Abstract
Background
Pulmonary vasodilators in general and prostacyclin therapy in particular, have markedly improved the outcome of patients with pulmonary arterial hypertension (PAH). As endothelial dysfunction is a key feature of PAH, and as endothelial progenitor cells (EPC) may contribute to vascular repair in PAH, we suspected that prostacyclin therapy might enhance EPC numbers and functions. In the present study, objectives were to determine whether EPC may contribute to vasodilator treatment efficacy in PAH.
Methods
We quantified CD34+ cells, CFU-Hill and ECFC (endothelial colony forming cells) in peripheral blood from children with idiopathic PAH (n = 27) or PAH secondary to congenital heart disease (n = 52). CD34+ were enumerated by flow cytometry, CFU-Hill and ECFC by a culture assay. ECFC grown ex vivo were tested for their angiogenic capacities before and after prostacyclin analog therapy (subcutaneous treprostinil).
Results
ECFC counts were significantly enhanced in the 8 children treated with treprostinil, while no change was observed in children receiving oral therapy with endothelin antagonists and/or PDE5 inhibitors. CD34+ cell and CFU-Hill counts were unaffected. ECFC from patients treated with treprostinil had a hyperproliferative phenotype and showed enhanced angiogenic potential in a nude mouse preclinical model of limb ischemia.
Conclusions
ECFC may partly mediate the clinical benefits of prostanoids in pulmonary arterial hypertension.
Keywords: Pulmonary hypertension, Congenital heart disease, Vasodilator treatment, Treprostinil, EPC
Introduction
Pulmonary vascular disease (PVD) can cause pulmonary arterial hypertension (PAH), usually leading to right heart failure and death if left untreated [1]. Endothelial cell dysfunction is a hallmark of both idiopathic PAH and PAH secondary to congenital heart disease [2–4].
A role of endothelial progenitor cells (EPC) in vascular repair and new vessel formation has been described [5–7]. EPC mobilized from bone marrow and/or resident locally in the lung, are thought to be important in maintaining vascular homeostasis; and there is a growing interest in the potential therapeutic or diagnostic use of EPC during PAH. Experimental and clinical studies have examined the possible contribution of EPC to the pathogenesis of PAH, but reported EPC counts in patients with pulmonary hypertension have been inconsistent [8–11]. Several types of EPC are defined, depending on the method used (flow cytometry or culture) and their characteristics. At least two populations of EPC have been described [5, 12]. “Early” EPC are spindle-shaped and express both endothelial and leukocyte markers. Quantification of this cell population, as described by Hill, utilizes a commercial kit that identifies so-called “CFU-Hill” [13]. The number of CFU-Hill in peripheral blood has been reported to correlate inversely with cardiovascular risk factors [13]. “Late” EPC, also called endothelial colony-forming cells (ECFC) [7, 14], develop after 1–3 weeks of culture and have the characteristics of precursor cells committed to the endothelial lineage. Both EPC subtypes have therapeutic potential but in vivo, cells that merge into neovessels have an ECFC phenotype [6, 7].
EPC transplantation was recently shown to improve pulmonary hypertension in a rat model [15, 16], while Wang et al. [17] found that EPC transplantation improved exercise capacity and pulmonary hemodynamics in patients with idiopathic PAH , and a contemporary open-label, non-randomized pilot trial showed that EPC transplantation led to significant improvements in exercise capacity, New York Heart Association functional class, and pulmonary hemodynamics in children with idiopathic PAH [18].
“Pulmonary vasodilator” therapy has greatly improved the prognosis of patients with PAH [19, 20]. In particular, parenteral prostacyclin improves the outcome of patients with PVD, not only by inducing pulmonary vasodilation but also by altering pulmonary vascular structure and function during long-term administration. Intravenous prostacyclin is currently recommended for patients of all ages with WHO functional class IV disease, and as add-on therapy for patients remaining in class III despite correctly dosed treatment with endothelin-receptor antagonists (ERA) and phosphodiesterase-5 inhibitors (PDE5) [19, 20]. Subcutaneous treprostinil, a parenteral prostanoid, is sometimes preferred to an intravenous prostacyclin in children, especially to avoid the long-term risks associated with chronic intravenous therapy.
Given the possible relationship between EPC and treatment efficacy, we therefore examined the impact of treprostinil therapy on the number and functional capacity of EPC in children with advanced PAH.
Patients and methods
Study population (Table 1)
Table 1.
Characteristics of the patients
| Reversible PAH (n = 28) | Irreversible PAH (n = 22) | Idiopathic PAH (n = 27) | P value reversible versus irreversible PAH | P value reversible versus Idiopathic PAH | P value irreversible versus Idiopathic PAH | |
|---|---|---|---|---|---|---|
| Age (y) | 2 (2.7–6.5) | 8 (6.1–16.4) | 6 (4.5–8.4) | 0.01* | 0.16 | 0.06 |
| Saturation (%) | 96 (89.2–95.3) | 86 (79.8–91.3) | 97.5 (93.5–98.4) | 0.02* | 0.07 | 0.0008* |
| Mean PAP | 55 (50–66.2) | 60 (50.3–66.5) | 53 (46–56.5) | 0.13 | 0.15 | 0.95 |
Data are expressed as medians and confidence intervals (95%CI). Baseline characteristics were compared with Wilcoxon’s rank sum test for non normally distributed variables (age) and Student’s unpaired test otherwise
* P < 0.05, reversible versus irreversible PAH
This prospectively designed study was aimed at determining the numbers and functional capacities of EPC before and after vasodilator treatment, and especially after treprostinil therapy. Blood samples were collected during outpatient visits from patients already on single- or dual-agent therapy. In the 8 treprostinil-treated patients, EPC were studied before treatment initiation, 2 and 5 days later and monthly thereafter.
The study was approved by the institutional ethics committee of Necker-Enfants Malades hospital, and the parents gave their signed informed consent. Fifty-two consecutive patients with CHD and PAH, and 27 consecutive patients with idiopathic PAH (Table 1) were enrolled between February 2008 and 2010. The 27 patients with idiopathic PAH had a median age of 6 years. Among the patients with CHD, 28 (median age 2 years) had reversible PAH, as defined by normal pulmonary pressure 6 months after surgery, while 24 (median age 8 years) had irreversible PAH, based on persistently elevated pulmonary artery pressure and pulmonary vascular resistance. Children with irreversible PAH were older than children with reversible and idiopathic PAH (P = 0.01 and P = 0.06, respectively) and had lower oxygen saturation values (P = 0.02 and P = 0.0008, respectively). Pulmonary artery pressure was similar in the three groups (Table 1). Patients with familial PAH associated with BMPR2 mutations were not included in this study, because of a possible impact of BMP on EPC angiogenic properties [6, 21, 22]. Indeed, BMPR2 mutations have been reported to affect both early [23] and late EPC, the latter showing a hyperproliferative phenotype and an impaired capacity to form vascular networks [22]. Because of adverse effects reported with IV epoprostenol and inhaled prostanoids, subcutaneous treprostinil was used as first-line add-on therapy for 8 young children who deteriorated while receiving combined oral therapy with an endothelin receptor agonist and a PDE5 inhibitor. Three of them had idiopathic PAH and five patients had PAH associated with a congenital heart defect. The decision to add subcutaneous tresprostinil was based on their clinical status (change in functional class), including right ventricular dysfunction (hepatomegaly, increase in tricuspid regurgitation volume, dilation of hepatic veins and the inferior vena cava) in seven patients. Two of them also had syncope. One patient in functional class II had severe complications associated with the central venous line used to deliver epoprostenol. All the patients had right heart catheterization prior to treprostinil treatment. Treprostinil administration was initiated at hospital, through a subcutaneous catheter in the outer leg. All the patients initially received a fixed dose of 1.25 ng/kg/min. The dose was then daily increased by 1.25 ng/kg/min, reaching an average of 20 ng/kg/min at hospital discharge. During treprostinil treatment, site pain was evaluated every 6 h using standard pain scales adapted to age. After hospital discharge the treprostinil dose was adjusted at monthly out-patient visits and reached an average of 40 ng/kg/min. Technical assistance was provided at home by specialized nurses trained in the management of parenteral prostanoid therapy in PAH patients. All patients had experienced a clinical improvement performed, for oldest children by 6MWT, and they all showed an improvement in oxygen saturation and functional status, as they were all in functional class I or II. In five patients who had right heart catheterization after treprostinil, pulmonary arterial pressure did not change but cardiac output increased and pulmonary vascular resistance decreased.
Flow cytometric quantification of CD34+ hematopoietic progenitor cells (HPC)
Circulating CD34+ cells were counted by flow cytometry (FC500 Cytometer; Beckman Coulter, Villepinte, France) after staining of whole blood with a fluorescein isothiocyanate (FITC)-labeled monoclonal mouse antihuman CD45 antibody, a phycoerythrin (PE)-labeled monoclonal mouse antihuman-CD34 antibody, and 7AAD (Stemkit®; Beckman Coulter). Absolute numbers of CD34+ cells μl−1 were determined by using calibration beads, as recommended by the manufacturer.
EPC quantification by cell culture
Blood was diluted 1:1 with PBS, 0.2 M EDTA and overlaid on Histopaque-1077 (Sigma–Aldrich, Saint-Quentin Fallavier, France). Cells were centrifuged at 100g for 20 min. Mononuclear cells (MNC) were collected and washed 3 times in PBS, 0.2 M EDTA. CFU-Hill were cultured with the EndoCult® Liquid Medium kit (StemCell Technologies, Vancouver, BC, Canada) according to the manufacturer’s instructions. Briefly, MNC were resuspended in complete EndoCult® medium and seeded at 5 × 106 cells/well in fibronectin-coated tissue culture plates (BD, Becton–Dickinson Biosciences). After 48 h, to obtain CFU-Hill, nonadherent cells were collected and plated in Endocult® buffer at 106 cells/well in 24-well fibronectin-coated plates. CFU-Hill colonies were counted after another 3 days, as recommended by the manufacturer. As previously described [7, 13, 24], these cells did not replicate in vitro and gradually disappeared 20 days after plating (Fig. 1b). To obtain ECFC, adherent cells at 48 h were kept in 6-well fibronectin-coated plates in EGM2 medium (Lonza, Saint-Beauzire, France) composed of endothelial cell basal medium-2 (EBM2), 5% fetal bovine serum (FBS) and growth factors. ECFC appeared between 7 and 30 days of culture and consisted of well-circumscribed cobblestone monolayers (Fig. 1d). Colonies were counted with an inverted microscope at ×20 magnification. The colonies were then harvested, trypsinized and reseeded in 6-well plates for complementary studies.
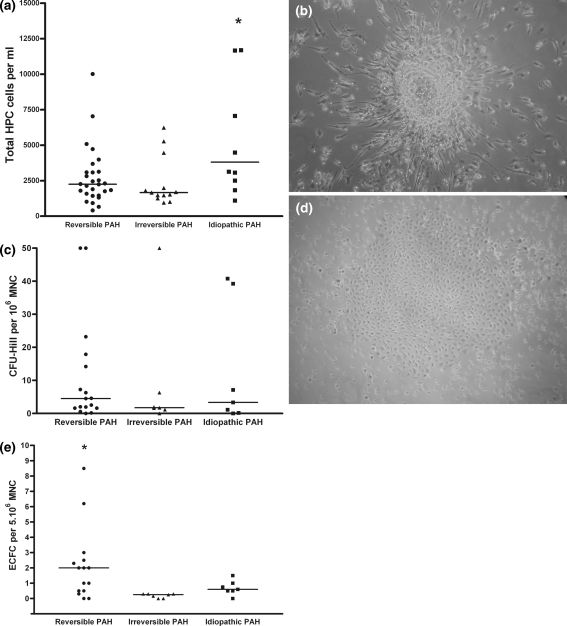
Fig. 1.
Basal HPC, CFU-Hill and ECFC counts in the children with PAH. a NUMBER of CD34+ hematopoietic progenitor cells (HPC) determined by FACS analysis. HPC is significantly higher in idiopathic PAH group. b Representative phase-contrast photomicrograph of CFU-Hill colony obtained with Endocult®. Note the central core of rounded cells with spindle-shaped cells sprouting through the periphery. c Number of CFU-Hill counted with cell culture Endocult® assay. No difference was observed between the different patient groups. d Representative phase-contrast photomicrograph of an ECFC colony (mag. ×20). e Number of ECFC by cell culture. ECFC is significantly higher in the reversible PAH group
ECFC proliferation assay
After 16 h of serum and growth-factor privation, ECFC were incubated for 48 h in EBM medium, 5% FBS. Proliferation was examined after 48 h by measuring cell phosphatase activity based on the release of paranitrophenol (pNPP) (Sigma) measured at OD 405 nm (Fluostar optima; BMG labtech, Champigny-sur-Marne, France). Control cells were ECFC from cord blood [6, 25–27].
In vitro matrigel tube formation assay
After 16 h of serum and growth-factor privation, ECFC (3 × 104 cells/well) were seeded on growth factor-reduced Matrigel (200 μl) (BD Biosciences) in EBM2 medium and cultured for 18 h at 37°C with 5% CO2. Capillary-like structures were observed by phase-contrast microscopy and networks formed by ECFC were quantified with Videomet software version 5.4.0.
Wound healing assay
A reproducible circular “wound” was made with a tip in confluent monolayers of ECFC cultured in 12-well plates. The surface area of the wound was measured at ×20 magnification under an inverted microscope, then the medium was removed and the cells were further incubated for 4 or 18 h with EBM, 5% FBS and VEGF 50 ng/ml. Wound repair activity was calculated by expressing the area of the wound after 4 or 18 h of incubation as a percentage of the initial area.
ECFC angiogenic potential in a mouse model of hind-limb ischemia
Nude mice underwent surgery to induce unilateral hindlimb ischemia as described elsewhere [6, 28] and were randomized to receive an intravenous injection of 1 × 105 ECFC derived from the two treated patient groups or 1 × 105 ECFC derived from cord blood. Two independent groups of 20 mice were realized with, in each experiment, 5 mice injected with PBS, 5 mice with cord blood ECFC, 5 mice with ECFC from patients with oral therapy and 5 mice with ECFC from patients with SC-treprostinil. After 2 weeks, the ischemic/normal limb blood flow ratio was determined with a laser Doppler perfusion imaging system.
Statistical analysis
Baseline characteristics were compared between the groups by using Wilcoxon’s rank sum test for non normally distributed variables and Student’s unpaired test otherwise. Student’s paired t test was used to compare progenitor cells from the same subjects before and after vasodilator treatment. Independent-samples t tests were used to compare late EPC functional capacity in vitro and in mice. StatView or SAS statistical software (Cary, NC 27513, USA) was used for all statistical analyses, and 2-tailed P values below 0.05 were considered to denote significant differences.
Results
HPC, CFU-Hill and ECFC counts before treatment
We found no difference in HPC counts between patients with reversible and irreversible PAH secondary to CHD [29], while HPC counts were significantly higher in patients with idiopathic PAH (Fig. 1a) (P = 0.02 vs. patients with reversible PAH and 0.01 vs. patients with irreversible PAH) in agreement with the data published by Diller et al. [10]. CFU-Hill counts after 5 days of culture did not differ across the three patient groups (Fig. 1c). By contrast, ECFC counts were significantly higher in reversible PAH than in irreversible and idiopathic PAH (Fig. 1e).
ECFC numbers are increased by prostanoid treatment
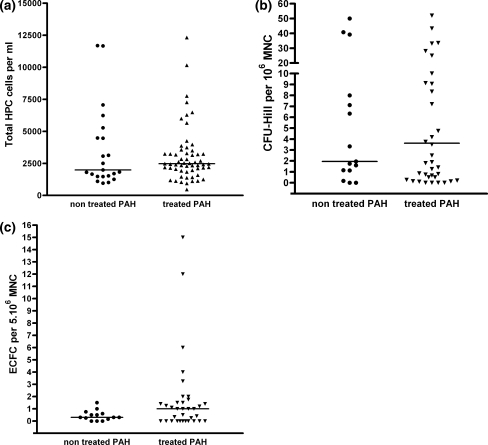
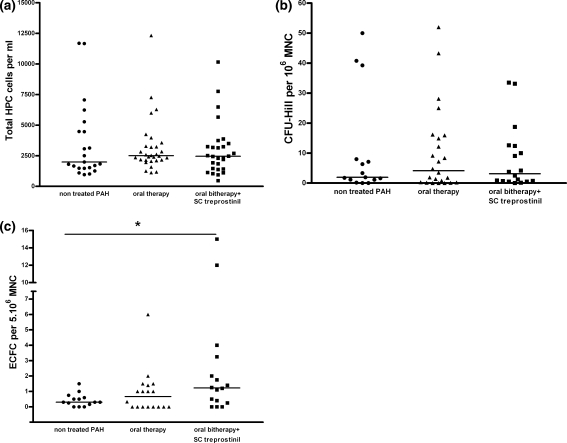
No significant change in HPC, CFU-Hill and ECFC were observed when all treated patients versus non treated patients were compared (Fig. 2a, b, c respectively, with P = 0.80, 0.89 and 0.15). Oral treatment (monotherapy or bitherapy) did not modify HPC, CFU-Hill and ECFC counts count (respectively, P = 0.52, 0.64 and 0.22, Fig. 3). No change in HPC or CFU-Hill counts (P = 0.76 and P = 0.19, respectively, Fig. 3a, b) were observed after prostanoid therapy (SC treprostinil), while in contrast, this led to a significant increase in ECFC counts (P = 0.04, Fig. 3c).
Fig. 2.
HPC, CFU-Hill and ECFC counts in peripheral venous blood of patients with pulmonary hypertension, with and without treatment. a Number of CD34+ hematopoietic progenitor cells (HPC) determined by FACS analysis according to patient group. No difference is observed between the treated and non treated patients (P = 0.80). b Number of CFU-Hill colonies determined by cell culture according to patient group. No difference is observed between the treated and non treated patients (P = 0.89). c Number of ECFC determined by cell culture according to patient group. No difference is observed between the treated and non treated patients (P = 0.15)
Fig. 3.
HPC, CFU-Hill and ECFC counts in peripheral venous blood of patients with pulmonary hypertension according to treatment subtype. a No difference is observed in HPC according to treatment subtype (oral mono and/or bitherapy with or without subcutaneous treprostinil with respectively, a P = 0.52 and 0.76 vs. not treated PAH). b No difference is observed in CFU-Hill colonies determined by cell culture according to treatment subtype (oral mono and/or bitherapy with or without subcutaneous treprostinil with respectively, a P = 0.64 and 0.19 vs. not treated PAH). c No difference is observed in ECFC determined by cell culture after oral mono and/or bitherapy (P = 0.22) while a significant increase in ECFC was observed with subcutaneous treprostinil treatment (* P = 0.04)
Treprostinil enhances the proangiogenic potential of ECFC
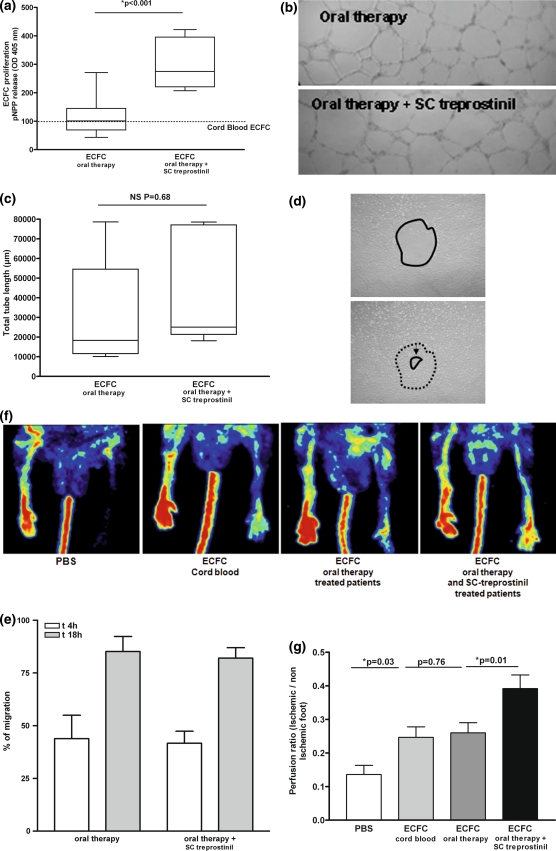
ECFC from patients receiving oral therapy, with or without SC treprostinil, were grown to confluence from single colonies. They retained the characteristic cobblestone aspect through numerous passages and stained positive for typical endothelial markers CD34, CD146, CD144, PAR-1 thrombin receptor and von Willebrand factor (data not shown). During the first 30 days of culture, cells cultured from patients receiving oral therapy plus SC treprostinil showed enhanced proliferation in EBM, 5% FBS compared to cells from patients receiving only oral therapy (Fig. 4a). In vitro vascular network formation in Matrigel was similar with ECFC from patients treated with or without treprostinil (Fig. 4b, c), as was VEGF-induced migration in the wound healing assay (Fig. 4d, e).
Fig. 4.
Angiogenic potential of ECFC isolated from patients with pulmonary hypertension receiving vasodilator treatment. Each patient-derived ECFC were individually analyzed for proliferation and angiogenic properties in vitro and in vivo. At least 4 different patients derived-ECFC were used in each case. a Cells from patients receiving oral therapy and SC treprostinil showed an increased proliferative potential (in EBM, 5% FBS) compared with those from patients receiving oral therapy. Results are presented normalized to ECFC from cord blood (100%). The mean and SEM of three experiments are shown P < 0.05. b, c ECFC isolated from patients receiving oral therapy, with or without SC treprostinil, form intact vascular networks with similar efficiency. The mean and SEM of three experiments are shown. d, e VEGF-stimulated migration of ECFC in vitro in a wound healing assay was similar in patients receiving oral therapy with or without SC treprostinil. The mean and SEM of three experiments are shown. f, g ECFC from patients receiving oral therapy, with or without SC treprostinil, were injected intravenously into nude mice having undergone femoral artery ligature. Injection of ECFC from patients receiving oral therapy only improved foot perfusion on day 14 to the same extent as control ECFC isolated from cord blood. ECFC from patients receiving oral therapy plus SC treprostinil improved foot perfusion on day 14 by 25% more than ECFC from patients receiving oral therapy alone (P = 0.012) and ECFC isolated from cord blood (P = 0.019)
In the preclinical model of hindlimb ischemia, 1 × 105 ECFC from each treatment group were injected intravenously into nude mice with femoral artery ligature. Injection of ECFC from patients on oral therapy or from cord blood improved foot perfusion to a similar extent on day 14, while ECFC from treprostinil-treated patients improved foot perfusion on day 14 compared to ECFC from patients on oral therapy without SC treprostinil (P = 0.012) and ECFC from cord blood (P = 0.019, Fig. 4f, g).
Discussion
Subcutaneous treprostinil markedly enhanced the number and functional capacity of ECFC isolated from children with severe PAH. As these cells are involved in angiogenesis and endothelial repair, this finding provides important insights into the mechanism of action of prostacyclin therapy in this setting.
The endothelium plays a central role in pulmonary vascular regulation, and endothelial dysfunction is increasingly viewed as critical for disease initiation and progression [3, 30]. We suspected that pharmacological treatment efficacy could be due, at least in part, to the endothelial repair capacity of ECFC. Irreversible and idiopathic PAH are associated with vascular remodeling and with smooth muscle and endothelial cell proliferation. Plexiform lesions have a similar histological aspect in idiopathic and irreversible PAH. We recently observed neoangiogenesis in lung surgical biopsy samples from patients with irreversible PAH due to CHD. This was associated to a proliferative endothelial phenotype with resistance to apoptosis [4]. These findings are consistent with a compensatory adaptive response to increased pulmonary blood flow and arterial pressure [31, 32], in which ECFC are likely to play an important role. Standard methods used to assess endothelial function in the pulmonary circulation are invasive and complex [33], but recently developed ex vivo evaluations of endothelial biology have the potential to provide important insights [34].
In the past 20 years, pulmonary “vasodilator” therapy has greatly improved the prognosis of patients with PAH [19, 20], it is now clear that these agents do more than simply dilate pulmonary arterioles. Indeed, such treatments have been found to enhance revascularization and/or EPC recruitment in preclinical studies [35–37] and more recently in patients with critical limb ischemia [38]. Although phosphodiesterase 5 (PDE5) inhibitors [39–41] and endothelin receptor antagonists (ERA) [42–44] improve hemodynamic parameters, they have not been shown to significantly reduce mortality of PAH patients, contrary to prostanoids.
This difference between prostanoid, PDE5 inhibitors and ERA therapy in terms of mortality could result, at least in part, from a prostanoid-induced enhancement of EPC numbers and functional capacity, leading to improved vascular repair and/or new vessel formation. Two clinical studies recently showed that transplantation of angiogenic cells improved exercise capacity and pulmonary hemodynamics in adults and children with idiopathic pulmonary hypertension [17, 18]. In patients with critical leg ischemia, we recently showed that bone marrow mononuclear cell therapy induced the formation of new vessels containing endothelial cells with a ECFC phenotype [6]. Moreover, Yoder et al. [7] have shown that transplanted ECFC acquire a complete endothelial phenotype, maintain a high proliferative potential, and participate in endothelial healing and angiogenesis. In this study, we showed an increase of foot perfusion in the preclinical model of hindlimb ischemia of ECFC isolated from patients treated with treprostinil. Since human cells are hardly detectable in the muscle vasculature, we cannot exclude a paracrine effect of ECFC isolated from patients receiving treprostinil. Indeed, ECFC have been described to secrete several angiogenic pathways that modulate their ability to increase foot perfusion in this preclinical model [27].
The main result of our study is that prostanoid therapy (contrary to PDE5 inhibitors and ERA therapy) increased the numbers and proliferative capacity of ECFC. ECFC have been shown to possess all the characteristics of true endothelial progenitors, based on the clonal relation between EPC and hematopoietic stem cells in patients with myeloproliferative disorders. Indeed, ECFC lack disease markers expressed by early EPC (CFU-Hill or CFU-EC), supporting the concept that CFU-Hill belong to the hematopoietic lineage. This suggests that, in patients with chronic myeloproliferative disorders, ECFC have an origin distinct from that of the hematopoietic malignant clone [7, 45], and probably have true vasculogenic potential [7]. Here we explored ECFC in prostanoid-treated and -untreated patients with PAH, a well-characterized vascular disorder, and found that only ECFC were modified by prostanoid treatment, the only therapy shown to reduce mortality among adults and children with PAH [46–49]. Our results confirm importance of ECFC in PAH. Indeed, Toshner et al. [22] describe in patients with PAH and BMPRII mutations that ECFC had a hyperproliferative phenotype and an impaired capacity to form vascular networks, despite an absence of difference in ECFC numbers.
In the present study, CFU-Hill numbers did not differ between the three groups of patients, and did not change during treatment. Results of early-EPC or CFU-Hill counts in PAH are controversial. Asosingh et al. [8] found significantly increased numbers of early EPC in PAH patients compared with controls. In contrast, Junhui et al. [9] found reduced numbers of early EPC, with functional defects, in idiopathic PAH patients compared with controls, a finding confirmed by Diller et al. [10].
One limitation of our study is that we did not study the functional status of ECFC from healthy children. In addition, we did not attempt to confirm our findings with another prostanoid, such as IV prostacyclin, that has also been shown to improve survival in this setting.
The higher counts and enhanced angiogenic properties of ECFC in children treated with treprostinil indicate that these cells could contribute to the compensatory adaptive response to increased pulmonary blood flow and/or pressure. It is thus tempting to speculate that ECFC expanded ex vivo might be beneficial in pediatric PAH, especially given the higher counts and functional capacities of ECFC in children compared with adults. Indeed, despite the lack of data on normal ECFC values in children, we found that the ECFC yield in culture for the two older groups of children (with idiopathic and irreversible PAH) was similar to that found in adults [0.2 and 0.6 colonies per 5 × 106 MNC, respectively, in irreversible and idiopathic PAH, vs 2.0 in reversible PAH and 0.3 in healthy adults (D. Smadja, personal data)]. These results are in line with those of Yoder’s group, who observed a hierarchy of proliferative potential between cord blood and adult blood. This result can reasonably be extrapolated to children less than 5 years old, as was the case of our patients with reversible PAH (median age 2 years).
In conclusion, this study suggests that prostanoids enhance the number and proliferative capacities of ECFC in children with pulmonary hypertension, an effect that may contribute to endothelial repair and/or new vessel formation and, thus, to the observed clinical benefits. The potential interest of ECFC count as a surrogate marker of prostanoid treatment efficacy is currently being investigated.
Acknowledgments
We thank Isabelle Zezepanski, Blandine Dizier, Sébastien Bertil, Florence Desvard, Yolande Daigneau, Florence Dao, Yann Burnel and Evelyne Galtier for their excellent technical assistance. This work was supported by research grants from the Leducq TransAtlantic Network of Excellence on Atherothrombosis Research (Grant 04CVD01), Leg Poix (Paris, France) and ARCFA (association pour la recherche en cardiologie du foetus à l’adulte).
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, Zadigue P, Dartevelle P, Simonneau G, Adnot S, Maitre B, Raffestin B, Eddahibi S. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. 2009;119:512–523. doi: 10.1172/JCI35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M, Montani D, Perros F, Dorfmuller P, Adnot S, Eddahibi S. Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vascul Pharmacol. 2008;49:113–118. doi: 10.1016/j.vph.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Levy M, Maurey C, Celermajer DS, Vouhe PR, Danel C, Bonnet D, Israel-Biet D. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol. 2007;49:803–810. doi: 10.1016/j.jacc.2006.09.049. [DOI] [PubMed] [Google Scholar]
- 5.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Smadja DM, Bieche I, Silvestre JS, Germain S, Cornet A, Laurendeau I, Duong-Van-Huyen JP, Emmerich J, Vidaud M, Aiach M, Gaussem P. Bone morphogenetic proteins 2 and 4 are selectively expressed by late outgrowth endothelial progenitor cells and promote neoangiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:2137–2143. doi: 10.1161/ATVBAHA.108.168815. [DOI] [PubMed] [Google Scholar]
- 7.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C, Comhair SA, Xu W, Licina L, Huang L, Anand-Apte B, Yoder MC, Tuder RM, Erzurum SC. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am J Pathol. 2008;172:615–627. doi: 10.2353/ajpath.2008.070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junhui Z, Xingxiang W, Guosheng F, Yunpeng S, Furong Z, Junzhu C. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir Med. 2008;102:1073–1079. doi: 10.1016/j.rmed.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Diller GP, van Eijl S, Okonko DO, Howard LS, Ali O, Thum T, Wort SJ, Bedard E, Gibbs JS, Bauersachs J, Hobbs AJ, Wilkins MR, Gatzoulis MA, Wharton J. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008;117:3020–3030. doi: 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- 11.Smadja DM, Mauge L, Sanchez O, Silvestre JS, Guerin C, Godier A, Henno P, Gaussem P, Israel-Biet D (2010) Distinct patterns of circulating endothelial cells in pulmonary hypertension. Eur Respir J (in press) [DOI] [PubMed]
- 12.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 13.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 14.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 15.Nagaya N, Kangawa K, Kanda M, Uematsu M, Horio T, Fukuyama N, Hino J, Harada-Shiba M, Okumura H, Tabata Y, Mochizuki N, Chiba Y, Nishioka K, Miyatake K, Asahara T, Hara H, Mori H. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation. 2003;108:889–895. doi: 10.1161/01.CIR.0000079161.56080.22. [DOI] [PubMed] [Google Scholar]
- 16.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96:442–450. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 17.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 18.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. 2008;12:650–655. doi: 10.1111/j.1399-3046.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 19.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–1263. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 20.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, McGregor K, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas P, Widimsky P, Sechtem U, Al Attar N, Andreotti F, Aschermann M, Asteggiano R, Benza R, Berger R, Bonnet D, Delcroix M, Howard L, Kitsiou AN, Lang I, Maggioni A, Nielsen-Kudsk JE, Park M, Perrone-Filardi P, Price S, Domenech MT, Vonk-Noordegraaf A, Zamorano JL. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 21.Dewachter L, Adnot S, Guignabert C, Tu L, Marcos E, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Eddahibi S. Bone morphogenetic protein signalling in heritable versus idiopathic pulmonary hypertension. Eur Respir J. 2009;34:1100–1110. doi: 10.1183/09031936.00183008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, Yang J, Suntharalingam J, Soon E, Exley A, Stewart S, Hecker M, Zhu Z, Gehling U, Seeger W, Pepke-Zaba J, Morrell NW. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res. 2006;98:209–217. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 24.Smadja DM, Bieche I, Susen S, Mauge L, Laurendeau I, d’Audigier C, Grelac F, Emmerich J, Aiach M, Gaussem P. Interleukin 8 is differently expressed and modulated by PAR-1 activation in early and late endothelial progenitor cells. J Cell Mol Med. 2009;13:2534–2546. doi: 10.1111/j.1582-4934.2008.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smadja DM, Bieche I, Helley D, Laurendeau I, Simonin G, Muller L, Aiach M, Gaussem P. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with up-regulation of integrin alpha(6) J Cell Mol Med. 2007;11:1149–1161. doi: 10.1111/j.1582-4934.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smadja DM, Bieche I, Uzan G, Bompais H, Muller L, Boisson-Vidal C, Vidaud M, Aiach M, Gaussem P. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arterioscler Thromb Vasc Biol. 2005;25:2321–2327. doi: 10.1161/01.ATV.0000184762.63888.bd. [DOI] [PubMed] [Google Scholar]
- 27.Smadja DM, d’Audigier C, Weiswald LB, Badoual C, Dangles-Marie V, Mauge L, Evrard S, Laurendeau I, Lallemand F, Germain S, Grelac F, Dizier B, Vidaud M, Bieche I, Gaussem P (2010) The Wnt antagonist Dickkopf-1 increases endothelial progenitor cell angiogenic potential. Arterioscler Thromb Vasc Biol (in press) [DOI] [PubMed]
- 28.Foubert P, Silvestre JS, Souttou B, Barateau V, Martin C, Ebrahimian TG, Lere-Dean C, Contreres JO, Sulpice E, Levy BI, Plouet J, Tobelem G, Le Ricousse-Roussanne S. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117:1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smadja DM, Gaussem P, Mauge L, Israel-Biet D, Dignat-George F, Peyrard S, Agnoletti G, Vouhe PR, Bonnet D, Levy M. Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation. 2009;119:374–381. doi: 10.1161/CIRCULATIONAHA.108.808246. [DOI] [PubMed] [Google Scholar]
- 30.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Lam CF, Peterson TE, Croatt AJ, Nath KA, Katusic ZS. Functional adaptation and remodeling of pulmonary artery in flow-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2005;289:H2334–H2341. doi: 10.1152/ajpheart.00375.2005. [DOI] [PubMed] [Google Scholar]
- 32.Black SM, Fineman JR, Steinhorn RH, Bristow J, Soifer SJ. Increased endothelial NOS in lambs with increased pulmonary blood flow and pulmonary hypertension. Am J Physiol. 1998;275:H1643–H1651. doi: 10.1152/ajpheart.1998.275.5.H1643. [DOI] [PubMed] [Google Scholar]
- 33.Celermajer DS, Cullen S, Deanfield JE. Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation. 1993;87:440–446. doi: 10.1161/01.cir.87.2.440. [DOI] [PubMed] [Google Scholar]
- 34.Fadini GP, Avogaro A, Ferraccioli G, Agostini C (2010) Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J 35:418–425 [DOI] [PubMed]
- 35.Dussault S, Maingrette F, Menard C, Michaud SE, Haddad P, Groleau J, Turgeon J, Perez G, Rivard A. Sildenafil increases endothelial progenitor cell function and improves ischemia-induced neovascularization in hypercholesterolemic apolipoprotein E-deficient mice. Hypertension. 2009;54:1043–1049. doi: 10.1161/HYPERTENSIONAHA.109.139451. [DOI] [PubMed] [Google Scholar]
- 36.Iglarz M, Silvestre JS, Duriez M, Henrion D, Levy BI. Chronic blockade of endothelin receptors improves ischemia-induced angiogenesis in rat hindlimbs through activation of vascular endothelial growth factor-no pathway. Arterioscler Thromb Vasc Biol. 2001;21:1598–1603. doi: 10.1161/hq1001.097065. [DOI] [PubMed] [Google Scholar]
- 37.Sun CK, Lee FY, Sheu JJ, Yuen CM, Chua S, Chung SY, Chai HT, Chen YT, Kao YH, Chang LT, Yip HK. Early combined treatment with cilostazol and bone marrow-derived endothelial progenitor cells markedly attenuates pulmonary arterial hypertension in rats. J Pharmacol Exp Ther. 2009;330:718–726. doi: 10.1124/jpet.109.154328. [DOI] [PubMed] [Google Scholar]
- 38.Di Stefano R, Barsotti MC, Melillo E, Iorio M, Santoni T, Armani C, Dell’omodarme M, Ristori C, De Caterina R, Balbarini A. The prostacyclin analogue iloprost increases circulating endothelial progenitor cells in patients with critical limb ischemia. Thromb Haemost. 2008;100:871–877. [PubMed] [Google Scholar]
- 39.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 40.Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 41.Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch DB. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 42.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 43.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 44.Humbert M, Barst RJ, Robbins IM, Channick RN, Galie N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24:353–359. doi: 10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 45.Piaggio G, Rosti V, Corselli M, Bertolotti F, Bergamaschi G, Pozzi S, Imperiale D, Chiavarina B, Bonetti E, Novara F, Sessarego M, Villani L, Garuti A, Massa A, Ghio R, Campanelli R, Bacigalupo A, Pecci A, Viarengo G, Zuffardi O, Frassoni F, Barosi G. Endothelial colony forming cells (ECFCs) from patients with chronic myeloproliferative disorders lack the disease-specific molecular clonality marker. Blood. 2009;14:3127–3130. doi: 10.1182/blood-2008-12-190991. [DOI] [PubMed] [Google Scholar]
- 46.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The primary pulmonary hypertension study group. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 47.Galie N, Humbert M, Vachiery JL, Vizza CD, Kneussl M, Manes A, Sitbon O, Torbicki A, Delcroix M, Naeije R, Hoeper M, Chaouat A, Morand S, Besse B, Simonneau G. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. 2002;39:1496–1502. doi: 10.1016/S0735-1097(02)01786-2. [DOI] [PubMed] [Google Scholar]
- 48.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 49.Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]