Abstract
We report the genetic organisation of six prophages present in the genome of Lactococcus lactis IL1403. The three larger prophages (36–42 kb), belong to the already described P335 group of temperate phages, whereas the three smaller ones (13–15 kb) are most probably satellites relying on helper phage(s) for multiplication. These data give a new insight into the genetic structure of lactococcal phage populations. P335 temperate phages have variable genomes, sharing homology over only 10–33% of their length. In contrast, virulent phages have highly similar genomes sharing homology over >90% of their length. Further analysis of genetic structure in all known groups of phages active on other bacterial hosts such as Escherichia coli, Bacillus subtilis, Mycobacterium and Streptococcus thermophilus confirmed the existence of two types of genetic structure related to the phage way of life. This might reflect different intensities of horizontal DNA exchange: low among purely virulent phages and high among temperate phages and their lytic homologues. We suggest that the constraints on genetic exchange among purely virulent phages reflect their optimal genetic organisation, adapted to a more specialised and extreme form of parasitism than temperate/lytic phages.
INTRODUCTION
Interest in lactococcal phages originally arose from the economical impact of their attacks on Lactococcus lactis strains that are used for the manufacture of fermented dairy products. Large numbers of strains and phages have been collected worldwide, over an extended time period, and characterised to some extent. Lactococcal phages fall into three prevalent groups of DNA homology (1,2). Two of these groups, designated 936 and c6A, are composed of virulent phages and one, designated P335, is mainly composed of temperate phages despite some rare virulent individuals that have been described. The large size of dairy plants and the manufacturing processes used create a strong selective pressure on both bacteria and phages. Lactococcal phages therefore constitute an interesting model to study the genetic organisation of phages, the structure of their population and ultimately their mode of evolution.
The DNA sequences of five lactococcal phages have been determined (3–7). Two belong to group 936, two to group c6A and one to group P335. We present here sequence analysis of six prophages carried by the L.lactis strain IL1403, and comparison of these sequences to those of lactococcal phages already available. We included in the comparison the sequence of the temperate L.lactis phage Tuc2009 (G.Fitzgerald and D.van Sinderen, personal communication). This analysis reveals a new type of lactococcal prophage, details the genetic structure of P335 prophages and indicates that temperate and virulent phage populations have different genetic structures.
MATERIALS AND METHODS
The sequence data presented here have been submitted to the DDBJ/EMBL/GenBank databases and appear under accession numbers AF323668–AF323673.
Bacterial strain
Lactococcus lactis subspecies lactis IL1403 (8) was grown at 30°C in M17 medium (9) in which lactose has been replaced by glucose.
Prophage induction
IL1403 prophages were induced by the addition of 1 µg/ml mitomycin C (Sigma Chemical Co.) to an early exponential-phase culture (OD600 = 0.1) of the strain. Incubation was continued at 30°C up to clarification of the culture (∼2 h).
DNA manipulations
Cellular DNA for PCR experiments was prepared using the Gene Releaser kit (BioVentures, Inc.), following the supplier’s instructions. Prophage DNA was extracted from the cell lysate by phenol/chloroform treatment and precipitated twice with isopropanol and ethanol, respectively (10).
PCR and sequencing
PCR reactions were performed using the DNA Thermal Cycler 9600 (Perkin-Elmer) and Taq polymerase (Promega). Pairs of oligonucleotides 1-2, 3-4, 5-6, 7-8, 9-10 and 11-12, complementary to prophage sequences were used to amplify forms of excised prophages bIL285, bIL286, bIL309, bIL310, bIL311 and bIL312, respectively. In case of the non-inducible prophage bIL311, two additional oligonucleotide pairs were used as a control. Pairs of oligonucleotides 1-14, 2-13, 3-16, 4-15, 5-18, 6-17, 7-20, 8-19, 9-22, 10-21, 11-23 and 12-24, complementary to prophage and chromosomal sequences were used to amplify chromosomal regions with integrated phages. The oligonucleotides had the following sequences:
1, 5′-GACACGCAAGTGTGGCTATC; 2, 5′-CTGCTCTTCGGAGCGGC; 3, 5′-GTTCAATATCGCCTAGGGCATGC;
4, 5′-CAAGACGGAACAATTAGCCCAG;
5, 5′-GCTCGGTCATAGTAGTTTG; 6, 5′-GTGAGAGAATTACAACGGAG;
7, 5′-GACACATACAGCCACCTTG; 8, 5′-CTCAGAAGTTGCAAGTCG;
9, 5′-GACGAGCAGACAGCGGAGC; 10, 5′-CTATACTCACATCTTGAGC;
11, 5′-GTAGGGCATAAGGATGGCGG; 12, 5′-GAAGGTCAACGTGGTCTTC;
13, 5′-GACTGATCATAAACCAAGC; 14, 5′-GTGCTTGTCTGATGTTGAGC;
15, 5′-CGTGAAGTGGATCTGTATCTG; 16, 5′-CGAAAACAGGGAGTTTTGTATAG; 17, 5′-CGGATAGGATATCTGAACCTG; 18, 5′-GGTGACTATGGTCGGGCAGC; 19, 5′-GAGAATTAAACGATCGTAAGC; 20, 5′-CTCGCAAGTGTACACAGTTC; 21, 5′-CACCGACTTCACTTTCAAAC; 22, 5′-CGAACTTTCTTACGAGCTTC;
23, 5′-CGAGCACAACTTCGCAGC;
24, 5′-GTGGTTGCCATTGTTGAAG. PCR products were purified using the Wizard PCR Preps DNA Purification System (Promega). The sequence was determined in a cycle extension reaction with dye terminator cycle-sequencing ready reaction (Applied Biosystems) and AmpliTaq DNA polymerase (Perkin Elmer) on a 373 DNA sequencer (Applied Biosystems).
Computer analysis
Open reading frames (ORFs) identification was based on the presence of a start codon (AUG, UUG or GUG), preceded in most cases by a ribosome binding site (RBS) complementary to the 3′ end of the 16SrRNA of L.lactis (3′-UCUUUCCUCCA-5′) (11), without length limitation. The search for sequence homology was carried out using FASTA (12), BLAST (13) and BLAST 2 sequences (14). tRNA genes were searched for in phage genome sequences using tRNAscan-SE (15).
RESULTS
Identification of prophages on the IL1403 genome
IL1403 is a plasmid-free derivative of L.lactis subspecies lactis strain IL594 (8). Two phages were previously identified in this strain following UV or mitomycin C induction (16). Recently, the entire sequence of the IL1403 genome has been determined (A.Bolotin et al., manuscript in preparation), making it possible to further characterise the prophages it contains. In a preliminary analysis of the IL1403 genome, five putative prophages were identified on the basis of frequent homology of the ORFs in these regions to known phage proteins (17). Detailed analysis of these regions and of a few others with less significant homology confirmed the existence of five prophages and suggested the existence of a sixth (Table 1). To identify the exact prophage ends and the attachment (att) core sequence, we induced the phages with mitomycin C and amplified the junctions of the phage arms by PCR (see Materials and Methods). PCR products were obtained for all phages except bIL311, indicating that their DNA is able to circularise. The sequence of the PCR products was compared to the sequence of the junction regions between phage and chromosomal DNA, and the phage ends thus identified. The genome length and the att core sequence of each phage are presented in Table 1.
Table 1. Position, orientation, length and att core sequences of the prophages in the IL1403 chromosome.
| Phage |
Start |
End |
Genome size
(bp) |
att core sequence |
Sequence accession number |
| bIL285 | 1 036 549 | 1 072 086 | 35 538 | TTTTTCATG | AF323668 |
| bIL286 | 1 457 116 | 1 415 283 | 41 834 | ACTCCCCTCGCCTCCATTGAATGGATTTTATAGAATCCAAT AGTATTAGAAATCGCTCAATAGAGCGGTTTTTGTTTTCT | AF323669 |
| bIL309 | 447 143 | 484 092 | 36 949 | CGAGGTTTGGCACCATGATCCGAGGGGGA | AF323670 |
| bIL310 | 49 863 | 34 907 | 14 957 | CAAAAAAACACTGATTGAATGCCGTATG | AF323671 |
| bIL311 | 2 011 247 | 2 025 756 | 14 510 | AAACTGTCTATTCTATTATATa | AF323672 |
| bIL312 | 502 595 | 517 773 | 15 179 | GAAAGACGCAGTTAAATAATTATAGCTAT | AF323673 |
abIL311 being non-inducible, a putative att core sequence has been determined by searching for perfect direct repeats on the chromosome sequence in the vicinity of the phage genome.
The six prophages appear to be localised at random on the chromosome and four of them are integrated into non-coding sequences. One, bIL309, is integrated into a tRNAArg gene and another, bIL312, into the 5′ end of the yfbM gene (A.Bolotin et al., unpublished). In both cases, integration reconstitutes an intact copy of the gene. The six prophages have a GC content ranging from 34.2 to 35.8%, similar to the value of 35.4% calculated for the IL1403 chromosome (17).
Identification and putative function of the ORFs
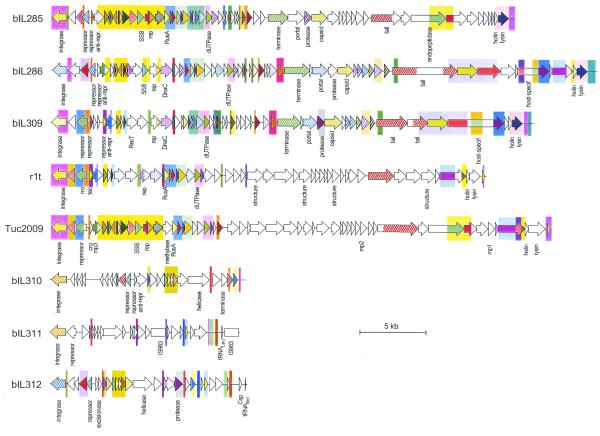
The genome map of each of the six prophages is presented in Figure 1. There are 62, 61, 56, 28, 27 and 21 ORFs for phage bIL285, bIL286, bIL309, bIL310, bIL312 and bIL311, respectively. Two phages specify a tRNA gene. Among the 256 ORFs of the prophages, 96 (37.5%) had no homology with proteins in the data banks, 96 (37.5%) were homologous to bacterial or phage proteins of unknown function and 64 (25%) were homologous to proteins with a putative or experimentally determined function. Similarities of the IL1403 prophage gene products to known protein sequences are presented as Supplementary Material. Homologies were most frequent with Gram-positive phages, but were also observed with Gram-negative phages, mobile genetic elements and bacterial genes. We identified genes putatively involved in the main steps of prophage life cycle: integration into the chromosome, lysogenic/lytic switch, DNA replication, recombination, morphogenesis and cell lysis.
Figure 1.
Structure of all known lactococcal prophage genomes. Genome extremities correspond to attB sites. ORFs are shown as arrows, oriented in the direction of their transcription, with designation below. Arrows with identical colour indicate proteins with >70% sequence identity (plain colour), or 20–70% sequence identity (hatched colour). Colour rectangles in the background indicate DNA regions with >60% sequence identity. A white box indicates IS983. Sequences are taken from van Sinderen et al. (5) (r1t), G.Fitzgerald and D.van Sinderen (personal communication; Tuc2009) and this study (bIL285, bIL286, bIL309, bIL310, bIL311 and bIL312).
Genome organisation
The genome of the six prophages is organised in two divergent clusters of ORFs, one small and the other large. The two are separated on the one side by the attachment site and on the other by an intergenic region, most probably involved in the genetic switch between the lysogenic and lytic state of the phages (Fig. 1). The small cluster comprises genes involved in integration and lysogeny maintenance, which are transcribed in one orientation, while the large cluster includes genes participating in the lytic development, which are transcribed divergently. Lytic genes are ordered as follows: repression of the lysogenic cycle, recombination, DNA replication, morphogenesis and cell lysis. This organisation is similar to that previously described for phage r1t (5) and also observed in Tuc2009 (Fig. 1). Two IS983 elements are inserted in phage bIL311, the only phage unable to circularise upon mitomycin C treatment of the cells. In conclusion, genes controlling related functions are highly clustered and the order of the clusters is conserved in lactococcal prophages. These features are frequently observed in double-stranded DNA-tailed bacteriophages (18).
Comparison of IL1403 prophages with temperate lactococcal phages
The genome map of IL1403 prophages is compared to that of temperate phage r1t and Tuc2009 in Figure 1. Two subgroups are apparent: one composed of five phages with a large genome (33–42 kb; bIL285, bIL286, bIL309, Tuc2009 and r1t) and one composed of three phages with a small genome (14–15 kb; bIL310, bIL311 and bIL312). The five large prophages have similar genome organisation, are homologous to each other and share homology with partially sequenced temperate lactococcal phages TP901-1 (accession numbers X84706 and Y14232) and BK5-T (accession number L44593). However, the homologous regions cover only 10–33% of the phage genome length. These genomes are thus formed by a mosaic of conserved sequences, interspersed by non-homologous regions. As a result, a given function can be performed by different types of proteins, depending on the phage. One type of replication protein, two types of integrase and immunity repressor and three types of terminase, portal protein, capsid protein and lysis modules are identifiable. Within the same type of protein, the level of amino acid identity is highly variable, ranging from 26 to 100%. Therefore, these lactococcal temperate phages are related but demonstrate a high amount of genetic variation.
The three smaller IL1403 prophages differ from the former by their surprisingly short size for double-stranded DNA phages, and the apparent absence of genes required for phage morphogenesis and lysis of the host cell. These features are also present in the Escherichia coli satellite phage P4, which depends on helper phage(s) for the morphogenetic and lytic functions needed for lytic development (for a review see 19,20). We suggest that the three small lactococcal prophages are satellites relying on phages from the P335 group for multiplication. However, the alternative possibility that the small phages are cryptic remnants of a yet unknown group of large phages cannot be ruled out at present. The construction and study of IL1403 derivatives, cured of the different P335 prophages, would allow to distinguish these possibilities. Interestingly, the helicase of the small prophage bIL312 shares identity with Orf11 of the pathogenicity island SalPl from Staphylococcus aureus (21) (see Supplementary Material). SalP1 shares homology with S.aureus phages, can integrate into the chromosome and, with the help of a functional phage, can excise and replicate its DNA (21). It is therefore possible that the SalP1 element represents another example of a satellite phage, a class of genetic elements whose frequency could have been underestimated.
DISCUSSION
The description of three new large L.lactis temperate phages and their comparison with r1t (5) and Tuc2009 (G.Fitzgerald and D.van Sinderen, personal communication) reveals that they share the same genetic organisation. However, the genomes are only homologous over 10–33% of their length. The genome of these phages thus appears to be a mosaic of conserved sequences interspersed by non-homologous regions. A similar structure has been previously observed in lambdoid phages, suggesting that they are formed by the combination of functional modules for which different versions exist in the population (18,22). The concept of quasi-species has been proposed to accommodate such groups of related phages in which individuals may have little sequence similarity but retain the same gene order and can exchange genetic information (18). We therefore propose that phages bIL285, bIL286, bIL309, r1t and Tuc2009 all belong to the same quasi-species, which corresponds to the P335 group (1).
The genetic structure of the P335 phages has important implications for their identification and classification. The fact that DNA homology between the phages of this group covers only 10–33% of their genome length indicates that the current classification of the lactococcal phages, based on hybridisation (1), may have to be re-examined. For example, phages 1358 and 1483, initially considered as representative of two different groups, in fact share homology with phage P335 (A.W.Jarvis, personal communication). Similarly, phage BK-5T, initially proposed to belong to a different group, shares homology with phages of the P335 group (see Results). Our results therefore allow to define more precisely the structure of the P335 group and indicate that there may well be fewer lactococcal phage groups than thought previously.
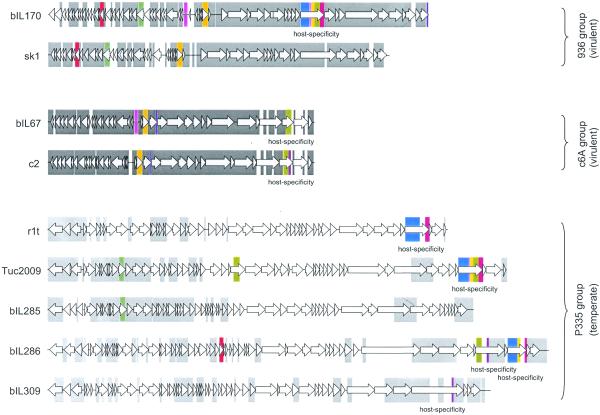
The genetic structure observed for L.lactis P335 phages is very different from that observed in virulent L.lactis phages (Fig. 2). Virulent phages bIL170 (7) and sk1 (6) of the 936 group, as well as phages bIL67 (3) and c2 (4) of the c6A group, differ only by point mutations, insertion and/or deletion of short sequences, sharing homology over 90–92% of their genome length. Heteroduplex studies performed on five other phages of the group of 936 also revealed that they were homologous over 81–94% of their genome length (23,24). Virulent lactococcal phages therefore have highly homologous genomes, in sharp contrast with the temperate P335 phages, whose genomes are only homologous over 10–33% of their length (Fig. 2). Our results therefore suggest the existence of two types of lactococcal phage population. One corresponds to virulent phages, which have very similar genomes, and the other to temperate phages, which share very different levels of genome homology, depending on which particular gene combination they contain. Very interestingly, a similar conclusion can be drawn from the study of E.coli phages. Indeed, virulent T-phages are homologous over 80–94% of their genome length (25–28), whereas temperate lambdoid phages genomes are much more variable (18,22).
Figure 2.
DNA sequence conservation among genomes of lactococcal phages. Lactococcal phage groups are indicated. Intra-group and inter-group DNA conservation (>60% sequence identity) are indicated by grey and colour rectangles, respectively. Sequences are taken from Crutz-Le Coq et al. (7; bIL170), Chandry et al. (6; sk1), Schouler et al. (3; bIL67), Lubbers et al. (4; c2), van Sinderen et al. (5; r1t), G.Fitzgerald and D.van Sinderen (personal communication; Tuc2009) and this study (bIL285, bIL286 and bIL309).
To examine how general a difference between the genetic structure of temperate and virulent phage populations may be, it is necessary to consider that most groups of temperate phages also include lytic individuals. Their existence led to the early notion that there is no basic difference between temperate and virulent phages (29,30). However, recent characterisation of lytic phages active on Streptococcus thermophilus, Lactobacillus delbrueckii or Mycobacterium established that they differ from their temperate homologues mainly by inactivation of the lysogeny module (31–33). It has been speculated that the loss of the lysogenic pathway may advantageously extend the phage host range, by avoiding the superinfection control systems of hosts that carry homologous temperate phages (34). We therefore propose that there are two types of virulent phages. Phages of the first type have temperate homologues, from which they differ by the absence of the functional lysogeny module. It is conceivable that they could readily return to the temperate status by re-acquisition of this module via recombination with such a homologue. These phages would therefore only be temporarily virulent and would behave, like temperate phages, on an evolutionary time scale. We designate such phages as lytic thereafter. Phages of the second type have no temperate homologues and consequently cannot become temperate. Examples of this type of phage include, in addition to lactococcal phages of groups 936 and c6A, Bacillus subtilis phages related to phi-29 or SP01, and the E.coli T-phages. These phages were designated as virulent.
We examined the genetic structure of all phage groups (a total of 10), for which more than one phage genome has been entirely sequenced. Genome homology was calculated between all pairs of individuals in a group and the range of homologies is presented in Table 2. Genomes of virulent phages are all highly similar, the homology extending over at least 82% of the genome. In contrast, the genomes of temperate or lytic phages are much more variable, the length of homology between pairs of individuals extending from 0% to a high value of 93% of the total genome length. We conclude that there is a perfect correlation between the phage lifestyle and the range of the homology over the genome. This range is narrow and at the high end of the scale (>82%) for virulent phages, while it is broad and starts at a rather low level of the scale (0–27%) for the temperate/lytic phages.
Table 2. DNA homology within groups of phages, according to their virulent or temperate status.
| Host |
Phages |
Group |
Phage way of life |
Length of genome homology between pairs of
phages within a group (%)a |
| L.lactis | bIL285, bIL286, bIL309, r1t, Tuc2009 | P335 | temperate/lytic | 10–33 |
| bIL310, bIL311, bIL312 | temperate | 7–10 | ||
| bIL67, c2 | c6A | virulent | 90 | |
| bIL170, sk1 | 936 | virulent | 92 | |
| S.thermophilus | 7201, DT1, O1205, Sfi11, Sfi19, Sfi21 | temperate/lytic | 27–93 | |
| B.subtilis | B103, phi-29, PZA | virulent | 86–100 | |
| Mycobacterium | D29, L5 | temperate/lytic | 86 | |
| E.coli | T3, T7 | virulent | 82 | |
| 933W, H-19B, HK022, HK97, λ, N15, P22, VT2-Sa | lambdoid | temperate | 0–91 | |
| 186, P2 | temperate | 59 |
aPairs of phage within the group were compared using BLAST 2 sequences (14), and homology was expressed by the ratio (length of homology × 100)/total length of the shorter genome.
Accession numbers are as follows: 186, U32222; 7201, AF145054; 933W, AF125520; B103, X99260; bIL67, L33769; bIL170, AF009630; bIL285, bIL286, bIL309, bIL310, bIL311 and bIL312, this paper; c2, L48605; D29, AF022214; DT1, AF085222; H-19B, AF034975; HK022, AF069308; HK97, AF069529; L5, Z18946; λ, J02459; N15, AF064539; O1205, U88974; P2, AF063097; P22, AF217253; phi-29, M14430, V01155, M14782; PZA, M11813; r1t, U38906; Sfi11, AF158600; Sfi19, AF115102; Sfi21, AF115103; sk1, AF011378; T3, X17255; T7, V01146; Tuc2009 (G.Fitzgerald and D.van Sinderen, personal communication); VT2-Sa, AP000363.
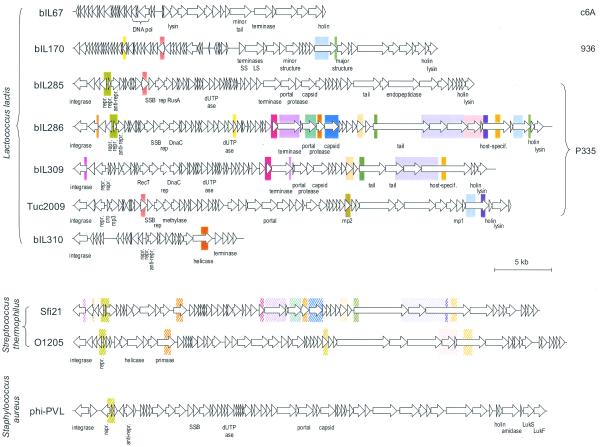
The difference in the genetic structure of the populations may reflect a different intensity of horizontal exchange of genetic material in virulent and temperate/lytic phages. The former exchange little, despite the opportunity to do so. For instance, lactococcal strains can be coinfected by virulent lactococcal phages of the c6A and 936 groups, but members of one group share essentially no DNA homology with members of the other (Fig. 2). Similarly, exchanges between virulent lactococcal phages and resident prophages are very rare. The only region of homology common to the three phage groups lies within putative tail-fibre genes, likely to determine host specificity (Fig. 2; see also Supplementary Material). Interestingly, conservation of DNA segments among otherwise unrelated E.coli phages has also been observed within tail-fibre genes (for a review see 35). These observations suggest that DNA exchange of virulent phages outside their group is limited to determinants extending their host spectrum, whose acquisition therefore confers a strong selective advantage. Finally, virulent phages show no significant DNA homology with any organism in the databases. In contrast, the mosaic structure of the temperate phages in the P335 group provides clear evidence that horizontal exchange of genetic material is a major component of evolution for these phages (36). Moreover, the DNA of these phages shares homology with that of temperate/lytic phages active on a different host species. For instance some segments of bIL286 and bIL309 have ∼60% DNA homology with S.thermophilus phages, such as Sfi19 and Sfi21 (Fig. 3). This high similarity could be due to the phylogenetic proximity of L.lactis and S.thermophilus, since homology of these lactococcal phages with the temperate phage PVL (37), active on the more distant S.aureus, is lower. This is in agreement with the proposal by Hendrix et al. (36) that the intensity of horizontal exchanges most probably depends on the phylogenetic distances between bacterial phage hosts.
Figure 3.
DNA sequence conservation among genomes of L.lactis phages of the P335, 936 and c6A groups, and phages active on different bacterial species. Colour rectangles indicate DNA regions with >70% sequence identity (plain colour), or 50–70% sequence identity (hatched colour). Sequences are taken from Schouler et al. (3; bIL67), Crutz-Le Coq et al. (7; bIL170), Lucchini et al. (33; Sfi21) and Kaneko et al. (37; PVL).
We formulate the following hypothesis to account for the difference in the intensity of genetic exchange for the two phage types. Virulent phage particles cannot last for a long time in the environment. Their survival therefore depends on their capacity to engage into frequent and productive cycles of multiplication. All phages of the same group thus have genomes optimally adapted to this necessity and are, by consequence, highly similar. Introduction of extraneous genetic material would most frequently distract from the optimum and be counter-selected. Only genes conferring a selective advantage, like those extending the phage host spectrum, can be acquired. A well-documented example concerns tail-fibre genes of E.coli phages (35). In contrast, temperate/lytic phages have the possibility to survive integrated in the bacterial chromosome for long periods. They can therefore tolerate much higher genome flexibility, and have many more evolutionary possibilities.
In conclusion, our results suggest the existence of two modes of genetic evolution, depending on the phage way of life: (i) temperate/lytic phages, as proposed by Hendrix et al. (36), would evolve by horizontal DNA exchanges, the frequency of the exchange depending on the taxonomic proximity of the bacterial hosts; (ii) virulent phages would not exchange DNA outside their group. We suggest that the constraints on genetic exchange reflect the optimal genetic organisation of virulent phages, adapted to a more specialised and extreme form of parasitism than temperate/lytic phages.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Acknowledgments
ACKNOWLEDGEMENTS
We gratefully acknowledge Douwe van Sinderen and Gerald Fitzgerald for their kind communication of the complete nucleotide sequence of phage Tuc2009 prior to publication.
DDBJ/EMBL/GenBank accession nos AF323668–AF323673
References
- 1.Jarvis A.W., Fitzgerald,G.F., Mata,M., Mercenier,A., Neve,H., Powell,I.A., Ronda,C., Saxelin,M. and Teuber,M. (1991) Species and type phages of lactococcal bacteriophages. Intervirology, 32, 2–9. [DOI] [PubMed] [Google Scholar]
- 2.Moineau S. (1999) Applications of phage resistance in lactic acid bacteria. Antonie Van Leeuwenhoeck, 76, 377–382 [PubMed] [Google Scholar]
- 3.Schouler C., Ehrlich,S.D. and Chopin,M.C. (1994) Sequence and organization of the lactococcal prolate-headed bIL67 phage genome. Microbiology, 140, 3061–3069. [DOI] [PubMed] [Google Scholar]
- 4.Lubbers M.W., Waterfield,N.R., Beresford,T.P.J., LePage,R.W.F. and Jarvis,A.W. (1995) Sequencing analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of structural genes. Appl. Environ. Microbiol., 61, 4348–4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Sinderen D., Karsens,H., Kok,J., Terpstra,P., Ruiters,M.H.J., Venema,G. and Nauta,A. (1996) Sequence analysis and moleclar characterization of the temperate lactococcal bacteriophage r1t. Mol. Microbiol., 19, 1343–1355. [DOI] [PubMed] [Google Scholar]
- 6.Chandry P.S., Moore,M.C., Boyce,J.D., Davidson,E.E. and Hillier,A.J. (1997) Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol., 26, 49–64. [DOI] [PubMed] [Google Scholar]
- 7.Crutz-Le Coq A.M., Cesselin,B., Commissaire,J., Anba,J., Kyriakidis,S. and Chopin,M.C. (1998) EMBL accession no. AF009630.
- 8.Chopin A., Chopin,M.C., Moillo-Batt,A. and Langella,P. (1984) Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid, 11, 260–263. [DOI] [PubMed] [Google Scholar]
- 9.Terzaghi B.E. and Sandine,W.E. (1975) Improved medium for lactic streptococci and their bacteriophages. Appl. Environ. Microbiol., 29, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maniatis T., Fritsch,E.F. and Sambrook,J. (1982) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 11.Ludwig W., Seewaldt,E., Klipper-Bâlz,R., Schleiffer,K.H., Magrum,L., Woese,C.R., Fox,G.E. and Stackebrandt,E. (1985) The phylogenic position of Streptococcus and Enterococcus. J. Gen. Microbiol., 131, 543–551. [DOI] [PubMed] [Google Scholar]
- 12.Pearson W.R. and Lipman,D.J. (1988) Improved tools for biological sequence comparison. Proc. Natl Acad. Sci. USA ., 85, 2444–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altschul S.F., Madden,T.L., Schäffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tatusova T.A. and MaddenT.L. (1999) Blast 2 sequences—a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett., 174, 247–250. [DOI] [PubMed] [Google Scholar]
- 15.Lowe T.M. and Eddy,S.R. (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res., 25, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chopin M.-C., Chopin,A., Rouault,A. and Galleron,N. (1989) Insertion and amplification of foreign genes in the Lactococcus lactis subsp. lactis chromosome. Appl. Environ. Microbiol., 55, 1769–1774. [DOI] [PMC free article] [PubMed]
- 17.Bolotin A., Mauger,S., Malarme,K., Ehrlich,S.D. and Sorokin,A. (1999) Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Van Leeuvenhoeck, 76, 27–76. [PubMed] [Google Scholar]
- 18.Casjens S., Hatfull,G. and Hendrix,R. (1992) Evolution of dsDNA tailed-bacteriophages genomes. Semin. Virol ., 3, 383–397. [Google Scholar]
- 19.Bertani L.E. and Six,E.W. (1988) The P2-like phages and their parasite, P4. In Calendar,R. (ed.), The Bacteriophages. Plenum Press, New York and London, pp. 73–143.
- 20.Lindqvist B.H., Deho,G. and Calendar,R. (1993) Mechanisms of genome propagation and helper exploitation by satellite phage P4. Microbiol. Rev., 57, 683–702. [DOI] [PMC free article] [PubMed]
- 21.Lindsay J.A., Ruzin,A., Ross,H.F., Kurepina,N. and Novick,R.P. (1998) The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol. Microbiol., 29, 527–543. [DOI] [PubMed] [Google Scholar]
- 22.Juhala R.J., Ford,M.E., Duda,R.L., Youlton,A., Hatfull,G.F. and Hendrix,R.W. (2000) Genomic sequences of bacteriophages HK97 and KH022 : pervasive mosaicism in the lambdoid bacteriophages. J. Mol. Biol., 299, 27–51. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis A.W. and Meyer,J. (1986) Electron microscopic heteroduplex study and restriction endonuclease cleavage analysis of the DNA genomes of three lactic streptococcal bacteriophages. Appl. Environ. Microbiol., 51, 566–571. [DOI] [PMC free article] [PubMed]
- 24.Loof M. and Teuber,M. (1986) Heteroduplex analysis of the genomes of Streptococcus lactis ‘subsp. diacetylactis’ bacteriophages of the P008-type isolated from german cheese factories. System. Appl. Microbiol., 8, 226–229. [DOI] [PubMed]
- 25.Kim J.S. and Davidson,N. (1974) Electron microscope heteroduplex study of sequence relations of T2, T4, and T6 bacteriophage DNAs. Virology, 57, 93–111. [DOI] [PubMed] [Google Scholar]
- 26.Repoila F., Tétart,F., Bouet,J.Y. and Krisch,H.M. (1994) Genomic polymorphism in the T-even bacteriophages. EMBO J., 13, 4181–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tétart F., Repoila,F., Monod,C. and Krisch,H.M. (1996) Bacteriophage T4 host range is expanded by duplications of a small domain of the tail fiber adhesin. J. Mol. Biol., 258, 726–731. [DOI] [PubMed] [Google Scholar]
- 28.Davis R.W. and Hyman,R.W. (1971) A study in evolution : the DNA base sequence homology between coliphages T7 and T3. J. Mol. Biol., 62, 287–301. [DOI] [PubMed] [Google Scholar]
- 29.Bertani G. (1958) Lysogeny. Adv. Virus Res., 5, 151–193. [DOI] [PubMed]
- 30.Hayes W. (1965) The Genetics of Bacteria and their Viruses. J. Wiley and Sons Inc., New York, NY.
- 31.Mikkonen M., Dupont,L., Alatossava,T. and Ritzenthaller,P. (1996) Defective site-specific integration elements are present in the genome of virulent bacteriophage LL-H of Lactobacillus delbrueckii. Appl. Environ. Microbiol., 62, 1847–1851. [DOI] [PMC free article] [PubMed]
- 32.Ford M.E., Sarkis,G.J., Belanger,A.E., Hendrix,R.W. and Hatfull,G.F. (1998) Genome structure of mycobacteriophage D29: implications for phage evolution. J. Mol. Biol., 279, 143–164. [DOI] [PubMed] [Google Scholar]
- 33.Lucchini S., Desiere,F. and Brussow,H. (1999) The genetic relationship between virulent and temperate Streptococcus thermophilus bacteriophages: a whole genome comparison of cos-site phages Sfi19 and Sfi21. Virology, 260, 232–243. [DOI] [PubMed] [Google Scholar]
- 34.Lucchini S., Desiere,F. and Brussow,H. (1999) Comparative genomics of Streptococcus thermophilus phage species supports a modular evolution theory. J. Virol., 73, 8647–8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandmeier H. (1994) Acquisition and rearrangement of sequence motifs in the evolution of bacteriophage tail fibres. Mol. Microbiol., 12, 343–350. [DOI] [PubMed] [Google Scholar]
- 36.Hendrix R.W., Smith,M.C.M., Burns,R.N., Ford,M.E. and Hatfull,G.F. (1999) Evolutionary relationships among diverse bacteriophages and prophages: all the world’s a phage. Proc. Natl Acad. Sci. USA, 96, 2192–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko J., Kimura,T., Narita,S., Tomita,T. and Kamio,Y. (1998) Complete nucleotide sequence and molecular organization of the temperate staphylococcal bacteriophage wPVL carrying panton-valentine leukocidin genes. Gene, 215, 57–67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.