Argos collars determined animal locations using the Doppler-shift method through the Argos satellite system, the GPS-Argos collars used the Argos system only for data transfer of GPS locations. For animal welfare reasons and to allow collar retrieval, all units were equipped with pre-programmed drop-off devices (CR-2a, Telonics).
Table 1.
Location parameters for 18 wild asses in the Mongolian Gobi.
| Monitoring period |
N |
MCPb (km2) | % Location days |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collar ID | Sex | Age | Start | End | LOCsa | LOC days | Outside of PAsc | <5 km of fence | Biomassd>250 gC/m2/a | Biomass <100 gC/m2/a | On slopes >5° | On slopes >20° | |
| Dzungarian Gobi | |||||||||||||
| 11525⁎ | Female | 2 | 24.06.02 | 01.08.04 | 489 | 337 | 6991 | 3.9 | 0 | 2.1 | 0 | 4.7 | 0 |
| 16690⁎ | Female | 3 | 28.06.02 | 01.08.04 | 1243 | 549 | 7368 | 1.5 | 0 | 0 | 0 | 4.7 | 0 |
| 167161⁎ | Male | 2 | 28.06.02 | 15.03.03 | 153 | 101 | 6889 | 7.9 | 0 | 2.0 | 0 | 0 | 0 |
| 22929 | Male | 7 | 16.07.03 | 03.08.04 | 648 | 347 | 4889 | 4.0 | 0 | 3.2 | 0 | 1.4 | 0 |
| 223661 | Male | 4 | 16.07.03 | 27.06.04 | 360 | 221 | 5858 | 4.5 | 0 | 4.1 | 0 | 2.7 | 0 |
| 25915 | Male | 4 | 16.07.03 | 07.08.04 | 424 | 252 | 5121 | 0.4 | 0 | 0.8 | 0 | 2.0 | 0 |
| 167162⁎ | Male | 10 | 17.07.03 | 18.05.04 | 111 | 67 | 5180 | 3.0 | 0 | 1.5 | 0 | 0 | 0 |
| Transaltai Gobi⁎⁎ | |||||||||||||
| 588492 | Male | Adult | 04.07.06 | 15.09.06 | 20 | 12 | 316 | 0 | 0 | 0 | 25.0 | 0 | 0 |
| 223662 | Female | Young | 21.05.07 | 25.10.08 | 975 | 495 | 16,907 | 0.4 | 0 | 0 | 33.9 | 11.7 | 0.2 |
| 70349 | Male | Young | 25.05.07 | 04.03.08 | 19 | 12 | 4971 | 0 | 0 | 0 | 0 | 16.7 | 0 |
| 588481 | Male | 7–8 | 27.05.07 | 01.09.07 | 270 | 93 | 10,748 | 0 | 0 | 0 | 14.0 | 19.4 | 0 |
| 25731 | Female | 5–7 | 05.06.07 | 21.12.08 | 1208 | 560 | 14,695 | 4.5 | 0 | 0 | 25.7 | 30.9 | 0.7 |
| SE Gobi | |||||||||||||
| 58,851 | Male | 2–3 | 03.07.05 | 25.04.06 | 993 | 297 | 69,988 | 93.6 | 5.1 | 0.3 | 3.0 | 0 | 0 |
| 58,850 | Female | 7 | 03.07.05 | 19.04.06 | 147 | 70 | 29,910 | 75.7 | 12.9 | 0 | 5.7 | 0 | 0 |
| 58,848 | Female | 5 | 03.07.05 | 20.10.06 | 1570 | 472 | 41,091 | 75.0 | 16.7 | 0 | 14.6 | 0 | 0 |
| 58,854 | Female | 11 | 04.07.05 | 02.12.05 | 67 | 32 | 39,396 | 75.0 | 3.1 | 0 | 0 | 0 | 0 |
| 58,853 | Male | 5–6 | 05.07.05 | 03.05.06 | 129 | 59 | 18,186 | 62.7 | 8.5 | 0 | 1.7 | 0 | 0 |
| 588,491 | Male | 4 | 08.07.05 | 30.07.05 | 35 | 18 | 11,400 | 100 | 0 | 0 | 0 | 0 | 0 |
| 58,852 | Male | 4 | 09.07.05 | 08.08.06 | 1168 | 369 | 19,671 | 59.1 | 0 | 0 | 3.5 | 0.8 | 0 |
Asterisks mark animals tracked with Argos collars using the Doppler-shift method, all other animals were tracked using GPS technology.
Grey shading marks animals which were not included in the statistical comparison of home range sizes as they did not fulfill the criteria of having being monitored >5 months with >50 location days more or less evenly distributed over the monitoring period.
LOC = location.
MCP = 100% minimum convex polygon.
PA = protected area.
gC/m2/a = gram carbon per square meter and year.
Precisions of the GPS locations were in the range of ±15–100 m (P. Kaczensky unpublished data). For Argos locations we only used the three most precise location classes, where the expected error is ±150–1000 m (Hays et al. 2001). Because of multiple technical failures (Kaczensky et al., accepted for publication), individual collars collected from 19–1570 locations on 12–560 days (location days). The number of daily locations varied from 1–7, depending on collar type, performance, and duty cycle. On average, 2.1 locations were obtained per animal and location day (range 1.5–3.3).
For visualization and analysis of spatial data, we used ArcMap 9.1 (ESRI, Environmental Systems Research Institute, Inc., Redlands, California, USA) with the Hawth’s Analysis Tool extension (http://www.spatialecology.com/htools). We calculated home ranges expressed as 100% minimum convex polygons (MCPs) for all individuals. However, for statistical comparison among the three bio-geographic regions we only used the MCPs of animals which were located over at least 5 months with >8 locations summing up to at least 50 location days. To avoid problems of autocorrelation for the descriptive statistics, we used the mean value of all locations per day and put it in relation to the total number of location days. Statistical analysis was performed in SPSS 14.0 (Statistical Package for the Social Sciences; SPSS Inc., Chicago, Illinois, USA).
4. Results
4.1. Wild ass distribution and connectivity
Superimposing the current wild ass distribution range over the mean annual productivity layer shows that Asiatic wild asses have become almost exclusively confined to areas south of the 250 gC/m2/year productivity isoline (see Supporting Data Appendix S5). That the species once thrived in higher productivity areas is shown by the historic distribution range from the 19th century, where the distribution range reached much further north up to the productivity isoline of 500 gC/m2/year (see Supporting Data Appendix S5).
4.2. Population genetics
The Mongolian wild ass population showed a high level of overall microsatellite diversity with an average of 9.39 alleles per locus and a mean allelic richness of 0.83 and mean FIS of 0.129 across the three bio-geographic regions. Expected and observed heterozygosities were ⩾0.82 and ⩾0.70 for each area, respectively. Although the genetic variability within the three areas was quite homogeneous, the samples from the Dzungarian Gobi revealed a recognizably higher genetic variability than those from the other two areas (Table 2). A total of 14 private alleles were detected, eight in the Dzungarian Gobi, four in the Transaltai Gobi, and two in the southeastern Gobi.
Table 2.
Microsatellite diversity indices of Asiatic wild ass in Mongolia.
| Population | Na | Ab | ARc | APd | HEe | HOf | PHWg | FISh |
|---|---|---|---|---|---|---|---|---|
| Dzungarian Gobi | 19 | 9.55 | 9.0 | 8 | 0.84 | 0.77 | n.s. | 0.092 |
| Transaltai Gobi | 18 | 8.27 | 8.1 | 4 | 0.83 | 0.70 | n.s. | 0.155 |
| SE Gobi | 43 | 10.36 | 8.2 | 2 | 0.82 | 0.70 | n.s. | 0.141 |
| Mean | 9.39 | 8.43 | 4.67 | 0.83 | 0.72 | 0.129 |
n.s. = not significant (P > 0.05).
N = sample size.
A = average number of alleles per locus.
AR mean allelic richness.
AP = number of private alleles.
HE = expected heterozygosity.
HO = observed heterozygosity.
PHW = probability test for deviation from expected Hardy–Weinberg proportions.
FIS = relative inbreeding coefficient.
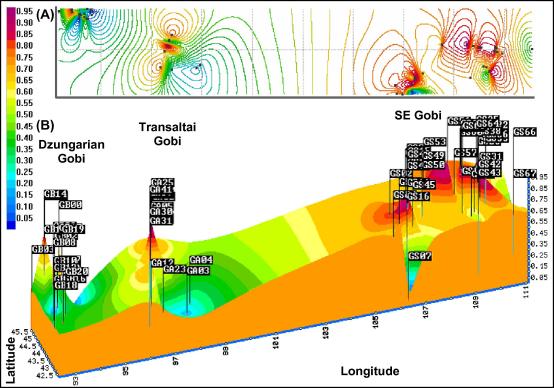
The highest FST value was observed between the Dzungarian and the southeastern Gobi (FST = 0.0191), the lowest between the Transaltai and the southeastern Gobi (FST = 0.0068), and an intermediate between the Dzungarian and the Transaltai Gobi (FST = 0.0088; all pairwise FST values were highly significant at P < 0.001). The Mantel analysis did not reveal an “isolation by distance” relationship (no significant positive correlation between genetic and geographical distances; r2 = 0.0115; P > 0.05). Individual multilocus genotypes-based STRUCTURE analyses clearly indicated the presence of a substructure, with the most likely grouping into two subpopulations. CLUMPP analysis showed that the samples from the Dzungarian and the southeastern Gobi cluster separately, whereas samples from the Transaltai Gobi were undefined (see Supporting Data Appendix S6). BARRIER analysis identified one genetic boundary with 89% of the bootstrap values between the Dzungarian and the Transaltai Gobi (Fig. 3, also see Supporting Data Appendix S6).
Fig. 3.
Synthesis map combining geographical and genetic data. To delineate the spatial organization of the populations, we combined geographical and genetic data. The synthesis map shows the average proportion of membership of each sample to the two subpopulations based on the CLUMPP analysis (dark red = 0% Dzungarian Gobi/100% southeast Gobi; dark blue = 100% Dzungarian Gobi/0% southeast Gobi). The samples are geo-referenced and the membership surface between the samples was interpolated using the kriging procedure available in the program SurGeE 1.4.0 (http://www.geocities.com/miroslavdressler/surgemain.htm). (A) 2-dimensional view of colour coded isolines of equal proportions, (B) 3-dimensional view with same colour coding and average proportions of membership for z-value (high z-values delineate samples with a high membership value for the southeast Gobi, low z-values delineate samples with a low membership value for the southeast Gobi). The numbers at the base of the graph provide the geographic coordinates.
4.3. Telemetry
Range size of wild asses showed a tendency to increase from west to east, but differences were not significant due to the small sample sizes and the rather large variation in range sizes within the three areas (ANOVA, P = 0.008, but post hoc comparisons with Tamhane correction for unequal variance all had P > 0.100). In the Dzungarian Gobi ranges for the seven animals averaged 5860 km2 (Kaczensky et al., 2008) and locations were almost completely confined to the Great Gobi B SPA (Fig. 1, Table 1). Locational data from the Transaltai Gobi is largely based on two individuals due to faulty collars. These animals roamed over large areas of 14,695–16,907 km2 and rarely ventured outside of the protected area (Fig. 1, Table 1). In the southeastern Gobi ranges for five animals varied from 18,186 to 69,988 km2. The ranges of two additional animals, followed over a rather short time period, were also large. Contrary to the two other areas, wild asses in the southeast Gobi spend the majority of time outside of protected areas (Table 1, Fig. 1).
Wild ass home ranges and locations largely fell below the 250 gC/m2/year productivity isoline and primarily fell into flat terrain (Table 1). Average productivity within the minimum convex polygon encompassing all wild ass locations averaged 164 gC/m2/year (SD = 41.8) in the Dzungarian Gobi, 111 gC/m2/year (SD = 27.6) in the Transaltai Gobi and 144 gC/m2/year (SD = 39.5) in the southeast Gobi. In the southeast Gobi mountains are rare and steep terrain is absent, but the Dzungarian and Transaltai Gobi both encompass mountain ranges. Whereas wild asses in the Dzungarian Gobi seem to stay away from mountainous terrain altogether, asses in the Transaltai Gobi do make use of mountainous terrain (Table 1).
In the Dzungarian and Transaltai Gobi, none of our collared Asiatic wild asses came close to any fence and thus we had no means of assessing the effect of fences. However, in the southeastern Gobi five animals stayed in the vicinity of the border fence for 1–79 days, and one animal (ID 58851) moved for 8 days (20–27 July 2005), 92 km within 0.09–10 km (mean: 3.8 km; N = 28) along the west side of the fenced Ulaanbaatar–Beijing railroad track (Fig. 1). Although the animals stayed close to the fence, none of them was able or willing to cross.
4.4. Gobi-wide assessment of the available wild ass habitat
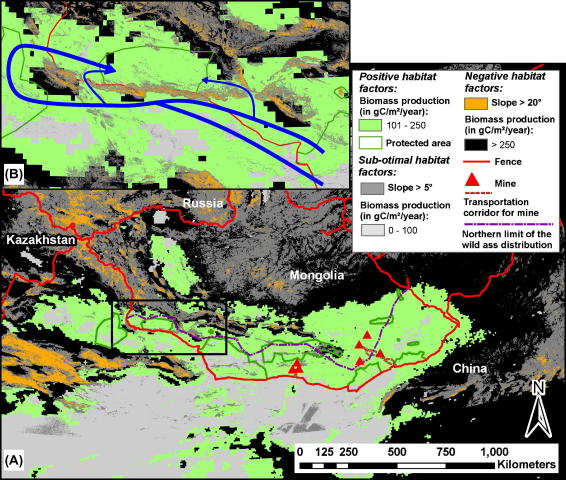
By combining the restraining effects of fences, mountains, and areas with a productivity >250 gC/m2/year, we mapped the remaining wild ass habitat over the entire Gobi (Fig. 4). The low wild ass population density in the Transaltai Gobi suggests that areas with a productivity <100 gC/m2/year and mountainous terrain likely constitute marginal habitat.
Fig. 4.
(A) Connectivity and extent of suitable wild ass habitat (light green) in the Gobi regions of Mongolian and northern China under the present land use intensity. Protected areas are marked with a green outline. Unsuitable areas, such as areas of high productivity (as a proxy for human/livestock density) are coloured black. Anthropogenic barriers are marked red and natural barriers in the form of steep slopes in orange. White- and light grey-areas delineate habitat believed to be marginal due to very low productivity and mountains, respectively. (B) Insert: The connectivity of wild ass habitat in the southwest could be enhanced by removing the border fence or by at least allowing for openings at strategic points. Declaring the border area an “ecological corridor” would link three large protected areas in central Asia which together cover 61,000 km2. Blue arrows mark the most likely movement corridors for Asiatic wild asses between the three protected areas.
In the Mongolian Gobi the best habitat, under the present human land-use pattern and intensity, stretches more or less continuously from the southeastern to the Transaltai Gobi, but connectivity between the Transaltai and Dzungarian Gobi is constrained by mountain ranges (Fig. 4). On the east side of the southeast Gobi the habitat is intersected by the fenced Ulaanbaatar–Beijing railway, which cuts off about 17,000 km2 of wild ass habitat. In the southwest, the suitable habitat stretches far into Xinjiang Province in China, but is presently cut off by the fence along the international border and over large areas also by high mountains. The habitat in northern Xinjing, including the Kalimalai Nature Reserve, encompasses around 100,000 km2. Southeast of the southeast Gobi, there is another 43,000 km2 area that seems to be suitable habitat for wild assess; it runs along a 50 km strip on the Chinese side of the border. Further west, habitat productivity is very low and likely constitutes only marginal habitat for wild asses (Fig. 4).
5. Discussion
5.1. One single large or several small subpopulations?
The Mongolian wild ass population has a high level of overall microsatellite diversity, both within and among the two subpopulations. The amount of heterozygosity examined in this study is consistent with previous studies on other equids using the corresponding microsatellite panel (Krüger et al., 2005). The Asiatic wild ass population in Mongolia shows no evidence of a recent bottleneck as reported for other wide-ranging animal species suffering from habitat fragmentation or range constriction (e.g. cougar Puma concolor; McRae et al., 2005). Although alleles in the Mongolian wild ass population are clearly structured in a non-random way, gene flow is still occurring, suggesting one Gobi population. From the number of private alleles, FST values, and Bayesian clustering, the wild ass population of the Gobi can be subdivided into 2, rather than 3, subpopulations. BARRIER analysis identified one main genetic boundary differentiating the subpopulation in the Dzungarian from those in the Transaltai and southeastern Gobi.
5.2. Range constriction due to human land use intensity
Since the 19th century, the Asiatic wild ass in Mongolia has probably lost as much as 70% of its original range because of human encroachment (Zevegmid and Dawaa, 1973; Denzau and Denzau, 1999). Spatially explicit data on human land use intensity are extremely difficult to collect over the entire expanse of the highly variable Gobi environment with its semi-nomadic human population. Wild ass distribution in the southeastern Gobi shows that human settlements per se do not seem to be a limiting factor for wild ass distribution (Kaczensky et al., 2006). Average biomass production, on the other hand, seems a good proxy for human/livestock presence. In the 19th century, when the human population was <500,000, wild asses ranged north into areas with a mean productivity <500 gC/m2/year. Today, with a human population numbering >2.6 million and livestock numbers >40 millions (National Statistical Office of Mongolia 2008), wild asses have become confined to areas south of the 250 gC/m2/year productivity isocline. This suggests that the present coexistence of wild asses with humans and their livestock in the Gobi is sensitive to increases in human/livestock densities. This negative relationship needs to be considered when discussing means to improve livestock grazing and access to remote Gobi pastures, for example by providing wells (Kaczensky et al. 2006). The low population density of wild asses in the Transaltai Gobi (Reading et al., 2001; Lhagvasuren, 2007), on the other hand, suggests that a mean productivity <100 gC/m2/year likely constitutes only marginal habitat for wild ass. Previous analysis suggests a high flexibility in respect to the use of different plant communities (Kaczensky et al., 2008); thus, vegetation type is unlikely to be of high importance for wild ass distribution.
Under the current land-use pattern and intensity, areas between 100 and 250 gC/m2/year seem to provide wild ass with the best available habitat. However, our assessment is based on observed use on the order of the distribution range (first order selection) and home range level (second order selection), rather than on statistical analysis on the third-order habitat selection within the home range (Johnson 1980). Consequently we cannot provide and never attempted to provide hard boundaries or a truly quantitative assessment of the effects of productivity, slope and fences. Rather our habitat and connectivity map is meant to provide a first large-scale assessment and planning tool. On a local scale, the availability of water, as well as socioeconomic and political factors, are likely to modify this general, landscape-level pattern. The northwestern part of the southeast Gobi receives a much higher human use (also see Supporting Data Appendix S5) than expected from the average productivity and asses seem to no longer occur in these areas. Mining activity and the availability of wells are a likely explanation for the disproportionately higher human presence. On the other hand, large portions of the Eastern Steppe have a much lower human population density than one would expect based on the productivity layer. The reasons for this deviation are the long distances to the nearest urban centers and the population exodus during the Russian–Japanese conflict in 1939. Thus available wild ass habitat might actually stretch much further east into the higher productivity areas of the Eastern Steppe than our 250 gC/m2/year limit would indicate.
5.3. Movement barriers
The population genetics data identified a potential barrier between the subpopulations in the Transaltai- and Dzungarian Gobi. Home ranges of radio-collared wild asses mainly encompassed flat terrain and few wild ass positions fell into steep terrain. In the Dzungarian Gobi wild asses even select against slope within their home ranges (Kaczensky et al. 2008). Thus, the barrier effect between the Dzungarian and Transaltai Gobi can be best explained by the topography between these two bio-geographic regions. However, we believe that the barrier effect by the mountains has likely been enhanced by the construction (in the 1970s) and the recent upgrading (in the 1980s and 1990s) of the border fence between Mongolia and China. Fences have been identified as serious movement barriers for Mongolian gazelles (Ito et al., 2005), and our telemetry data and direct observations (see Supporting Data Appendix S7) suggest the same barrier effect for Asiatic wild asses. Between the Transaltai and Dzungarian Gobi the border fence largely inhibits animals from moving south of the border mountains, from where they could reach the Great Gobi B SPA via China along large valleys from the south or through the plains from the west. Nowadays only the valleys on the east side of Great Gobi B SPA allow for population exchange between the Dzungarian and the rest of the Mongolian Gobi (Fig. 4).
In the east, the Ulaanbaatar–Beijing railway line cuts off about 17,000 km2 of suitable wild ass habitat, where asses have basically disappeared (P. Kaczensky, unpubl. data). Although the fence is interrupted by small under- and over-passes to allow herders and their livestock to cross, none of these crossing structures have been designed or positioned for wildlife use (see Supporting Data Appendix S7). Mongolian gazelles, which occur on both sides of the railway, seem largely unable to find or use these openings (Ito et al., 2008) and the long walk of one collared wild ass parallel to the fence suggests the same for wild asses (Fig. 1).
The lack of wildlife crossing structures makes re-colonization of the suitable habitat on the east side of the Ulaanbaatar–Beijing railway rather unlikely. Mitigation measures, like well-designed under- or over-passes, can reduce the barrier effect of fenced transportation routes (e.g. Luell et al., 2003; Clevenger and Huijser, 2009) and would be desirable for the Ulaanbaatar–Beijing railway. Such measures would not only help wild ass restoration east of the railway, but would also improve the connectivity among subpopulations of Mongolian gazelle (Ito et al., 2005; Mueller et al., 2008), and most likely would also benefit goitered gazelle and argali wild sheep.
5.4. Protected areas are an important piece of a larger picture
Protected areas cover 29% of the wild ass range in the Mongolian Gobi. The two strictly protected areas in the Dzungarian and Transaltai Gobi seem large enough to provide wild ass and other large ungulates with sufficient water and pasture year-round (Kaczensky et al., 2008). However, the situation is quite different in the southeastern Gobi, which likely houses the majority of the Mongolian wild ass population (Reading et al., 2001; Lhagvasuren 2007; B. Lkhagvasuren and S. Strindberg, unpubl. data). In this region individual ranges of the wild asses are very large. We believe that the driving force behind the observed large-scale wild ass movements are the strong spatio-temporal dynamics related to the availability of pastures, forage, and water in the southeastern Gobi (Kaczensky et al., 2006). A similar relationship has been shown for Mongolian gazelles (Ito et al. 2006; Mueller et al. 2008). Consequently, conservation of wild asses and other plain ungulates in the southeastern Gobi cannot focus on protected areas alone, but needs to incorporate the surrounding multi-use landscapes.
This is not an easy task because the southeastern Gobi is rich in mineral deposits, and exploration and mining activities are increasing (World Bank, 2006). In the area around the Small Gobi SPA, a single company holds 117,000 km2 of mineral concessions. To allow transport to China, roads and parallel railway track are either being upgraded or under construction in at least two locations (Fig. 4; Ivanhoe Mines, 2010). These transportation corridors cut through prime wild ass habitat and will likely result in the separation of the Small Gobi A from the Small Gobi B SPA (Kaczensky et al., 2006) and on a larger scale will inhibit, or greatly reduce, movements from the southeast Gobi west into the Transaltai and Dzungarian Gobi. Thus, without imposition of appropriate mitigation measures, these transport corridors threaten to disrupt one of the few remaining intact ecosystems allowing for mass migrations of large plain ungulates in central Asia.
5.5. Transboundary protected area network
Although the Dzungarian Gobi constitutes a rather distinct bio-geographic unit surrounded by natural movement barriers, the border fence further aggravates population exchange of plains ungulates with the rest of the Mongolian Gobi (via corridors that pass through China). Furthermore, northern Xinjiang and especially Kalimalai Nature Reserve seem to still house a wild ass population, possibly numbering several thousand individuals (Yang 2007, Yang unpubl. data). According to our wild ass habitat assessment, northern Xinjiang has about 100,000 km2 of suitable wild ass habitat continuous with the Mongolian Gobi. Xinjiang Province is home to a large Uigur minority and for fear of riots firearm ownership is strictly regulated and controlled. This largely inhibits poaching, which seems to be a major problem in the adjacent Chinese province of Inner Mongolia further east (Wang and Schaller, 1996; Reading et al., 1998).
Although wild asses have been observed on the Chinese side of the fence (W. Yang, unpubl. data) and crossings have been documented (P. Kaczensky unpubl. data), an exchange with the Mongolian population seems severely restricted to times when the fence is breached (e.g., by smugglers/poachers) or to certain locations where the fence is not continuous (e.g., in steep terrain; see Supporting Data Appendix S8). The continuity and spatial extant of the wild ass population in the Gobi would certainly profit from a coordinated transboundary conservation strategy. The border areas in northern Xinjiang are almost uninhabited, and they link three large important protected areas totaling 70,300 km2: Kalimalai Nature Reserve in Xinjiang China, and Great Gobi A and B SPA in Mongolia. The entire border strip should be given the status of a “transboundary ecological corridor” and should ideally be managed as a peace park (Ali, 2007). Opening the fence, at least in places, would most likely also allow for the expansion or re-connection of other rare mammal populations like wild Bactrian camels or re-introduced Przewalski’s horses.
Acknowledgments
This research was conducted within the framework of the Przewalski’s horse re-introduction project of the International Takhi Group, in cooperation with the Mongolian Ministry of Nature and Environment and the National University of Mongolia. Funding was provided by the Austrian Science Foundation (FWF project P14992 and P18624) and the World Bank’s Netherlands–Mongolia Trust Fund for Environmental Reform. We thank R. Samjaa, D. Lkhagvasuren, N. Enkhsaikhaan, O. Ganbaatar, B. Mijiddorj, Y. Adiya, D. Enkhbileg, G. Dorvchindorj, Y. Nyambayar, D. Sheehy, T. Whitten, H. von Wehrden, and the local rangers and their families for their much-needed support. The Norwegian Institute for Nature Research (NINA) in Norway provided office space during the submission phase of the manuscript and John Linnell provided helpful corrections and suggestions.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.biocon.2010.12.013.
Appendix A. Supplementary material
References
- Ali S.H. Introduction: a natural connection between ecology and peace? In: Ali S.H., editor. Peace Parks: Conservation and Conflict Resolution. MIT Press; Cambridge, MA: 2007. pp. 1–18. [Google Scholar]
- Berger J. The last mile: how to sustain long-distance migration in mammals. Conservation Biology. 2004;18:320–331. [Google Scholar]
- Bolger D.T., Newmark W.D., Morrison T.A., Doak D.F. The need for integrative approaches to understand and conserve migratory ungulates. Ecological Letters. 2007;11:63–77. doi: 10.1111/j.1461-0248.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Clark E.L., Munkhbat J., Dulamtseren S., Baillie J.E.M., Batsaikhan N., Samiya R., Stubbe M. Zoological Society of London; London: 2006. Mongolian Red List of Mammals. Regional Red List Series, vol. 1. [Google Scholar]
- Clevenger A.P., Huijser M.P. Federal Highway Administration (FHWA); Washington, DC, USA: 2009. Handbook for Design and Evaluation of Wildlife Crossing Structures in North America. < http://www.westerntransportationinstitute.org/documents/reports/425259_Final_Report.pdf>. [Google Scholar]
- CMS, 2002. Convention on Migratory Species. Appendix II. <http://www.cms.int/documents/appendix/cms_app2.htm>.
- Denzau G., Denzau H. Jan Thorbecke Verlag; Stuttgart, Germany: 1999. Wildesel. (in German) [Google Scholar]
- Dixon J.D., Oli M.K., Wooten M.C., Eason T.H., McCown J.W., Paetkau D. Effectiveness of a regional corridor in connecting two Florida black bear populations. Conservation Biology. 2006;2:155–162. doi: 10.1111/j.1523-1739.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- Epps C.W., Wehausen J.D., Bleich V.C., Torres S.G., Brashares J.S. Optimizing dispersal and corridor models using landscape genetics. Journal of Applied Ecology. 2007;44:714–724. [Google Scholar]
- Evanno G., Regnaut S., Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Ferguson S.H., Elkie P.C. Seasonal movement patterns of woodland caribou. Journal of Zoology. 2004;262:125–134. [Google Scholar]
- Fernandez-Gimenez M.E. Reconsidering the role of absentee herd owners: a view from Mongolia. Human Ecology. 1999;27:1–27. [Google Scholar]
- Fernandez-Gimenez M.E., Batbuyan B. Law and disorder: local implementation of Mongolia’s land law. Development and Change. 2004;35:141–165. [Google Scholar]
- Frair J.L., Merrill E.H., Beyer H.L., Morales J.M. Thresholds in landscape connectivity and mortality risks in response to growing road networks. Journal of Applied Ecology. 2008;45:1504–1513. [Google Scholar]
- Frankham R. Genetics and extinction. Biological Conservation. 2005;126:131–140. [Google Scholar]
- Geist J., Kuehn R. Genetic diversity and differentiation of Central European freshwater pearl mussel (Margaritifera margaritifera) populations: Implications for conservation and management. Molecular Ecology. 2005;14:425–439. doi: 10.1111/j.1365-294X.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- Goudet, J., 2001. Fstat, a program to estimate and test gene diversities and fixation indices, version 2.9.3. <http://www2.unil.ch/popgen/softwares/fstat.htm>.
- Grayson R. Anatomy of the people’s gold rush in modern Mongolia. World Placer Journal. 2007;7:1–66. < http://www.mine.mn/WPJ7_1_Peoples_Gold_Rush.pdf>. [Google Scholar]
- Harrington J.L., Conover M.R. Characteristics of ungulate behavior and mortality associated with wire fences. Wildlife Society Bulletin. 2006;34:1295–1305. [Google Scholar]
- Hays G.C., Akesson S., Godley B.J., Luschi P., Santidrian P. The implications of location accuracy for the interpretation of satellite-tracking data. Animal Behaviour. 2001;61:1035–1040. [Google Scholar]
- Hilbig W. SPB Academic Publishing; Amsterdam, Netherlands and New York, USA: 1995. The vegetation of Mongolia. [Google Scholar]
- Holderegger R., Wagner H.H. Landscape genetics. BioScience. 2008;58:19–207. [Google Scholar]
- Ito T.Y., Mura N., Lhagvasuren B., Enkhbileg D., Takasuki S., Tsunekawa A., Jiang Z. Preliminary evidence of a barrier effect of a railroad on the migration of Mongolian gazelles. Conservation Biology. 2005;19:945–948. [Google Scholar]
- Ito T.Y., Miura N., Lhagvasuren B., Enkhbileg D., Takasuki S., Tsunekawa A., Jiang Z. Satellite tracking of Mongolian gazelles (Procapra gutturosa) and habitat shifts in their seasonal ranges. Journal of Zoology. 2006;269:291–298. [Google Scholar]
- Ito T.Y., Buuveibaatar B., Lhagvasuren B., Takatsuki S., Tsunekawa A. One-sided barrier impact of an international railroad on Mongolian gazelles. Journal of Wildlife Management. 2008;72:940–943. [Google Scholar]
- Ivanhoe Mines, 2009. Oyu Tolgoi (copper – gold), Mongolia. <http://www.ivanhoemines.com/s/Oyu_Tolgoi.asp?ReportID=379189>.
- Ivanhoe Mines, 2010. Oyu Tolgoi Project. Technical Report June 2010. Available at: <http://www.ivanhoemines.com/i/pdf/IDP10_June062010.PDF>.
- Jakobsson M., Rosenberg N.A. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Johnson D.H. The comparison of usage and availability measurements for evaluating resource preference. Ecology. 1980;61:65–71. [Google Scholar]
- Kaczensky, P., Sheehy, D.P., Johnson, D.E., Walzer, C., Lhkagvasuren, D., Sheehy, C.M., 2006. Room to roam? The threat to khulan (Wild Ass) from human intrusion. Mongolia Discussion Papers, East Asia and Pacific Environment and Social Development Departure. Washington, DC, World Bank.
- Kaczensky P., Ganbaatar O., von Wehrden H., Walzer C. Resource selection by sympatric wild equids in the Mongolian Gobi. Journal of Applied Ecology. 2008;45:1762–1769. [Google Scholar]
- Kaczensky, P., Ito, T.Y., Walzer, C., accepted for publication. Satellite telemetry of large mammals in Mongolia: what expectations should we have for collar function? Wildlife Biology in Practice. [DOI] [PMC free article] [PubMed]
- Kramer-Schadt S., Revilla E., Wiegand T., Breitenmoser U. Fragmented landscapes, road mortality and patch connectivity: modelling influences on the dispersal of Eurasian Lynx. Journal of Applied Ecology. 2004;41:711–723. [Google Scholar]
- Krüger K., Gaillard C., Stranzinger G., Rieder S. Phylogenetic analysis and species allocation of individual equids using microsatellite data. Journal of Animal Breeding and Genetics. 2005;122:78–86. doi: 10.1111/j.1439-0388.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- Kuehn R., Kaczensky P., Lkhagvasuren D., Pietsch S., Walzer C. Differentiation of meat samples from domestic horses (Equus caballus) and Asiatic wild asses (Equus hemionus) using a species specific restriction site in the mitochondrial cytochrome b region. Mongolian Journal of Biological Science. 2006;4:55–60. doi: 10.22353/mjbs.2006.04.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankester K., van Apeldoorn R., Meelis E., Verboom J. Management perspectives for populations of the Eurasian badger (Meles meles) in a fragmented landscape. Journal of Applied Ecology. 1991;28:561–573. [Google Scholar]
- Lhagvasuren B. Population assessment of khulan (Equus hemionus) in Mongolia. Exploration into the Biological Resources of Mongolia. 2007;10:45–48. [Google Scholar]
- Luell, B., Bekker, G.J., Cuperus, R., Dufek, J., Fry, G., Hicks, C., Hlava, V., Keller, V., Rosell, C., Sangwine, T., Torslov, N., Wandall, B. le Maire (Eds)., 2003. Wildlife and Traffic: A European Handbook for Identifying Conflicts and Designing Solutions. European Co-operation in the Field of Scientific and Technical Research (COST Transport). Brussels, Belgium. <http://www.iene.info/cost-341/COST%20341-handbook.pdf>.
- Lovari S., Sforzi A., Scala C., Fico R. Mortality parameters of the wolf in Italy: does the wolf keep himself from the door? Journal of Zoology London. 2007;272:117–124. [Google Scholar]
- Manni F., Guérard E., Heyer E. Geographic patterns of (genetic, morphologic, linguistic) variation: how barriers can be detected by using Monmonier’s algorithm. Human Biology. 2004;76:173–190. doi: 10.1353/hub.2004.0034. [DOI] [PubMed] [Google Scholar]
- McRae B.H., Beier P., Dewald L.E., Huynh L.Y., Keim P. Habitat barriers limit gene flow and illuminate historical events in a wide-ranging carnivore, the American puma. Molecular Ecology. 2005;14:1965–1977. doi: 10.1111/j.1365-294x.2005.02571.x. [DOI] [PubMed] [Google Scholar]
- Milner-Gulland E.J., Bukreeva O.M., Coulson T., Lushchekina A.A., Kholodova M.V., Bekenov A.B., Grachev I.A. Reproductive collapse in saiga antelope harems. Nature. 2003;422:125. doi: 10.1038/422135a. [DOI] [PubMed] [Google Scholar]
- Monmonier M. Maximum-difference barriers: an alternative numerical regionalization method. Geographical Analysis. 1973;3:245–261. [Google Scholar]
- Mueller T., Olson K.A., Fuller T.K., Schaller G.B., Murray M.G., Leimgruber P. In search of forage: predicting dynamic habitats of Mongolian gazelles using satellite-based estimates of vegetation productivity. Journal of Applied Ecology. 2008;45:649–658. [Google Scholar]
- National Statistical Office of Mongolia, 2008. Yearbook 2007. <http://www.statis.mn/v3/index2.php?page=free_access>.
- Prince S.D., Goward S.N. Global primary production: a remote sensing approach. Journal of Biogeography. 1995;22:815–833. [Google Scholar]
- Pritchard J.K., Stephens M., Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui J. Riding on the roof of the world. Nature. 2007;449:398–402. doi: 10.1038/449398a. [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2005. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Raymond M., Rousset F. GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. Journal of Heredity. 1995;86:248–249. [Google Scholar]
- Raymond M., Rousset F. An exact test for population differentiation. Evolution. 1995;49:1283–1286. doi: 10.1111/j.1558-5646.1995.tb04456.x. [DOI] [PubMed] [Google Scholar]
- Reading R.P., Mix H., Lhagvasuren B., Tseveenmyadag N. The commercial harvest of wildlife in Dornod Aimag, Mongolia. Journal of Wildlife Management. 1998;62:59–71. [Google Scholar]
- Reading R.P., Mix H.M., Lhagvasuren B., Feh C., Kane D.P., Dulamtseren S., Enkhbold S. Status and distribution of khulan (Equus hemionus) in Mongolia. Journal of Zoology, London. 2001;254:381–389. [Google Scholar]
- Riley S.P.D., Pollinger J.P., Sauvajot R.M., York E.C., Bromley C., Fuller T.K., Wayne R.K. A southern California freeway is a physical and social barrier to gene flow in carnivores. Molecular Ecology. 2006;15:1733–1741. doi: 10.1111/j.1365-294X.2006.02907.x. [DOI] [PubMed] [Google Scholar]
- Taberlet P., Luikart G. Non-invasive genetic sampling and individual identification. Biological Journal of the Linnean Society. 1999;68:41–55. [Google Scholar]
- Thirgood S., Mosser A., Tham S., Hopcraft G., Mwangomo E., Mlengeya T., Kilewo M., Fryxell J., Sinclair A.R.E., Borner M. Can parks protect migratory ungulates? The case of the Serengeti wildebeest. Animal Conservation. 2004;7:113–120. [Google Scholar]
- van Noordwijk A.J. The interaction of inbreeding depression and environmental stochasticity in the risk of extinction of small populations. In: Loeschcke V., Tomiuk J., Jain S.K., editors. Conservation Genetics. Birkhäuser Verlag; Basel, Switzerland: 1994. pp. 131–146. [DOI] [PubMed] [Google Scholar]
- Vernesi C., Bruford M.W., Bertorelle G., Pecchioli R.A., Hauffe H.C. Where’s the conservation in conservation genetics? Conservation Biology. 2008;22:802–804. doi: 10.1111/j.1523-1739.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- von Wehrden H., Wesche K. Relationships between climate, productivity and vegetation in southern Mongolian drylands. Basic and Applied Dryland Research. 2007;1:100–120. doi: 10.1127/badr/1/2007/100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wehrden, H., Hanspach, J., Kaczensky, P., Wesche, K., submitted for publication. Testing the global validity of the non-equilibrium theory of rangeland science by evaluating field studies against a common climatic data base. Journal of Biogeography.
- Walzer C., Kaczensky P., Ganbaatar O., Lengger J., Enkhsaikhan N., Lkhagvasuren D. Capture and anesthesia of wild Mongolian equids - the Przewalski’s horse (E. ferus przewalskii) and khulan (E. hemionus) Mongolian Journal of Biological Sciences. 2006;4:19–28. [Google Scholar]
- Wang X., Schaller G.B. Status of large mammals in western inner Mongolia, China. Journal of East China Normal University. 1996;12:93–104. [Google Scholar]
- Weir B.S., Cockerham C.C. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- Wilcove D.S., Wikelski M. Going, going, gone: is animal migration disappearing? PLOS Biology. 2008;6(7):1361–1364. doi: 10.1371/journal.pbio.0060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard, J.R., Zahler, P., 2006. Silent Steppe: The illegal wildlife trade crisis. Mongolia Discussion Papers, East Asia and Pacific Environment and Social Development Department. World Bank, Washington, DC.
- Wolanski E., Gereta E., Borner M., Mduma S. Water, migration and the Serengeti ecosystem. American Scientist. 1999;87:526–533. [Google Scholar]
- World Bank, 2003. From Goats to Coats: Institutional Reform in Mongolia’s Cashmere Sector. Report No. 26240-MOG. East Asia and Pacific Environment and Social Development Department. World Bank, Washington, DC.
- World Bank, 2006. A review of Environmental and Social Impacts in the Mining Sector. Mongolia Discussion Papers, East Asia and Pacific Environment and Social Development Department. World Bank, Washington, DC.
- Yang W. An overview of the state of Equus hemionus in whole China. Exploration into the Biological Resources of Mongolia. 2007;10:155–158. [Google Scholar]
- Zevegmid D., Dawaa N. Die seltenen Großsäuger der Mongolischen Volksrepublik und ihr Schutz. Archiv für Naturschutz und Landschaftsforsch. 1973;13:87–106. (in German) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.