Abstract
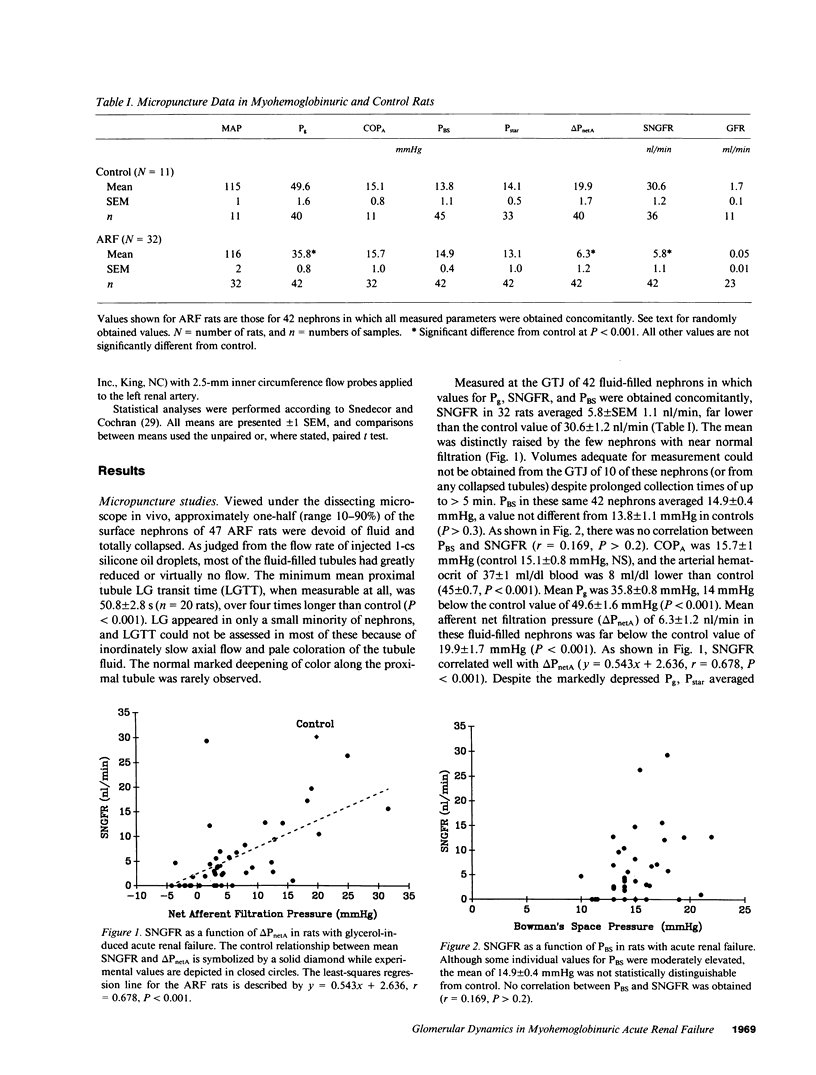
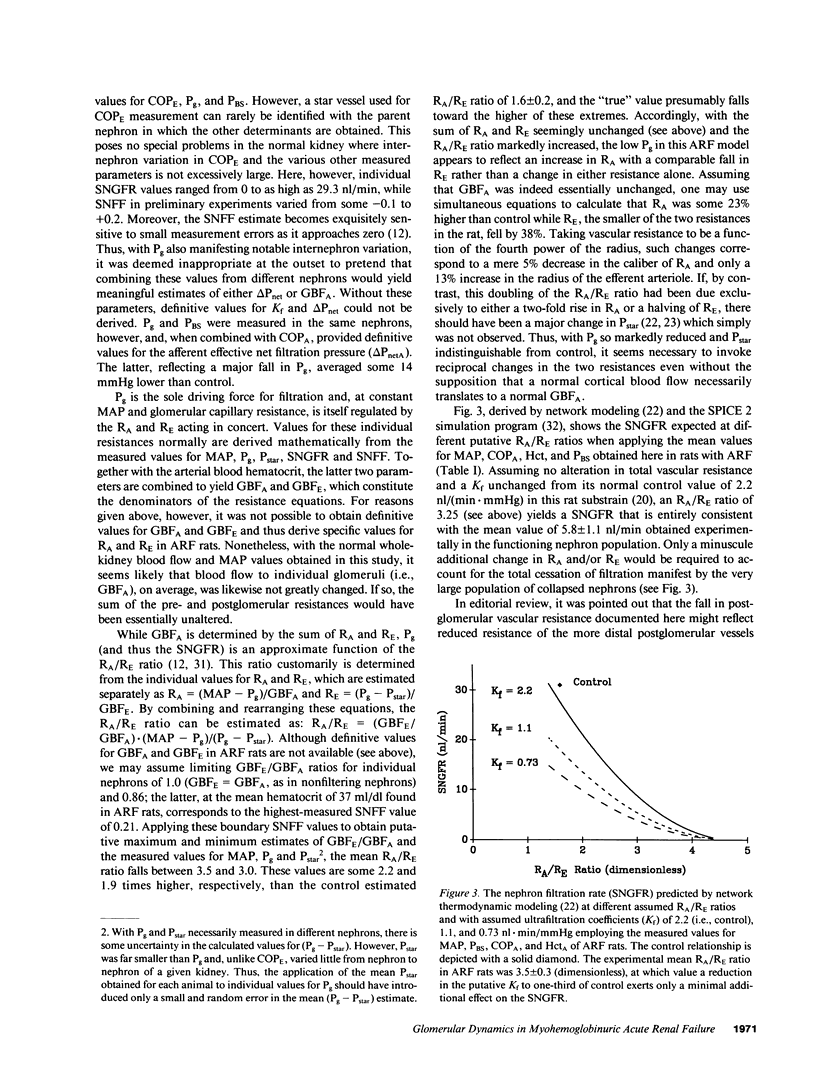
The glomerular dynamic correlates of failed filtration were studied in volume replete rats with established glycerol-induced acute renal failure (ARF). Over one-half of all nephrons formed virtually no filtrate, while the single nephron glomerular filtration rate (SNGFR) of fluid-filled nephrons, measured at the glomerulotubular junction to preclude the possibility of covert tubular leakage, averaged one-sixth of control (P less than 0.001). Even that low mean value was elevated by a few nephrons with a near normal SNGFR. Renal failure thus reflected both total filtration failure in the majority of nephrons and massively reduced filtration in most of the remainder. Glomerular capillary pressure (Pg) averaged some 14 mmHg below control (P less than 0.001), whereas the arterial colloid osmotic and Bowman's space pressures were not significantly altered. Renocortical and whole kidney blood flow were also unchanged. Marked internephron functional heterogeneity precluded estimates of the ultrafiltration coefficient. However, the fall in SNGFR correlated well with the markedly depressed Pg and afferent net filtration pressure (delta PnetA, P less than 0.001), which in turn were caused by increased preglomerular resistance and a reciprocal fall in efferent arteriolar resistance. This complex change in intrarenal resistances was largely, if not entirely, responsible for failed filtration in this ARF model.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUKLAND K., BOWER B. F., BERLINER R. W. MEASUREMENT OF LOCAL BLOOD FLOW WITH HYDROGEN GAS. Circ Res. 1964 Feb;14:164–187. doi: 10.1161/01.res.14.2.164. [DOI] [PubMed] [Google Scholar]
- Arendshorst W. J., Finn W. F., Gottschalk C. W. Pathogenesis of acute renal failure following temporary renal ischemia in the rat. Circ Res. 1975 Nov;37(5):558–568. doi: 10.1161/01.res.37.5.558. [DOI] [PubMed] [Google Scholar]
- Biber T. U., Mylle M., Baines A. D., Gottschalk C. W., Oliver J. R., MacDowell M. C. A study by micropuncture and microdissection of acute renal damage in rats. Am J Med. 1968 May;44(5):664–705. doi: 10.1016/0002-9343(68)90251-9. [DOI] [PubMed] [Google Scholar]
- Bidani A. K., Churchill P. C. Aminophylline ameliorates glycerol-induced acute renal failure in rats. Can J Physiol Pharmacol. 1983 Jun;61(6):567–571. doi: 10.1139/y83-087. [DOI] [PubMed] [Google Scholar]
- Blantz R. C. The mechanism of acute renal failure after uranyl nitrate. J Clin Invest. 1975 Mar;55(3):621–635. doi: 10.1172/JCI107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmer C. J., Collis M. G., Yates M. S. Effect of the adenosine antagonist 8-phenyltheophylline on glycerol-induced acute renal failure in the rat. Br J Pharmacol. 1986 May;88(1):205–212. doi: 10.1111/j.1476-5381.1986.tb09488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedru M. F., Baethke R., Oken D. E. Renal cortical blood flow and glomerular filtration in myohemoglobinuric acute renal failure. Kidney Int. 1972 Apr;1(4):232–239. doi: 10.1038/ki.1972.33. [DOI] [PubMed] [Google Scholar]
- Churchill S., Zarlengo M. D., Carvalho J. S., Gottlieb M. N., Oken D. E. Normal renocortical blood flow in experimental acute renal failure. Kidney Int. 1977 Apr;11(4):246–255. doi: 10.1038/ki.1977.37. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Robinette J. B., Guggenheim S. J. Effect of acetylcholine on the early phase of reversible norepinephrine-induced acute renal failure. Kidney Int. 1981 Mar;19(3):399–409. doi: 10.1038/ki.1981.32. [DOI] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Mercer P. F., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. V. Response to ischemic injury. J Clin Invest. 1974 Jan;53(1):105–116. doi: 10.1172/JCI107527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLANIGAN W. J., OKEN D. E. RENAL MICROPUNCTURE STUDY OF THE DEVELOPMENT OF ANURIA IN THE RAT WITH MERCURY-INDUCED ACUTE RENAL FAILURE. J Clin Invest. 1965 Mar;44:449–457. doi: 10.1172/JCI105158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamenbaum W., Huddleston M. L., McNeil J. S., Hamburger R. J. Uranyl nitrate-induced acute renal failure in the rat: micropuncture and renal hemodynamic studies. Kidney Int. 1974 Dec;6(6):408–418. doi: 10.1038/ki.1974.126. [DOI] [PubMed] [Google Scholar]
- Flamenbaum W., Kotchen T. A., Oken D. E. Effect of renin immunization on mercuric chloride and glycerol-induced renal failure. Kidney Int. 1972 Jun;1(6):406–412. doi: 10.1038/ki.1972.53. [DOI] [PubMed] [Google Scholar]
- Flamenbaum W., McDonald F. D., DiBona G. F., Oken D. E. Micropuncture study of renal tubular factors in low dose mercury poisoning. Nephron. 1971;8(3):221–234. doi: 10.1159/000179923. [DOI] [PubMed] [Google Scholar]
- Hägnevik K., Gordon E., Lins L. E., Wilhelmsson S., Forster D. Glycerol-induced haemolysis with haemoglobinuria and acute renal failure. Report of three cases. Lancet. 1974 Jan 19;1(7847):75–77. doi: 10.1016/s0140-6736(74)92291-0. [DOI] [PubMed] [Google Scholar]
- Jackson B., Oken D. E. Internephron heterogeneity of filtration fraction and disparity between protein- and hematocrit-derived values. Kidney Int. 1982 Feb;21(2):309–315. doi: 10.1038/ki.1982.23. [DOI] [PubMed] [Google Scholar]
- Jensen P. K., Steven K. Influence of intratubular pressure on proximal tubular compliance and capillary diameter in the rat kidney. Pflugers Arch. 1979 Nov;382(2):179–187. doi: 10.1007/BF00584220. [DOI] [PubMed] [Google Scholar]
- Oken D. E. An analysis of glomerular dynamics in rat, dog, and man. Kidney Int. 1982 Aug;22(2):136–145. doi: 10.1038/ki.1982.145. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Arce M. L., Wilson D. R. Glycerol-induced hemoglobinuric acute renal failure in the rat. I. Micropuncture study of the development of oliguria. J Clin Invest. 1966 May;45(5):724–735. doi: 10.1172/JCI105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken D. E., Cotes S. C., Flamenbaum W., Powell-Jackson J. D., Lever A. F. Active and passive immunization to angiotensin in experimental acute renal failure. Kidney Int. 1975 Jan;7(1):12–18. doi: 10.1038/ki.1975.2. [DOI] [PubMed] [Google Scholar]
- Oken D. E., DiBona G. F., McDonald F. D. Micropuncture studies of the recovery phase of myohemoglobinuric acute renal failure in the rat. J Clin Invest. 1970 Apr;49(4):730–737. doi: 10.1172/JCI106285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken D. E. Theoretical analysis of pathogenetic mechanisms in experimental acute renal failure. Kidney Int. 1983 Jul;24(1):16–26. doi: 10.1038/ki.1983.121. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Thomas S. R., Mikulecky D. C. A network thermodynamic model of glomerular dynamics: application in the rat. Kidney Int. 1981 Feb;19(2):359–373. doi: 10.1038/ki.1981.27. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Wolfert A. I., Laveri L. A., Choi S. C. Effects of intra-animal nephron heterogeneity on studies of glomerular dynamics. Kidney Int. 1985 Jun;27(6):871–878. doi: 10.1038/ki.1985.94. [DOI] [PubMed] [Google Scholar]
- Osswald H., Schmitz H. J., Heidenreich O. Adenosine response of the rat kidney after saline loading, sodium restriction and hemorrhagia. Pflugers Arch. 1975 Jun 26;357(3-4):323–333. doi: 10.1007/BF00585986. [DOI] [PubMed] [Google Scholar]
- STEINHAUSEN M. [A method for the differentiation of proximal and distal tubuli in the renal cortes in vivo and its use in determining tubular flow rates]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;277:22–35. [PubMed] [Google Scholar]
- Schor N., Ichikawa I., Rennke H. G., Troy J. L., Brenner B. M. Pathophysiology of altered glomerular function in aminoglycoside-treated rats. Kidney Int. 1981 Feb;19(2):288–296. doi: 10.1038/ki.1981.19. [DOI] [PubMed] [Google Scholar]
- Tanner G. A., Sloan K. L., Sophasan S. Effects of renal artery occlusion on kidney function in the rat. Kidney Int. 1973 Dec;4(6):377–389. doi: 10.1038/ki.1973.134. [DOI] [PubMed] [Google Scholar]
- Thiel G., McDonald F. D., Oken D. E. Micropuncture studies of the basis for protection of renin depleted rats from glycerol induced acute renal failure. Nephron. 1970;7(1):67–79. doi: 10.1159/000179809. [DOI] [PubMed] [Google Scholar]
- Thiel G., McDonald F. D., Oken D. E. Micropuncture studies of the basis for protection of renin depleted rats from glycerol induced acute renal failure. Nephron. 1970;7(1):67–79. doi: 10.1159/000179809. [DOI] [PubMed] [Google Scholar]
- Thiel G., Wilson D. R., Arce M. L., Oken D. E. Glycerol induced hemoglobinuric acute renal failure in the rat. II. The experimental model, predisposing factors, and pathophysiologic features. Nephron. 1967;4(5):276–297. doi: 10.1159/000179588. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Thiel G., Arce M. L., Oken D. E. The role of the concentration mechanism in the development of acute renal failure: micropuncture studies using diabetes insipidus rats. Nephron. 1969;6(2):128–139. doi: 10.1159/000179721. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Thiel G., Arce M. L., Oken D. E. The role of the concentration mechanism in the development of acute renal failure: micropuncture studies using diabetes insipidus rats. Nephron. 1969;6(2):128–139. doi: 10.1159/000179721. [DOI] [PubMed] [Google Scholar]
- Winston J. A., Safirstein R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am J Physiol. 1985 Oct;249(4 Pt 2):F490–F496. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]
- Wolfert A. I., Laveri L. A., Reilly K. M., Oken K. R., Oken D. E. Glomerular hemodynamics in mercury-induced acute renal failure. Kidney Int. 1987 Aug;32(2):246–255. doi: 10.1038/ki.1987.199. [DOI] [PubMed] [Google Scholar]



