Abstract
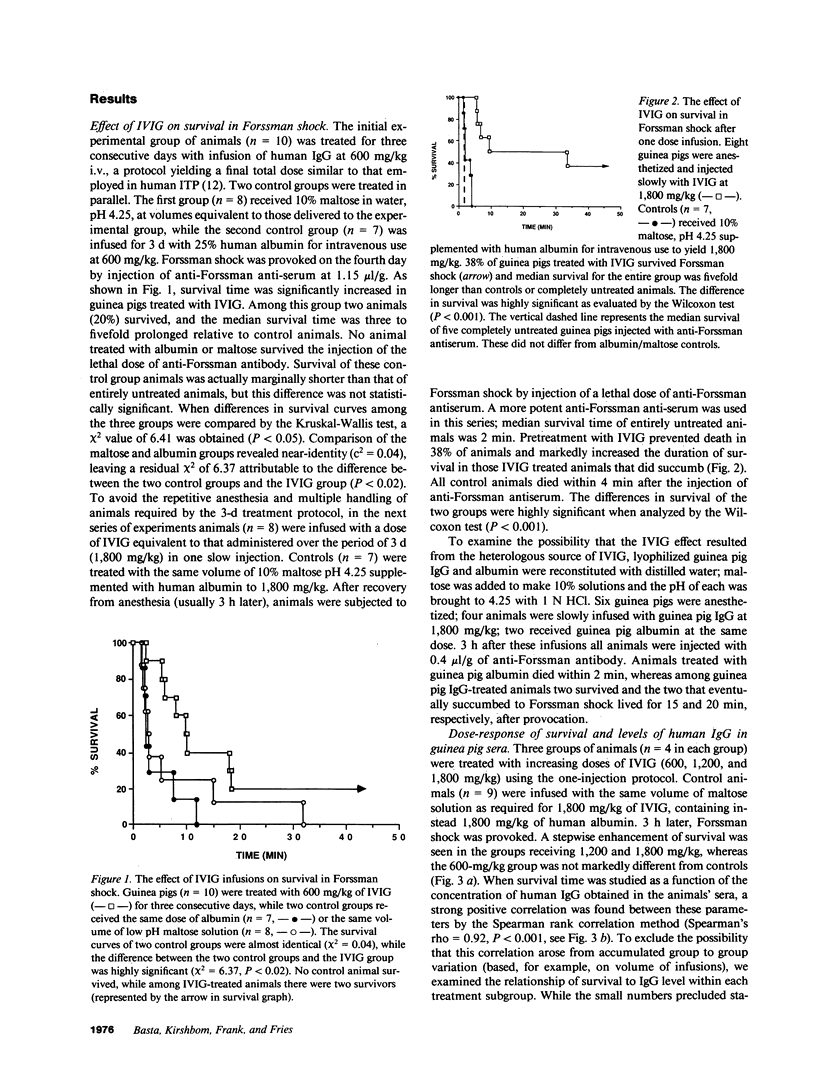
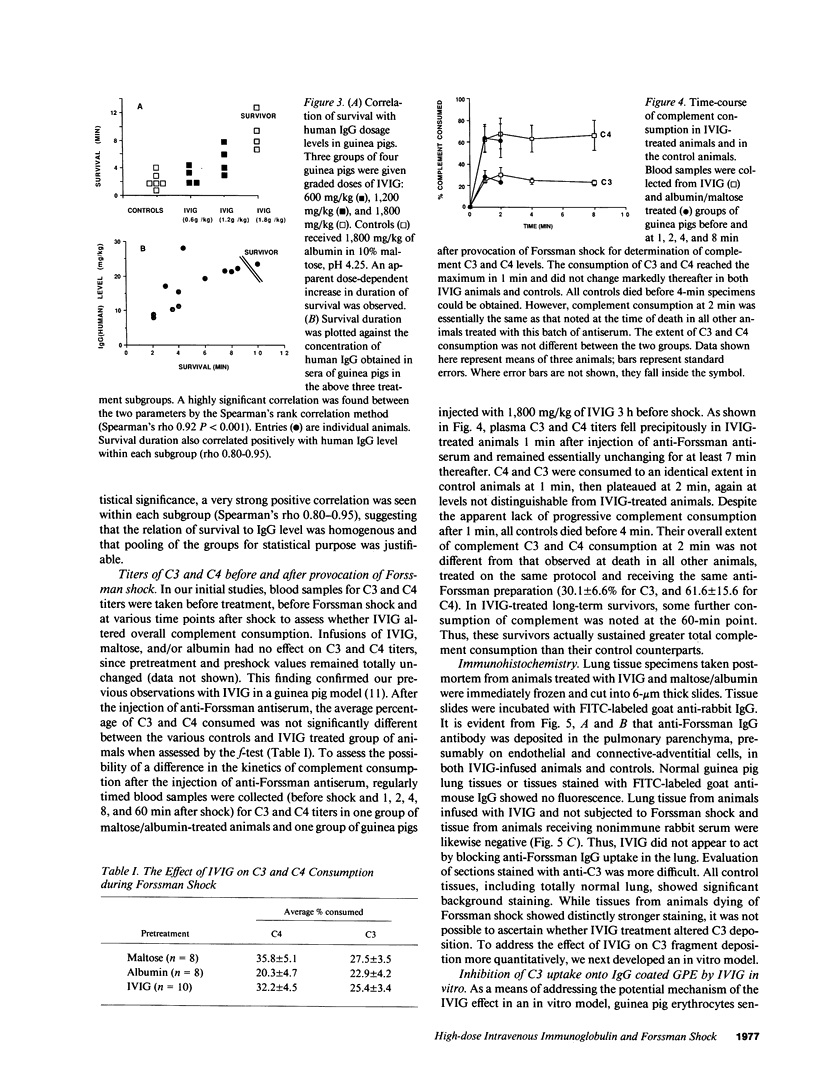
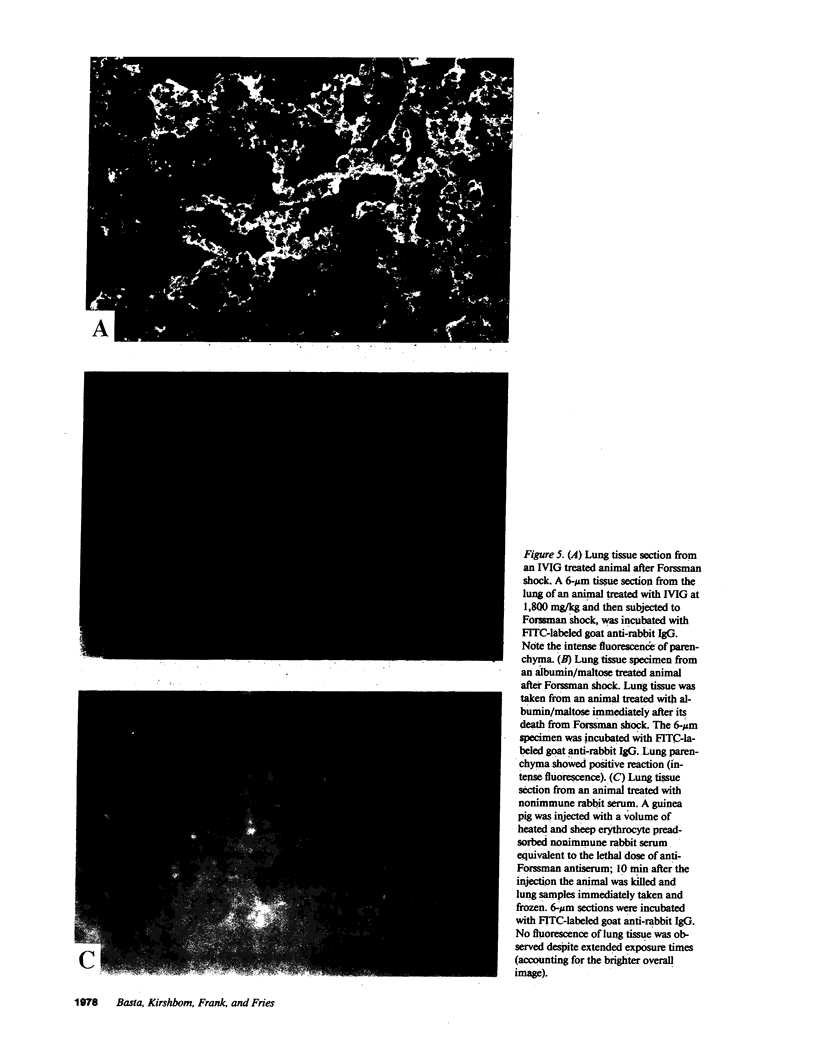
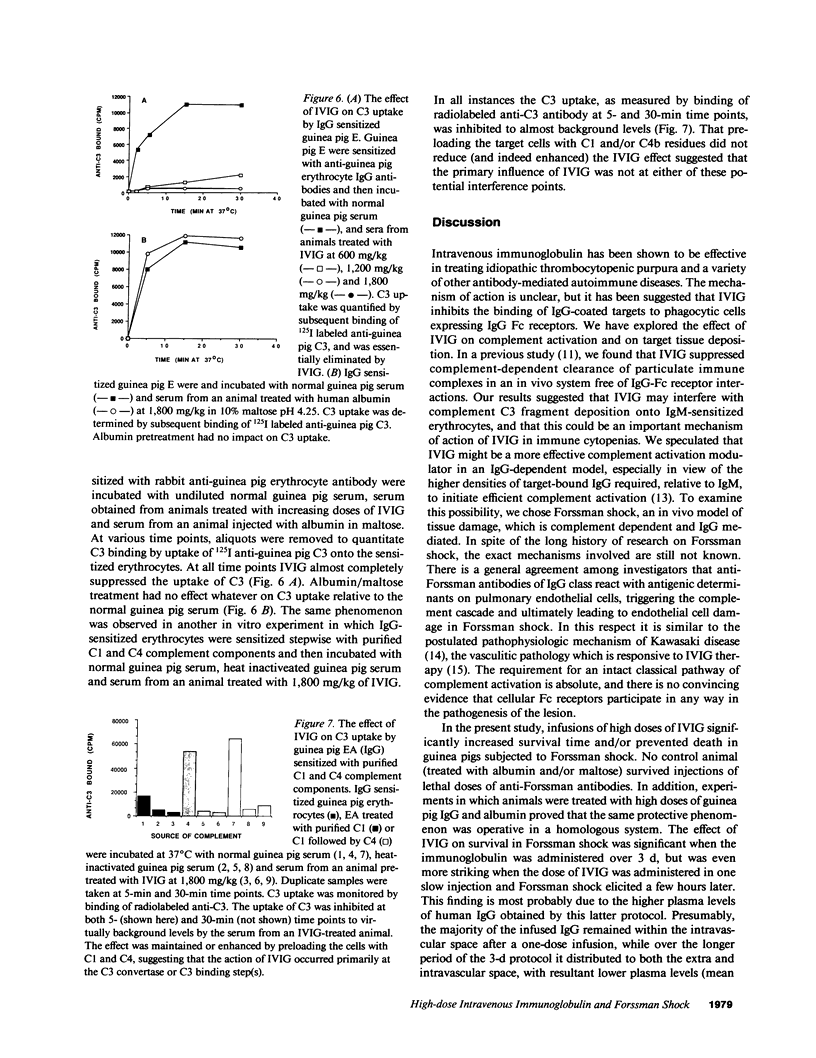
Studies were performed in in vitro and in vivo models to assess the effect of intravenous immunoglobulin (IVIG) on the development of acute complement-mediated tissue damage. IVIG significantly increased the duration of survival and frequently prevented the death of guinea pigs injected with anti-Forssman antiserum to cause lethal Forssman shock; no control animal treated with albumin and/or maltose vehicle survived. The most pronounced effect was achieve by delivering IVIG as one slow injection at 1,800 mg/kg 3 h before Forssman shock was elicited. Infusion of guinea pig IgG at the same dosage was similarly protective. A strong positive correlation was found between IgG plasma levels and survival time in guinea pigs treated with graded doses of IVIG. Therapy itself did not affect C3 and C4 levels nor the capacity to activate these components. In vitro studies showed almost complete inhibition of C3 uptake onto IgG-sensitized erythrocytes using serum from an IVIG-treated animal. We suggest that supraphysiologic levels of IVIG act in part by preventing active C3 fragments from binding to target cells. Infusion of high dose IVIG may be a rational approach to modulating acute, complement-dependent tissue damage in a variety of diseases in man.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basta M., Langlois P. F., Marques M., Frank M. M., Fries L. F. High-dose intravenous immunoglobulin modifies complement-mediated in vivo clearance. Blood. 1989 Jul;74(1):326–333. [PubMed] [Google Scholar]
- Bauman N., Elliott W. J. The relative efficacies of 7S and 19S Forssman antibody in producing lesions in the guinea pig. Proc Soc Exp Biol Med. 1972 Feb;139(2):670–672. doi: 10.3181/00379727-139-36212. [DOI] [PubMed] [Google Scholar]
- Berger M., Rosenkranz P., Brown C. Y. Intravenous and standard immune serum globulin preparations interfere with uptake of 125I-C3 onto sensitized erythrocytes and inhibit hemolytic complement activity. Clin Immunol Immunopathol. 1985 Feb;34(2):227–236. doi: 10.1016/0090-1229(85)90027-3. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Berger M., Joiner K. A., Frank M. M. Classical complement pathway activation by antipneumococcal antibodies leads to covalent binding of C3b to antibody molecules. Infect Immun. 1983 Nov;42(2):594–598. doi: 10.1128/iai.42.2.594-598.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries L. F., Gaither T. A., Hammer C. H., Frank M. M. C3b covalently bound to IgG demonstrates a reduced rate of inactivation by factors H and I. J Exp Med. 1984 Dec 1;160(6):1640–1655. doi: 10.1084/jem.160.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd K. J., Reid K. B. The binding of complement component C3 to antibody-antigen aggregates after activation of the alternative pathway in human serum. Biochem J. 1981 May 1;195(2):471–480. doi: 10.1042/bj1950471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Imbach P., Barandun S., d'Apuzzo V., Baumgartner C., Hirt A., Morell A., Rossi E., Schöni M., Vest M., Wagner H. P. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981 Jun 6;1(8232):1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- Jacobs R. J., Reichlin M. Generation of low m.w., C3-bearing immunoglobulin in human serum. J Immunol. 1983 Jun;130(6):2775–2781. [PubMed] [Google Scholar]
- Kulics J., Rajnavölgyi E., Füst G., Gergely J. Interaction of C3 and C3b with immunoglobulin G. Mol Immunol. 1983 Aug;20(8):805–810. doi: 10.1016/0161-5890(83)90076-7. [DOI] [PubMed] [Google Scholar]
- LEDUC E. H., TANAKA N. A study of the cellular distribution of Forssman antigen in various species. J Immunol. 1956 Sep;77(3):198–212. [PubMed] [Google Scholar]
- Leung D. Y., Collins T., Lapierre L. A., Geha R. S., Pober J. S. Immunoglobulin M antibodies present in the acute phase of Kawasaki syndrome lyse cultured vascular endothelial cells stimulated by gamma interferon. J Clin Invest. 1986 May;77(5):1428–1435. doi: 10.1172/JCI112454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. E., Frank M. M. Complement-mediated tissue damage: contribution of the classical and alternate complement pathways in the Forssman reaction. J Immunol. 1972 Jun;108(6):1517–1525. [PubMed] [Google Scholar]
- Nagai H., Kurimoto Y., Koda A. Immunopharmacological approach to Forssman shock. Microbiol Immunol. 1980;24(7):649–655. doi: 10.1111/j.1348-0421.1980.tb02866.x. [DOI] [PubMed] [Google Scholar]
- Newburger J. W., Takahashi M., Burns J. C., Beiser A. S., Chung K. J., Duffy C. E., Glode M. P., Mason W. H., Reddy V., Sanders S. P. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986 Aug 7;315(6):341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- Stiehm E. R., Ashida E., Kim K. S., Winston D. J., Haas A., Gale R. P. Intravenous immunoglobulins as therapeutic agents. Ann Intern Med. 1987 Sep;107(3):367–382. doi: 10.7326/0003-4819-107-2-367. [DOI] [PubMed] [Google Scholar]
- Tsai C. C., Taichman N. S., Pulver W. H., Schönbaum E. Heterophile antibodies and tissue injury. 3. A role for platelets in the development of lethal vascular injury during Forssman shock in guinea pigs. Am J Pathol. 1973 Aug;72(2):179–200. [PMC free article] [PubMed] [Google Scholar]