Abstract
Radiation therapy is a potentially curative, important treatment option in localized prostate cancer. However, at 8 years after radiation therapy, even in the best risk subset of patients, approximately 10% of patients will experience clinical disease recurrence. The identification of molecular markers of treatment success or failure may allow for the development of strategies to further improve treatment outcomes. Herein, we investigated five molecular markers of DNA repair. 513 patients with castrate-resistant prostate cancer (CRPC), including 284 patients who received radiotherapy, 229 patients without radiotherapy and 152 healthy individuals were genotyped for five polymorphisms in DNA excision repair genes: ERCC1 N118N (500C>T), XPD K751Q (2282A>C), XRCC1 R194W (685C>T), XRCC1 R399Q (1301G>A) and PARP1 V762A (2446T>C). The distribution of genetic polymorphisms in the patients with CRPC and in healthy controls was compared, and the association between the polymorphisms and overall survival was investigated. The polymorphisms evaluated did not show differences between the patient group and the healthy controls, nor did they show a trend toward an association with survival. However, in the radiation treated subgroup, the median survival time was associated with the XRCC1 haplotype. The median survival time was 11.75 years for patients with the R399Q AA /R194W CC haplotype, 12.17 years for patients with the R399Q AG/R194W CC haplotype, 6.665 years for patients with the R399Q AG/R194W CT haplotype, and 6.21 years for patients with the R399Q GG/R194W CT haplotype (p = 0.034). This association was not found when all patients were investigated. We conclude that the genetic polymorphisms in XRCC1 may affect the outcome in patients who received radiotherapy for localized prostate cancer.
Key words: DNA excision repair, BER, XRCC1, prostate cancer, radiotherapy, genetic polymorphism, haplotype
Introduction
Radiation therapy is an important treatment option for patients with localized, early stage prostate cancer. In patients with T1 to T3 lesions, without nodal or distant metastases, similar clinical results are obtained through surgery (radical prostatectomy) or radiation therapy. Radiation therapy can be delivered by any of several approaches: external beam, brachytherapy, and intensity modulated radiation therapy (IMRT). However, with surgery or with radiation therapy, a percentage of patients with well-documented localized disease will experience the return of their malignancy.
In patients with low risk localized prostate cancer, treated with modern IMRT, actuarial PSA relapse-free survival is 85–89%. In unfavorable risk localized prostate cancer, the actuarial PSA relapse-free survival is 59–72%.1 Therefore, even in the group of patients with the best clinical features and the most favorable prognosis, 11–15% of these patients have intra-tumor characteristics that lead to relapse of disease. One question is whether there are intra-tumor considerations for DNA repair pathways that may make some prostate cancer cells more resistant to radiation therapy, and therefore make those tumors more likely to clinically recur.
Though considerable inter-patient differences in response to radiotherapy occur, the mechanisms behind these different responses are not well understood. A variety of patient, tumor, treatment and molecular factors contribute to the various outcome of radiotherapy. The understanding of this mechanism may increase the predictability of outcome and selection of the optimal treatment. The work published by the Radiation Therapy Oncology Group (RTOG) investigated a total of 11 potential prognostic markers, and only p53 and DNA ploidy showed association with overall survival.2 Since ionizing radiation acts through creating various types of DNA damage, the inter-individual radiosensitivity may influence the patient's response to such therapy. The genetic polymorphisms in DNA repair genes were believed to serve as the genetic basis for such inter-individual differences. It was also found that the genetic polymorphisms in DNA repair genes were differently distributed in ethnic groups and might contribute to the ethnic disparity of sensitivity to DNA-damaging chemotherapy.3
The types of DNA damage induced by radiation include DNA base damage and both single- and double-strand DNA breaks.4 Such lesions, if inadequately repaired, can lead to cell death by lethal chromosomal aberrations or apoptosis, the desired outcome of radiation therapy. Multiple DNA repair pathways are involved to maintain the genomic integrity, and the homologous recombination (HR) and non-homologous end-joining (NHEJ), nucleotide excision repair (NER) and base excision repair (BER) pathways are thought to contribute heavily to remove the damage caused by ionizing radiation.4,5
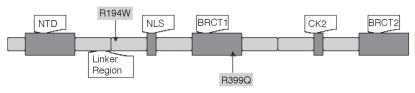
XRCC1 was the first human gene cloned in the BER pathway, and cells lacking this gene product are hypersensitive to ionizing radiation.6 XRCC1 works as a stimulator and scaffold protein for other enzymes involved in this pathway. Polymorphisms have been previously identified in XRCC1 that correlate with phenotypic changes.7 One important polymorphism in XRCC1 is R194W, located in the linker region separating the NH2-terminal domain (NTD) from the central BRCT1 (BRCA1 C-terminus) domain, as illustrated in Figure 1. The linker region was also suggested to be a potential binding domain of several interactive proteins, and is rich in basic amino acids. The substitution of arginine to hydrophobic tryptophan may affect the protein binding efficiency. According to a review by Goode et al.8 the R194W polymorphism was related to reduced risk to cancer, and this was confirmed by two later association studies.9,10 However, another study showed a highly significant association (p = 0.0005) of R194W with the increased risk of head and neck cancer in a Korean population.11 The possible reasons for these confounding results include that the epidemiological studies could be misleading and this polymorphism might not directly associate, but link to another relevant polymorphism to form haplotypes.7 The second XRCC1 polymorphism, R399Q, is a well-studied single nucleotide polymorphism (SNP) located in the BRCT1 domain, which is essential for PARP1 binding. Cells carrying this mutation have been shown to be defective in responding to both X-ray radiation and UV light.12 Studies correlated the polymorphisms in XRCC1 with either adverse effects13 or protective effects resulting from radiotherapy,14,15 or favorable response to therapeutic radiation16–18 in several cancers.
Figure 1.
Structure of XRCC1 domains and locations of the single nucleotide polymorphisms (SNPs) genotyped in this study. NTD, N-terminal domain; NLS, nuclear localization signal domain; BRCT, BRCA C-terminus domain; CK2, Ck2 phosphorylation sites, modified from.7
PARP1, another important gene in DNA repair, assists by recruiting XRCC1 after sensing DNA damage. The variation, V762A in PARP1, causes the loss of two methyl groups that in turn increases the distance between 762 and its closest neighbor in the active site. This steric change looses the binding of NAD+ and reduces the enzymatic activity nearly two fold.19 As a consequence, the variant enzyme may be less able to sense the damage in DNA and reduce the recruitment of XRCC1 and other proteins involved in the repair process. Since PARP1 also plays an important role in repairing radiation inflicted lesions, several PARP1 inhibitors have been tested in clinical trials to try to increase the effectiveness of ionizing radiation in the treatment of cancer.20–22
In addition to BER, the NER pathway also plays a role in removing multiple types of DNA damage, including those caused by UV light and platinum-containing chemotherapy agents. Important genes in the NER, ERCC1 and XPF, are essential for the 5′ incision into the DNA strand that releases bulky DNA lesions.23,24 XPD is a 5′-3′ helicase that participates in DNA strand separation prior to the 5′ incision step performed by the ERCC1-XPF heterodimer.25
The aim of this study is to investigate the genetic polymorphisms in the DNA repair pathways that are involved in repairing radiation induced DNA damage, and will focus on the NER and BER pathways.
Results
Five hundred and thirteen patients with CRPC were assayed for five single nucleotide polymorphisms (SNPs): ERCC1 N118N (500C>T), XPD K751Q (2282A>C), XRCC1 R399Q (1301G>A), XRCC1 R194W (685C>T), and PARP1 V762A (2446T>C). The distribution of these SNPs among the 513 patients studied was compared to the 152 healthy volunteer controls. Statistical analyses of the genotype prevalence for all five polymorphisms revealed no evidence of any differences between the two groups (Table 1). All of the genotype distributions were in Hardy-Weinberg equilibrium in both cases and controls.
Table 1.
Distribution of polymorphisms among healthy controls and patients
| SNP | Genotype | Control* | Patients | OR | 95% CI | p value |
| ERCC1 | CC | 23 (0.21) | 91 (0.21) | Referent | - | - |
| N118N | CT | 53 (0.49) | 197 (0.46) | 0.940 | 0.5426–1.627 | 0.8899 |
| (500C>T) | TT | 32 (0.30) | 143 (0.33) | 1.129 | 0.6218–2.052 | 0.7595 |
| XPD | AA | 49 (0.42) | 186 (0.43) | Referent | - | - |
| K751Q | AC | 56 (0.47) | 178 (0.42) | 0.837 | 0.5419–1.294 | 0.4399 |
| (2282A > C) | CC | 13 (0.11) | 64 (0.15) | 1.297 | 0.6608–2.546 | 0.5129 |
| XRCC1 | CC | 120 (0.87) | 402 (0.89) | Referent | - | - |
| R194W | CT | 17 (0.12) | 43 (0.09) | 0.755 | 0.4154–1.372 | 0.3399 |
| (685C>T) | TT | 1 (0.01) | 7 (0.02) | 2.090 | 0.2544–17.16 | 0.6893 |
| XRCC1 | GG | 49 (0.46) | 145 (0.41) | Referent | - | - |
| R399Q | AG | 47 (0.44) | 151 (0.43) | 1.086 | 0.6850–1.721 | 0.8144 |
| (1301G>A) | AA | 10 (0.10) | 56 (0.16) | 1.892 | 0.8967–3.994 | 0.1248 |
| PARP1 | TT | 80 (0.67) | 315 (0.70) | Referent | - | - |
| V762A | CT | 32 (0.27) | 123 (0.27) | 0.976 | 0.6163–1.546 | 0.9068 |
| (2446T>C) | CC | 7 (0.06) | 15 (0.03) | 0.544 | 0.2147–1.380 | 0.1873 |
OR, odds ratio; CI, exact confidence interval.
Values are number (percentage).
We determined whether the polymorphisms were associated with overall survival using the univariate method. None of the polymorphisms evaluated showed a trend toward an association with survival individually. The results are shown in Table 2.
Table 2.
Median survival, and two-tailed log-rank test p values
| SNP | Genotype | Median survival (years) | Median survival radiation group (years) | Median survival non-radiation group (years) |
| ERCC1 | CC | 8.21 | 9.72 | 6.915 |
| N118N | CT | 7.84 | 10.35 | 4.781 |
| (500C>T) | TT | 8.33 | 8.86 | 6.381 |
| p value | 0.7622 | 0.9649 | 0.4028 | |
| XPD | AA | 8.13 | 8.86 | 6.7 |
| K751Q | AC | 8.21 | 10.33 | 5.32 |
| (2282A>C) | CC | 7.155 | 9.22 | 4.15 |
| p value | 0.9925 | 0.9325 | 0.6019 | |
| XRCC1 | GG | 8.17 | 9.22 | 5.88 |
| R399Q | AG | 7.77 | 10.41 | 5.41 |
| (1301G>A) | AA | 11.12 | 11.75 | 8.305 |
| p value | 0.5256 | 0.8456 | 0.6261 | |
| XRCC1 | CC | 8.06 | 9.66 | 5.88 |
| R194W | CT | 6.52 | 6.81 | 4.24 |
| (685C>T) | TT | 9.22 | 9.22 | 10.595 |
| p value | 0.5493 | 0.3361 | 0.8515 | |
| PARP1 | TT | 8.17 | 9.55 | 5.9 |
| V762A | CT | 7.69 | 8.82 | 4.985 |
| (2446T>C) | CC | 5.88 | 11.675 | 3.9 |
| p value | 0.8469 | 0.6805 | 0.0949 |
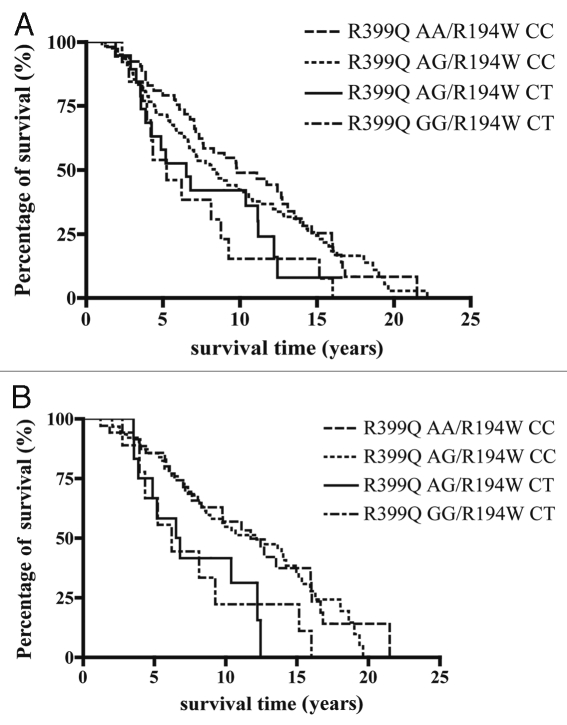
By comparing the individual median survival time in each genotype group, it was noted that the variant genotype (AA) of XRCC1 R399Q had the longest survival time (11.12 years), while the patients having the XRCC1 R194W heterozygous genotype CT had the shortest median survival time (6.52 years). Interestingly, in the group of patients who received radiotherapy as their treatment for the localized prostate cancer, the individuals with the XRCC1 R399Q AA or AG genotypes had median survival times as long as 10 years, while the individuals with the XRCC1 R194W CT genotype only had the median survival time of 6.81 years. Thus we investigated the intragenic association of the two polymorphisms with the overall survival. Patients having either the R399Q AA or AG genotypes and patients having the R194W CT genotype were included in this investigation and four haplotypes were found: R399Q AA/R194W CC, R399Q AG/R194W CC, R399Q AG/R914W CT and R399Q GG/R194W CT. It was noted that the XRCC1 R399Q AA genotype and the R194W CT genotype tend to be mutually exclusive. There is only one patient with this haplotype, and this patient is still living. When all patients were investigated, the median survival time was 9.81 years for the 53 patients with the R399Q AA/R194W CC genotype, 8.39 years for the 124 patients with the R399Q AG/R194W CC genotype, 6.52 years for the 19 patients with the R399Q AG/R194W CT genotype and 5.26 years for the 13 patients with the R399Q GG/R194W CT genotype, with a global two-tailed p-value of 0.14. The probability of survival over time is shown in Figure 2A. However, in the radiation treated subgroup, the median survival time showed an association with the XRCC1 haplotypes. The median survival time was 11.75 years for the 35 patients with the R399Q AA/R194W CC genotype, 12.17 years for the 63 patients with the R399Q AG/R194W CC genotype, 6.665 years for the 12 patients with the R399Q AG/R194W CT genotype and 6.21 years for the 9 patients with the R399Q GG/R194W CT genotype (p = 0.034). The probability of overall survival over time is depicted for these patients in Figure 2B. These results suggest that the haplotype of the BER gene XRCC1 serve as a prognostic marker for radiotherapy in prostate cancer.
Figure 2.
Kaplan-Meier overall survival curves in patients with CRPC according to XRCC1 R194W (685C>T) and R399Q (1301G>A) haplotypes. The duration of survival was computed from the date of prostate cancer diagnosis until the date of death or last follow-up. P values are adjusted for haplotype analysis. (A) All patients are divided into four haplotype groups, and the median survival time 9.81 years for R399Q AA /R194W CC (n=53), 8.39 years for R399Q AG/R194W CC (n=124), 6.52 years for R399Q AG/R194W CT (n=19) and 5.26 years for R399Q GG/R194W CT genotype (n=13), p= 0.14. (B) Patients who received radiotherapy are grouped into the same four subsets according to their XRCC1 haplotype. The median survival time was 11.75 years for R399Q AA /R194W CC (n=35), 12.17 years for R399Q AG/R194W CC genotype (n=63), 6.665 years for R399Q AG/R194W CT (n=12) and 6.21 years for R399Q GG/R194W CT (n=9), p=0.034.
In the NCI-60 cell line screening, the genotypes of the 5 SNPs: ERCC1 N118N (500C>T), XPD K751Q (2282A>C), XRCC1 R399Q (1301G>A), XRCC1 R194W (685C>T), and PARP1 V762A (2446T>C), did not show significant correlation to the sensitivity to DNA damaging chemotherapy agents cisplatin, carboplatin, oxaliplatin, and tetraplatin as reported previously.27
Discussion
The study presented here investigated the possible association between polymorphisms in NER and BER DNA repair genes and clinical outcome of radiotherapy in patients with prostate cancer. We observed several patterns with our data. First, all five SNPs assessed in this study were not associated with prostate cancer as compared to healthy volunteers. Second, there was a significant trend in patient survival to suggest the possibility that the XRCC1 R399Q genotype in combination with the XRCC1 R194W may have an impact on the outcome of radiotherapy in prostate cancer. Neither the XRCC1 R399Q nor the XRCC1 R194W was associated with overall survival individually (p = 0.5256 and 0.5493, respectively). However, the combination of R399Q and R194W genotypes showed correlation to the overall survival in the patients receiving radiotherapy in prostate cancer. Patients possessing at least one variant allele A of R399Q and wild-type CC of R194W had significantly longer survival time after radiotherapy, while patients having at least one wild-type allele G of R399Q and the heterozygous genotype CT of R194W had shorter survival time (p = 0.034). This outcome was not observed when patients received therapies other than radiation were included.
As suggested by our study, the genotype of XRCC1 R399Q may be a prognostic factor to radiation therapy in patients with prostate cancer, and this effect is modified by the R194W genotype.
Laboratory studies indicated that the variant genotype of XRCC1 R399Q is more sensitive to X-ray and UV-light than the other two genotypes within this codon.12 XRCC1 R399Q is located in the BRCT1 domain (Fig. 1), a critical region that is required for PARP1 mediated recruitment of XRCC1 upon DNA damage. This site is involved in survival after methylation damage.28 It was suggested that the substitution of an arginine to glutamine could cause the loss of a secondary structure feature such as an alpha helix that is important for correct protein-protein interactions in the BRCT1 domain, and thus compromising the DNA repair capability.29 Longer median survival was found in this study for patients possessing the variant genotype AA of the XRCC1 R399Q (11.12 years comparing to 7.77 years and 8.17 years for the other two genotypes), though not statistically significant (p = 0.5256). A study showed that the number of variant alleles in APE1 D148Q and XRCC1 R399Q genotypes was significantly correlated with prolonged cell cycle delay following ionizing radiation (IR), which resulted in IR hypersensitivity in breast cancer cases (p = 0.001).30 Theoretically, the variant allele of the XRCC1 R399Q may impair the interaction between XRCC1 and other proteins, resulting in inefficient removal of radiation induced DNA damage and prolonged cell cycle arrest, which delivers favorable response to radiotherapy.
The polymorphism of R194W is located in a linker region (residues 158–310) between the NTD and the central BRCT domain of XRCC1 (Fig. 1), enriched in basic amino acids. The high pI and overall positive charge of this region was suggested to have an important role in proper secondary structure formation.31 This domain is also the potential protein-binding domain for several interactive protein partners (PCNA, APE1, etc.) of the XRCC1 protein. The transition from the positively charged arginine to a hydrophobic tryptophan could affect binding and DNA repair efficiency. An in silico study suggested that the presence of the variant allele of R194W might result in a damaging effect and an intolerant protein.7 We found a low frequency of the variant genotype TT of this SNP in our study population (1% in the healthy volunteers and 2% in the patient group). It is also noteworthy that in our patient group, the heterozygous genotype of the XRCC1 R194W tends to segregate from the variant homozygous genotype of R399Q, which may indicate that the wild-type allele of R399Q has a protective effect that compensates the compromised protein function of XRCC1 caused by R194W allele. A previous study showed that the variant allele of R194W had higher frequency in radiation-sensitive breast cancer cases (OR 1.98, 95% CI 0.92–4.17).32 Our study also showed longer survival time in the patients with the variant genotype of R194W (9.22 years comparing to 8.06 years and 6.52 years) but not statistically significant (p = 0.5493). However, in the haplotype analysis, as the result of it's tending to group with the wild-type allele of XRCC1 R399Q, the variant allele of R194W showed a protective effect on radiotherapy. This is consistent with another study showing that the wild-type allele G of R399Q along with the variant allele T of R194W, and the wild-type allele of XRCC1 R280H had shorter overall survival than other haplotypes in patients with lung cancer that received radiotherapy (p = 0.04).18 Though some epidemiological studies did suggest the variant allele of XRCC1 R194W confers reduced cancer risk,8 others suggested vice versa.11 Our data presented here seems to indicate that there may be a complicated intergenic interaction between the polymorphisms of XRCC1 R399Q and R194W. This intergenic interaction may be universal and extends to multiple DNA repair genes. As suggested by another study,33 possessing more than four SNPs in DNA repair genes resulted in hypersensitivity to radiation in cells obtained from patients with cancer (p < 0.001).
DNA repair pathways help to maintain genetic stability and prevent the development of cancer. However, they also represent a potential mechanism of resistance to DNA damaging chemotherapy and radiotherapy. The polymorphisms in DNA repair genes provide the genetic basis for various DNA repair capability. To identify radiosensitive cancer patients before treatment may allow tailored radiotherapy and optimize the effectiveness and toxicity of ionizing radiation in clinical practice.
Subjects and Methods
Five hundred and thirteen patients with castrate-resistant prostate cancer (CRPC) were analyzed in this study. These include 284 patients who received external beam radiotherapy (XRT) and/or brachytherapy and 229 patients with the same disease but did not receive radiotherapy. All patients were Caucasians and were enrolled in an institutional review board-approved clinical trial within the intramural program of the National Cancer Institute, and were arbitrarily assigned a number in our database to protect confidentiality. Informed consent was obtained from all subjects before trial participation. In addition, 152 male Caucasian control samples were analyzed. All volunteers had signed informed consent to allow their samples to be used for genotyping, and none had a diagnosis of cancer.
Genomic DNA was extracted from serum or white blood cell buffy coat layers of whole blood of patients, or NCI-60 cell pellets as previously described.26 Polymerase chain reaction (PCR) and direct nucleotide sequencing were performed as described previously.3
Confidence intervals for the odds ratios of the distributions of individual polymorphisms relative to the wild-type between controls and patients with cancer were determined using the exact method. The probability of survival as a function of time since diagnosis was determined by the Kaplan-Meier method. The statistical significance of the differences in survival among the genotypes was determined by the log-rank test. An adjustment was made to the p value comparing survival among patients with different haplotypes when the grouping was made after examining the data and selecting the better of the possible combinations. Except as noted, all p values are two-tailed and reported without adjustment for multiple comparisons.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/12172
References
- 1.DeVita VTJL, Jr, Theodore S, Steven AR, Ronald AD, Robert AW. Principles and Practice of Oncology. Lippincott Williams & Wilkins (LWW) 2008 [Google Scholar]
- 2.Roach M, 3rd, Waldman F, Pollack A. Predictive models in external beam radiotherapy for clinically localized prostate cancer. Cancer. 2009;115:3112–3120. doi: 10.1002/cncr.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao R, Price DK, Sissung T, Reed E, Figg WD. Ethnic disparities in Americans of European descent versus Americans of African descent related to polymorphic ERCC1, ERCC2, XRCC1 and PARP1. Mol Cancer Ther. 2008;7:1246–1250. doi: 10.1158/1535-7163.MCT-07-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorgensen TJ. Enhancing radiosensitivity: targeting the DNA repair pathways. Cancer Biol Ther. 2009;8:665–670. doi: 10.4161/cbt.8.8.8304. [DOI] [PubMed] [Google Scholar]
- 5.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 6.Churchill ME, Peak JG, Peak MJ. Repair of near-visible- and blue-light-induced DNA single-strand breaks by the CHO cell lines AA8 and EM9. Photochem Photobiol. 1991;54:639–644. doi: 10.1111/j.1751-1097.1991.tb02068.x. [DOI] [PubMed] [Google Scholar]
- 7.Ladiges WC. Mouse models of XRCC1 DNA repair polymorphisms and cancer. Oncogene. 2006;25:1612–1619. doi: 10.1038/sj.onc.1209370. [DOI] [PubMed] [Google Scholar]
- 8.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- 9.Hu Z, Ma H, Chen F, Wei Q, Shen H. XRCC1 polymorphisms and cancer risk: a meta-analysis of 38 case-control studies. Cancer Epidemiol Biomarkers Prev. 2005;14:1810–1818. doi: 10.1158/1055-9965.EPI-04-0793. [DOI] [PubMed] [Google Scholar]
- 10.Hung RJ, Brennan P, Canzian F, Szeszenia-Dabrowska N, Zaridze D, Lissowska J, et al. Large-scale investigation of base excision repair genetic polymorphisms and lung cancer risk in a multicenter study. J Natl Cancer Inst. 2005;97:567–576. doi: 10.1093/jnci/dji101. [DOI] [PubMed] [Google Scholar]
- 11.Tae K, Lee HS, Park BJ, Park CW, Kim KR, Cho HY, et al. Association of DNA repair gene XRCC1 polymorphisms with head and neck cancer in Korean population. Int J Cancer. 2004;111:805–808. doi: 10.1002/ijc.20338. [DOI] [PubMed] [Google Scholar]
- 12.Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect. 2003;111:1843–1850. doi: 10.1289/ehp.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burri RJ, Stock RG, Cesaretti JA, Atencio DP, Peters S, Peters CA, et al. Association of single nucleotide polymorphisms in SOD2, XRCC1 and XRCC3 with susceptibility for the development of adverse effects resulting from radiotherapy for prostate cancer. Radiat Res. 2008;170:49–59. doi: 10.1667/RR1219.1. [DOI] [PubMed] [Google Scholar]
- 14.De Ruyck K, Van Eijkeren M, Claes K, Morthier R, De Paepe A, Vral A, et al. Radiation-induced damage to normal tissues after radiotherapy in patients treated for gynecologic tumors: association with single nucleotide polymorphisms in XRCC1, XRCC3 and OGG1 genes and in vitro chromosomal radiosensitivity in lymphocytes. Int J Radiat Oncol Biol Phys. 2005;62:1140–1149. doi: 10.1016/j.ijrobp.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Chang-Claude J, Popanda O, Tan XL, Kropp S, Helmbold I, von Fournier D, et al. Association between polymorphisms in the DNA repair genes, XRCC1, APE1 and XPD and acute side effects of radiotherapy in breast cancer patients. Clin Cancer Res. 2005;11:4802–4809. doi: 10.1158/1078-0432.CCR-04-2657. [DOI] [PubMed] [Google Scholar]
- 16.Ho AY, Atencio DP, Peters S, Stock RG, Formenti SC, Cesaretti JA, et al. Genetic predictors of adverse radiotherapy effects: the Gene-PARE project. Int J Radiat Oncol Biol Phys. 2006;65:646–655. doi: 10.1016/j.ijrobp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Brem R, Cox DG, Chapot B, Moullan N, Romestaing P, Gerard JP, et al. The XRCC1-77T->C variant: haplotypes, breast cancer risk, response to radiotherapy and the cellular response to DNA damage. Carcinogenesis. 2006;27:2469–2474. doi: 10.1093/carcin/bgl114. [DOI] [PubMed] [Google Scholar]
- 18.Yoon SM, Hong YC, Park HJ, Lee JE, Kim SY, Kim JH, et al. The polymorphism and haplotypes of XRCC1 and survival of non-small-cell lung cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:885–891. doi: 10.1016/j.ijrobp.2005.07.951. [DOI] [PubMed] [Google Scholar]
- 19.Wang XG, Wang ZQ, Tong WM, Shen Y. PARP1 Val762Ala polymorphism reduces enzymatic activity. Biochem Biophys Res Commun. 2007;354:122–126. doi: 10.1016/j.bbrc.2006.12.162. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Hur E. Involvement of poly (ADP-ribose) in the radiation response of mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1984;46:659–671. doi: 10.1080/09553008414551891. [DOI] [PubMed] [Google Scholar]
- 21.Arundel-Suto CM, Scavone SV, Turner WR, Suto MJ, Sebolt-Leopold JS. Effect of PD 128763, a new potent inhibitor of poly(ADP-ribose) polymerase, on X-ray-induced cellular recovery processes in Chinese hamster V79 cells. Radiat Res. 1991;126:367–371. [PubMed] [Google Scholar]
- 22.Bowman KJ, White A, Golding BT, Griffin RJ, Curtin NJ. Potentiation of anti-cancer agent cytotoxicity by the potent poly(ADP-ribose) polymerase inhibitors NU1025 and NU1064. Br J Cancer. 1998;78:1269–1277. doi: 10.1038/bjc.1998.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Duin M, de Wit J, Odijk H, Westerveld A, Yasui A, Koken HM, et al. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986;44:913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- 24.van Duin M, Koken MH, van den Tol J, ten Dijke P, Odijk H, Westerveld A, et al. Genomic characterization of the human DNA excision repair gene ERCC-1. Nucleic Acids Res. 1987;15:9195–9213. doi: 10.1093/nar/15.22.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung P, Bailly V, Weber C, Thompson LH, Prakash L, Prakash S. Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature. 1993;365:852–855. doi: 10.1038/365852a0. [DOI] [PubMed] [Google Scholar]
- 26.Hamada A, Danesi R, Price DK, Sissung T, Chau C, Venzon D, et al. Association of a CYP17 polymorphism with overall survival in Caucasian patients with androgen-independent prostate cancer. Urology. 2007;70:217–220. doi: 10.1016/j.urology.2007.06.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, Fojo T. Oxaliplatin, tetraplatin, cisplatin and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol. 1996;52:1855–1865. doi: 10.1016/s0006-2952(97)81490-6. [DOI] [PubMed] [Google Scholar]
- 28.Levy N, Martz A, Bresson A, Spenlehauer C, de Murcia G, Menissier-de Murcia J. XRCC1 is phosphorylated by DNA-dependent protein kinase in response to DNA damage. Nucleic Acids Res. 2006;34:32–41. doi: 10.1093/nar/gkj409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monaco R, Rosal R, Dolan MA, Pincus MR, Brandt-Rauf PW. Conformational Effects of a Common Codon 399 Polymorphism on the BRCT1 Domain of the XRCC1 Protein. Protein J. 2007;26:541–546. doi: 10.1007/s10930-007-9095-y. [DOI] [PubMed] [Google Scholar]
- 30.Hu JJ, Smith TR, Miller MS, Lohman K, Case LD. Genetic regulation of ionizing radiation sensitivity and breast cancer risk. Environ Mol Mutagen. 2002;39:208–215. doi: 10.1002/em.10058. [DOI] [PubMed] [Google Scholar]
- 31.Marintchev A, Robertson A, Dimitriadis EK, Prasad R, Wilson SH, Mullen GP. Domain specific interaction in the XRCC1-DNA polymerase beta complex. Nucleic Acids Res. 2000;28:2049–2059. doi: 10.1093/nar/28.10.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moullan N, Cox DG, Angele S, Romestaing P, Gerard JP, Hall J. Polymorphisms in the DNA repair gene XRCC1, breast cancer risk and response to radiotherapy. Cancer Epidemiol Biomarkers Prev. 2003;12:1168–1174. [PubMed] [Google Scholar]
- 33.Azria D, Ozsahin M, Kramar A, Peters S, Atencio DP, Crompton NE, et al. Single nucleotide polymorphisms, apoptosis, and the development of severe late adverse effects after radiotherapy. Clin Cancer Res. 2008;14:6284–6288. doi: 10.1158/1078-0432.CCR-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]