Abstract
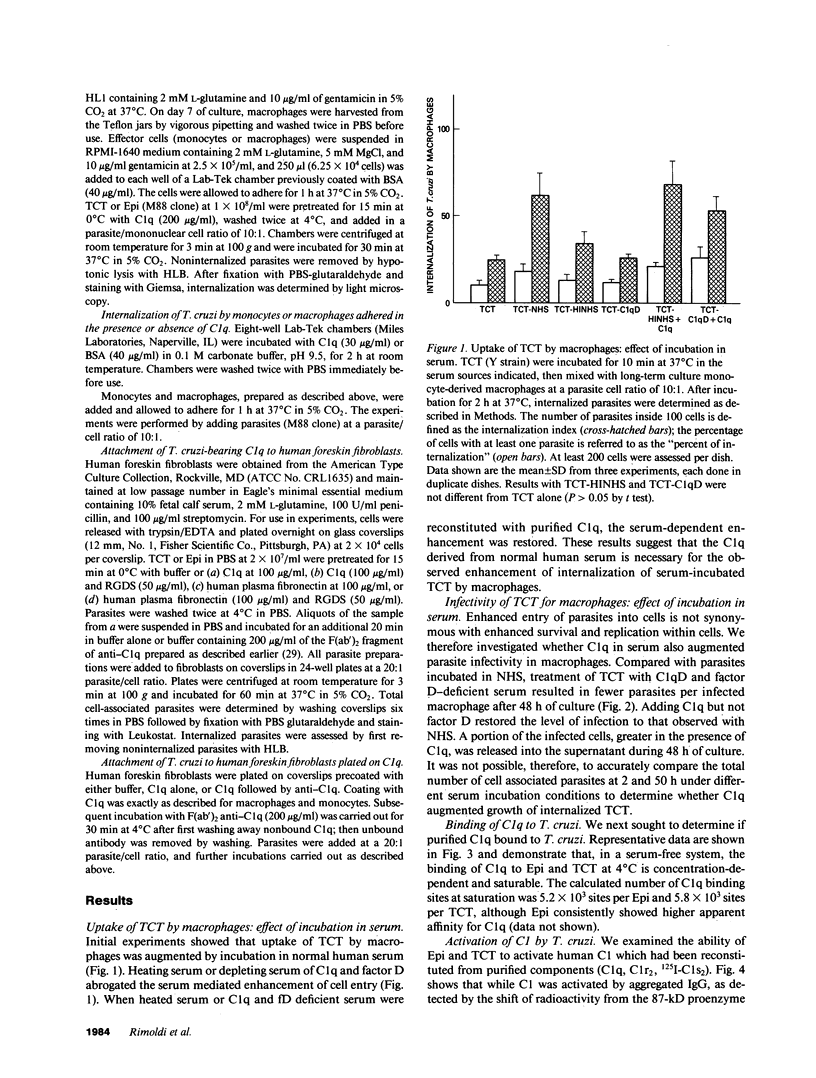
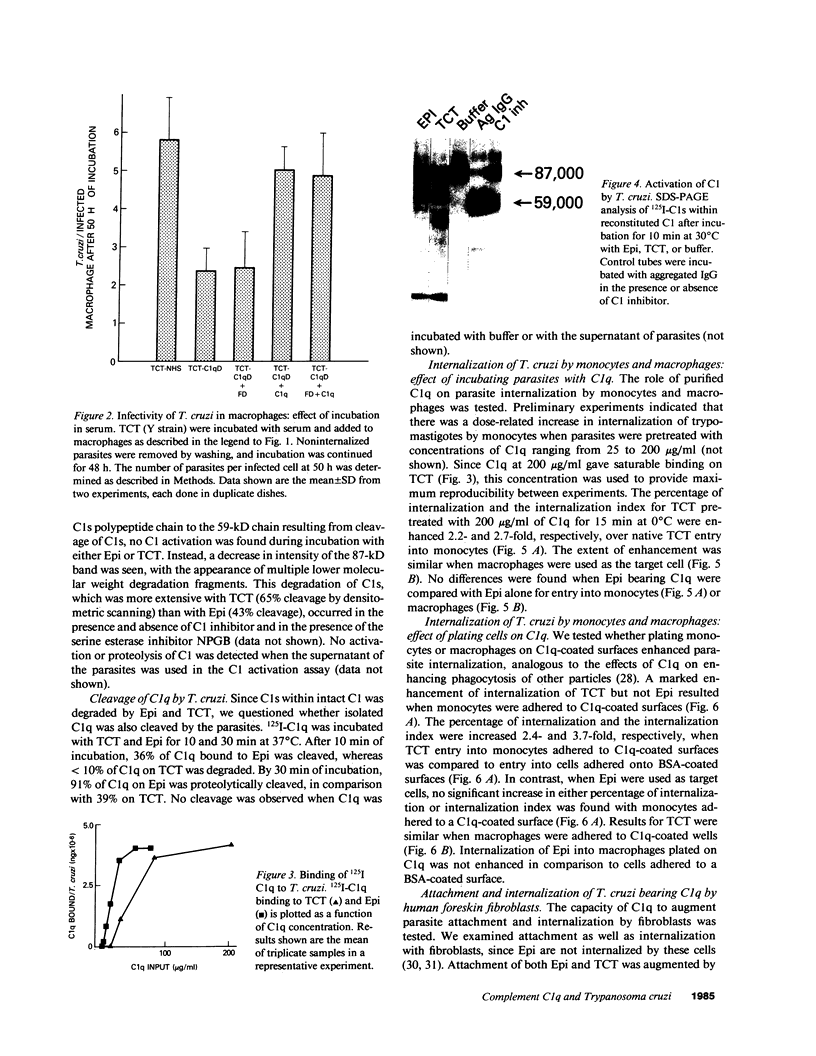
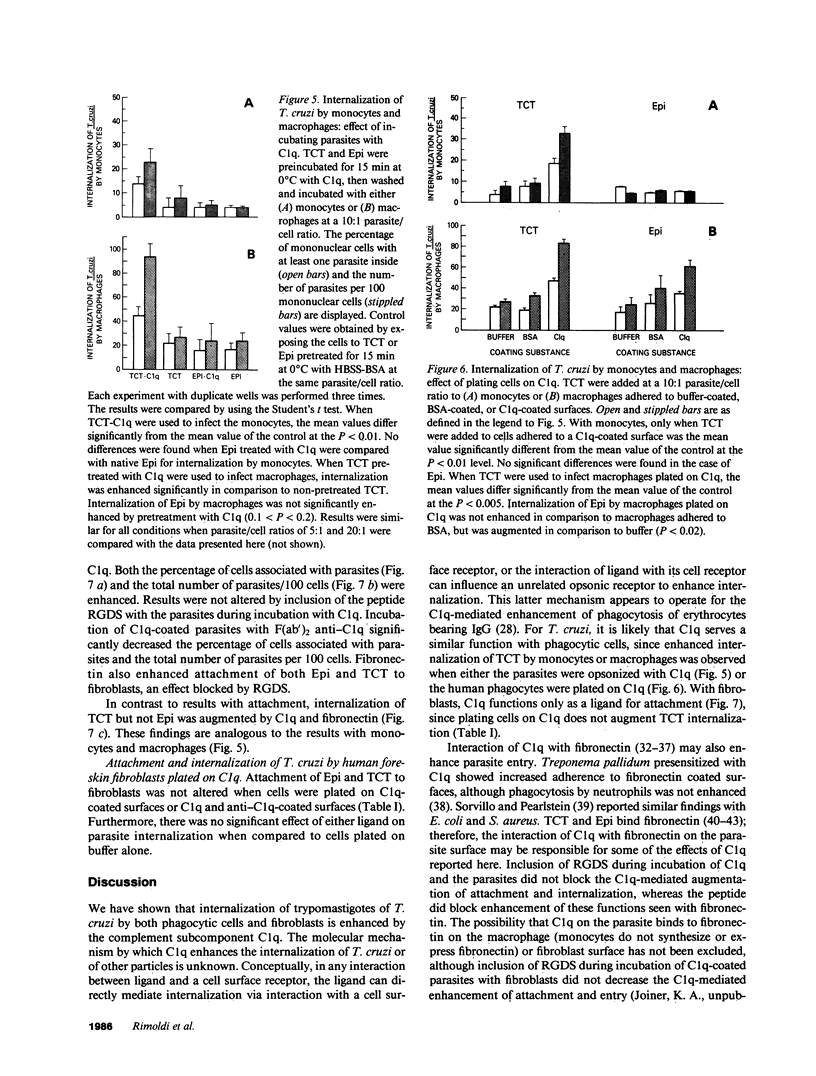
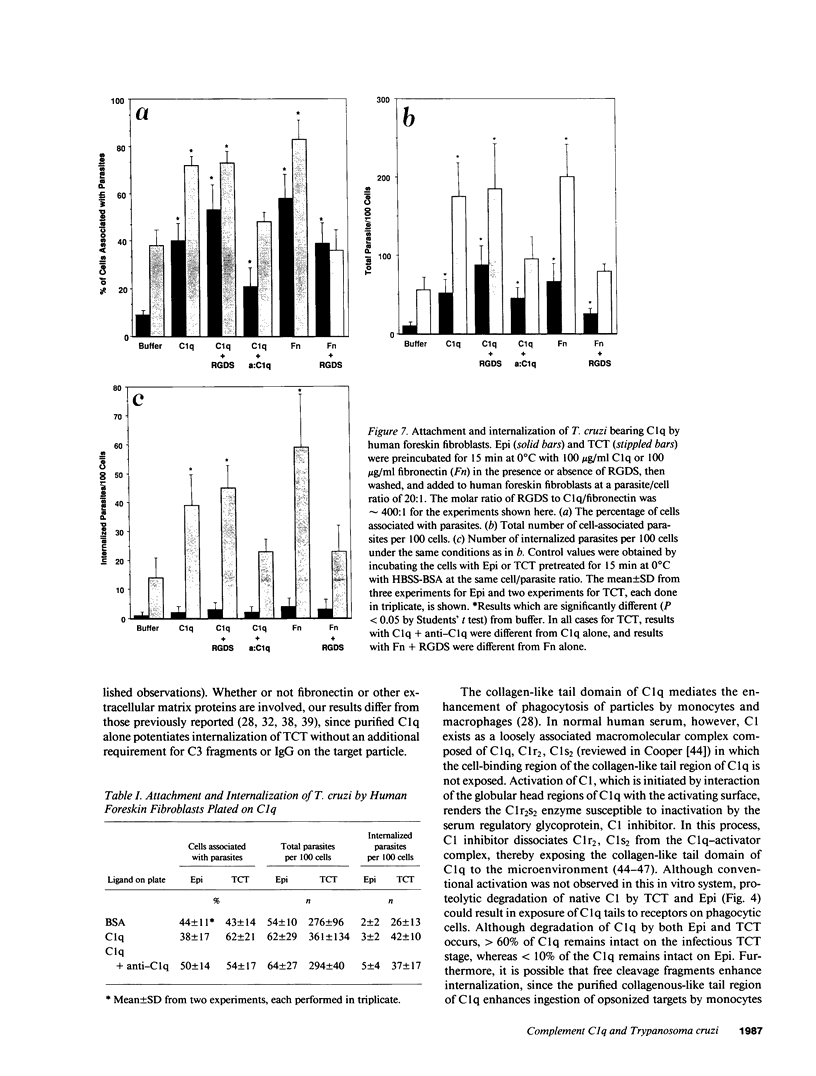
Internalization and infectivity of Trypanosoma cruzi trypomastigotes by macrophages is enhanced by prior treatment of parasites with normal human serum. Heating serum or removing C1q from serum abrogates the enhancement, but augmentation of attachment and infectivity is restored by addition of purified C1q to either serum source. Although both noninfective epimastigotes (Epi) and vertebrate-stage tissue culture trypomastigotes (TCT) bind C1q in saturable fashion at 4 degrees C, internalization by monocytes and macrophages of TCT but not Epi-bearing C1q is enhanced in comparison to untreated parasites. Adherence of human monocytes and macrophages to surfaces coated with C1q also induces a marked enhancement of the internalization of native TCT. C1q enhances attachment of both Epi and TCT to human foreskin fibroblasts, but only when C1q is on the parasite and not when the fibroblasts are plated on C1q-coated surfaces. Only TCT coated with C1q show enhanced invasion into fibroblasts. Although trypomastigotes produce an inhibitor of the complement cascade which limits C3 deposition during incubation in normal human serum, C1q binds to the parasite and enhances entry of trypomastigotes into target cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. W., Colli W. Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J Protozool. 1982 May;29(2):264–269. doi: 10.1111/j.1550-7408.1982.tb04024.x. [DOI] [PubMed] [Google Scholar]
- Baughn R. E. Antibody-independent interactions of fibronectin, C1q, and human neutrophils with Treponema pallidum. Infect Immun. 1986 Nov;54(2):456–464. doi: 10.1128/iai.54.2.456-464.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing D. H., Almeda S., Isliker H., Lahav J., Hynes R. O. Fibronectin binds to the C1q component of complement. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4198–4201. doi: 10.1073/pnas.79.13.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak D. A., Frank M. M., Tenner A. J. C1q acts synergistically with phorbol dibutyrate to activate CR1-mediated phagocytosis by human mononuclear phagocytes. Eur J Immunol. 1988 Dec;18(12):2001–2007. doi: 10.1002/eji.1830181220. [DOI] [PubMed] [Google Scholar]
- Bobak D. A., Gaither T. A., Frank M. M., Tenner A. J. Modulation of FcR function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J Immunol. 1987 Feb 15;138(4):1150–1156. [PubMed] [Google Scholar]
- Bullock W. E., Wright S. D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallesco R., Pereira M. E. Antibody to Trypanosoma cruzi neuraminidase enhances infection in vitro and identifies a subpopulation of trypomastigotes. J Immunol. 1988 Jan 15;140(2):617–625. [PubMed] [Google Scholar]
- Chesne S., Villiers C. L., Arlaud G. J., Lacroix M. B., Colomb M. G. Fluid-phase interaction of C1 inhibitor (C1 Inh) and the subcomponents C1r and C1s of the first component of complement, C1. Biochem J. 1982 Jan 1;201(1):61–70. doi: 10.1042/bj2010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly M. C., Kierszenbaum F. Increased host cell-Trypanosoma cruzi interaction following phospholipase D treatment of the parasite surface. Mol Biochem Parasitol. 1985 Nov;17(2):191–202. doi: 10.1016/0166-6851(85)90018-0. [DOI] [PubMed] [Google Scholar]
- Cooper N. R. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Ziccardi R. J. Reconstitution of C1 in native proenzyme form and its use in a quantitative C1 activation test. J Immunol. 1977 Nov;119(5):1664–1667. [PubMed] [Google Scholar]
- Da Silva R. P., Hall B. F., Joiner K. A., Sacks D. L. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989 Jul 15;143(2):617–622. [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyns C. F., Human H. J., Werely C. J., De Klerk D. P. The glycosaminoglycans of the gubernaculum during testicular descent in the fetus. J Urol. 1990 Mar;143(3):612–617. doi: 10.1016/s0022-5347(17)40040-1. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., daSilva W. D., Rimoldi M. T., Hammer C. H., Sher A., Kipnis T. L. Biochemical characterization of a factor produced by trypomastigotes of Trypanosoma cruzi that accelerates the decay of complement C3 convertases. J Biol Chem. 1988 Aug 15;263(23):11327–11335. [PubMed] [Google Scholar]
- Joiner K., Hieny S., Kirchhoff L. V., Sher A. gp72, the 72 kilodalton glycoprotein, is the membrane acceptor site for C3 on Trypanosoma cruzi epimastigotes. J Exp Med. 1985 May 1;161(5):1196–1212. doi: 10.1084/jem.161.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K., Sher A., Gaither T., Hammer C. Evasion of alternative complement pathway by Trypanosoma cruzi results from inefficient binding of factor B. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6593–6597. doi: 10.1073/pnas.83.17.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Kolb L. M., Podack E. R. C1q: isolation from human serum in high yield by affinity chromatography and development of a highly sensitive hemolytic assay. J Immunol. 1979 May;122(5):2103–2111. [PubMed] [Google Scholar]
- Laurell A. B., Johnson U., Mårtensson U., Sjöholm A. G. Formation of complexes composed of C1r, C1s, and C1 inactivator in human serum on activation of C1. Acta Pathol Microbiol Scand C. 1978 Dec;86C(6):299–306. doi: 10.1111/j.1699-0463.1978.tb02594.x. [DOI] [PubMed] [Google Scholar]
- Menzel E. J., Smolen J. S., Liotta L., Reid K. B. Interaction of fibronectin with C1q and its collagen-like fragment (CLF). FEBS Lett. 1981 Jun 29;129(1):188–192. doi: 10.1016/0014-5793(81)80787-9. [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Edelson P. J. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J Immunol. 1985 Oct;135(4):2785–2789. [PubMed] [Google Scholar]
- Mosser D. M., Edelson P. J. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. 1987 May 28-Jun 3Nature. 327(6120):329–331. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Chaplan S., Cohn Z. Trypanosoma cruzi. Factors modifying ingestion and fate of blood form trypomastigotes. J Exp Med. 1980 Aug 1;152(2):447–451. doi: 10.1084/jem.152.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouaissi M. A., Afchain D., Capron A., Grimaud J. A. Fibronectin receptors on Trypanosoma cruzi trypomastigotes and their biological function. Nature. 1984 Mar 22;308(5957):380–382. doi: 10.1038/308380a0. [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Cornette J., Capron A. Identification and isolation of Trypanosoma cruzi trypomastigote cell surface protein with properties expected of a fibronectin receptor. Mol Biochem Parasitol. 1986 Jun;19(3):201–211. doi: 10.1016/0166-6851(86)90002-2. [DOI] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E., Sorvillo J., Gigli I. The interaction of human plasma fibronectin with a subunit of the first component of complement, C1q. J Immunol. 1982 May;128(5):2036–2039. [PubMed] [Google Scholar]
- Peyrol S., Ouaissi M. A., Capron A., Grimaud J. A. Trypanosoma cruzi: ultrastructural visualization of fibronectin bound to culture forms. Exp Parasitol. 1987 Feb;63(1):112–114. doi: 10.1016/0014-4894(87)90084-1. [DOI] [PubMed] [Google Scholar]
- Piras M. M., Piras R., Henriquez D., Negri S. Changes in morphology and infectivity of cell culture-derived trypomastigotes of Trypanosoma cruzi. Mol Biochem Parasitol. 1982 Aug;6(2):67–81. doi: 10.1016/0166-6851(82)90066-4. [DOI] [PubMed] [Google Scholar]
- Prioli R. P., Ordovas J. M., Rosenberg I., Schaefer E. J., Pereira M. E. Similarity of cruzin, an inhibitor of Trypanosoma cruzi neuraminidase, to high-density lipoprotein. Science. 1987 Dec 4;238(4832):1417–1419. doi: 10.1126/science.3120314. [DOI] [PubMed] [Google Scholar]
- Prograis L. J., Jr, Hammer C. H., Katusha K., Frank M. M. Purification of C1 inhibitor. A new approach for the isolation of this biologically important plasma protease inhibitor. J Immunol Methods. 1987 May 4;99(1):113–122. doi: 10.1016/0022-1759(87)90039-1. [DOI] [PubMed] [Google Scholar]
- Puentes S. M., Sacks D. L., da Silva R. P., Joiner K. A. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. J Exp Med. 1988 Mar 1;167(3):887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. Proteins involved in the activation and control of the two pathways of human complement. Biochem Soc Trans. 1983 Jan;11(1):1–12. doi: 10.1042/bst0110001. [DOI] [PubMed] [Google Scholar]
- Rimoldi M. T., Sher A., Heiny S., Lituchy A., Hammer C. H., Joiner K. Developmentally regulated expression by Trypanosoma cruzi of molecules that accelerate the decay of complement C3 convertases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):193–197. doi: 10.1073/pnas.85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Wright S. D. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med. 1988 Jul 1;168(1):279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Güther M. L., Yoshida N. Mechanism of resistance to lysis by the alternative complement pathway in Trypanosoma cruzi trypomastigotes: effect of specific monoclonal antibody. J Immunol. 1986 Sep 1;137(5):1623–1628. [PubMed] [Google Scholar]
- Sher A., Hieny S., Joiner K. Evasion of the alternative complement pathway by metacyclic trypomastigotes of Trypanosoma cruzi: dependence on the developmentally regulated synthesis of surface protein and N-linked carbohydrate. J Immunol. 1986 Nov 1;137(9):2961–2967. [PubMed] [Google Scholar]
- Sorvillo J. M., Gigli I., Pearlstein E. The effect of fibronectin on the processing of C1q- and C3b/bi-coated immune complexes by peripheral blood monocytes. J Immunol. 1986 Feb 1;136(3):1023–1026. [PubMed] [Google Scholar]
- Sorvillo J. M., Pearlstein E. C1q, a subunit of the first component of complement, enhances binding of plasma fibronectin to bacteria. Infect Immun. 1985 Sep;49(3):664–669. doi: 10.1128/iai.49.3.664-669.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvillo J., Gigli I., Pearlstein E. Fibronectin binding to complement subcomponent C1q. Localization of their respective binding sites. Biochem J. 1985 Feb 15;226(1):207–215. doi: 10.1042/bj2260207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvillo J., Gigli I., Pearlstein E. Requirements for the binding of human plasma fibronectin to the C1q subunit of the first component of complement. J Immunol. 1983 Sep;131(3):1400–1404. [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Valet G., Cooper N. R. Isolation and characterization of the proenzyme form of the C1s subunit of the first complement component. J Immunol. 1974 Jan;112(1):339–350. [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Fibronectin enhances macrophage association with invasive forms of Trypanosoma cruzi. J Immunol. 1984 Jul;133(1):460–464. [PubMed] [Google Scholar]
- Wozencraft A. O., Sayers G., Blackwell J. M. Macrophage type 3 complement receptors mediate serum-independent binding of Leishmania donovani. Detection of macrophage-derived complement on the parasite surface by immunoelectron microscopy. J Exp Med. 1986 Oct 1;164(4):1332–1337. doi: 10.1084/jem.164.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Active disassembly of the first complement component, C-1, by C-1 inactivator. J Immunol. 1979 Aug;123(2):788–792. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Physicochemical and functional characterization of the C1r subunit of the first complement component. J Immunol. 1976 Feb;116(2):496–503. [PubMed] [Google Scholar]
- Zingales B., Colli W. Trypanosoma cruzi: interaction with host cells. Curr Top Microbiol Immunol. 1985;117:129–152. doi: 10.1007/978-3-642-70538-0_7. [DOI] [PubMed] [Google Scholar]