Abstract
Severe hypoxia has been demonstrated to induce a replication arrest which is associated with decreased levels of nucleotides. Chk1 is rapidly phosphorylated in response to severe hypoxia and in turn deactivates TLK1 through phosphorylation. Loss of Chk1 has been shown to sensitize cells to hypoxia/reoxygenation. After short (acute) exposure to hypoxia this is due to an increased rate of reoxygenation-induced replication restart and subsequent p53-dependent apoptosis. After longer (chronic) exposure to hypoxia S phase cells do not undergo reoxygenation-induced replication restart. Cells exposed to these levels of hypoxia are however sensitive to loss of Chk1. This suggests a new role for Chk1 in the cell cycle response to reoxygenation.
Key words: hypoxia, reoxygenation, replication restart, Chk1, TLK1
Introduction
Hypoxia is a physiologically relevant stress during embryonic development, wound healing and tumor progression. The significance of the hypoxia effect on tumor progression has been effectively highlighted by many reports demonstrating that hypoxia correlates with poor prognosis in cancer patients.1–3 This has been attributed to the finding that hypoxic cells are both more resistant to current therapies (chemo and radio) and are more aggressive i.e., more likely to be resistant to apoptosis and/or metastasize.4–6 Regions of hypoxia occur in tumors due to the demand for oxygen outstripping the supply.7–9 This is due, in part, to the poor tumor vasculature, which often includes aberrant structures. Hypoxic gradients exist in tumors, with the highest oxygen concentrations being found near functional vessels and the lowest surrounding necrotic regions. Vessel mismatch experiments have demonstrated that hypoxia is not necessarily constant within a tumor but fluctuates dramatically.7–11 The result of this is periods of hypoxia followed by reoxygenation/reperfusion.12
In our studies we have focused on severe hypoxic conditions (<0.1% O2), which exist adjacent to the necrotic areas in tumors. The cells which experience this almost complete lack of oxygen (anoxia) for the most part die through either necrosis, apoptosis or possibly autophagy.13,14 However, as mentioned the tumor vasculature is very dynamic with vessels opening and closing at surprising rates suggesting that reperfusion or reoxygenation of hypoxic regions can and does occur.9,11 Reoxygenation is also significant as a consequence of radio-therapy. Tumor cells at physiologically normal oxygen levels are more sensitive to radiation, therefore these cells are preferentially killed by radiation leaving the more hypoxic/resistant cells. These cells, as a direct consequence, may receive an improved oxygen supply post radiation i.e., they are reoxygenated. Our previous work has shown that cells exposed to severe hypoxia experience an S phase arrest or more accurately an inhibition of DNA synthesis.15 This should not be confused with a checkpoint response as it appears independent of both ATM and ATR-mediated signalling.16
Chronic Exposure to Hypoxia Inhibits Both Replication Restart and DNA Repair
In our recent study, we demonstrated that decreasing levels of nucleotides correlate with this block in DNA synthesis in response to hypoxia (<0.1% O2).17 We attributed this to the finding that the activity of ribonucleotide reductase which, is the chief enzyme responsible for generating nucleotides, is oxygen dependent.18 The principle question addressed in our study was, what happens to cells arrested in this way if they become reoxygenated? In order to address the possibility of reoxygenation-induced replication restart we made use of the DNA fiber technique to allow us to look at individual DNA strands and measure replication rates. In support of our previous data showing that nucleotides are limiting we found that replication is inhibited during both the initiation and elongation phases i.e., actively replicating forks stalled and origins failed to fire. In the conditions measured, this was found to be independent of the checkpoint kinase, Chk1, again supportive of it being the result of a lack of nucleotides versus DNA damage response signalling. We cannot however exclude the possibility that in response to either falling nucleotide levels or some other hypoxia-induced signal, DNA synthesis is also inhibited by additional stress response pathways. For example, the inhibition of DNA synthesis may be reenforced by checkpoint signalling. Our previous attempts to artificially maintain nucleotide levels during hypoxia have been unsuccessful, although it is unclear if this was for technical reasons or if alternative signalling pathways are active.15
We demonstrated that nucleotide levels return to normal very rapidly in response to reoxygenation suggesting that the materials for DNA synthesis become available. Replication restart was investigated after either acute (up to 8 hours) or chronic (12–24 hours) exposure to hypoxia. Using both BrdU incorporation and DNA fibers we demonstrated that reoxygenated cells after chronic hypoxia exposure do not undergo replication restart. We have attributed this to the disassembly of the replisome (machinery required for replication). Essential components of the replisome, such as the MCM complex, were repressed and/or relocalized from the chromatin in chronic hypoxia.19 In contrast after shorter, acute, periods of hypoxia cells did resume replication after reoxygenation and this correlated with no functional changes in the replication proteins investigated. These findings are significant for numerous reasons and especially when considered with the current literature which indicates that DNA repair is also repressed in hypoxic conditions.20 A number of reports indicate that repair pathways such as non homologous end joining (NHEJ) and mismatch repair (MMR) are repressed in response to hypoxia although the most studied response is in the homologous recombination (HR) pathway.21–25 Proteins essential to HR, such as BRCA1/2 and Rad51 have been shown to be repressed in response to hypoxia through a variety of mechanism involving for example E2F promoter occupancy and microRNAs.26,27 The emerging picture is that in response to severe hypoxia the cell turns off its ability to restart replication and repair DNA. The importance of this is highlighted by the finding that, although the repression of either pathway (replication and repair) may not have functional consequences until chronic periods of time have passed, the repression begins very early in hypoxia exposure. This is demonstrated in Figure 1 which shows the levels of Rad51 decreasing in response to hypoxia; after 2 hours the levels of Rad51 protein are down by 25%. This point was also highlighted in our recent manuscript which showed that the mRNA levels of MCM4, 5, 6 and 7 are repressed after just 4 hours of hypoxia.17 We found that MCM6 levels were repressed in hypoxic regions of both spheroids and xenograft tumor sections. Importantly, these regions were not necrotic areas but contained viable cells. These data indicate that during acute exposure to hypoxia, cellular decisions are made to repress replication re-start and DNA repair and that by the time the exposure is chronic neither of these pathways remain functional. The obvious unanswered question is why the cell would do this? The active inhibition of these pathways could represent a self-preservation energy-preservation shift for the cell, which speculatively, would resemble autophagy, also suggested by Glazer and colleagues.27 This is also supported by reports demonstrating that in response to hypoxia translation is abrogated.28
Figure 1.
Levels of the HR protein Rad51 drop rapidly in response to severe hypoxia. RKO cells were exposed to 0.02% O2 for the time periods indicated or as a positive control 1 mM HU. The protein levels of Rad51 and Actin are shown. Quantification of the levels of Rad51 normalized to Actin are also shown.
Cancer cells exposed to hypoxia for time periods sufficient to repress HR and collapse the replisome pose no threat to the host as they are unable to resume replication even if reoxygenation were to occur. However, we and others suggest that DNA repair can become compromised in response to hypoxia in conditions that still permit replication or replication-restart.17,29 These cells risk accumulating DNA replication associated damage and also ROS-mediated damage generated during periods of reoxygenation.30 These reoxygenated cells are replication restart competent but do so in the presence of DNA damage with impaired DNA repair capabilities. Our data indicated that in the presence of wild type p53 these cells are eliminated through the induction of apoptosis. However, as described in numerous reports, the majority of tumor cells have lost this ability, therefore raising the possibility that reoxygenated cells which have accumulated DNA damage may re-enter the cell cycle.31 It is our hypothesis that, although a rare event, these cells could contribute to genomic instability and therefore tumor development.
Chk1 Signalling in Hypoxia/Reoxygenation
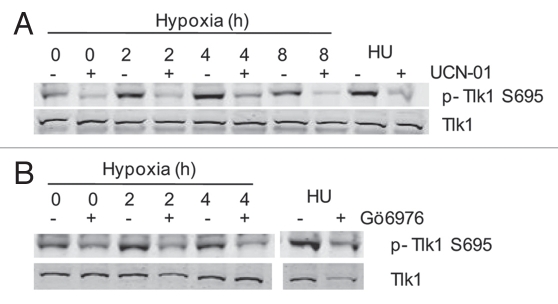
We have investigated the role of Chk1 in the response to both hypoxia and reoxygenation.32 Using BrdU or 3H-thymidine incorporation assays we have been unable to demonstrate a role for Chk1 in the cell cycle response to hypoxia.33 The DNA fiber technique demonstrated that both fork stalling and inhibition of origin firing occurred with normal kinetics in the response to hypoxia in the presence of a Chk1 inhibitor. This was somewhat surprising as we have shown previously that ATR phosphorylates Chk1 in response to hypoxia at serines 345 and 317 implying that it is active. To date, a downstream target of hypoxia-induced Chk1 has not been identified, raising the possibility that Chk1 is not actually active in these conditions. Here, we show that hypoxia-induced phosphorylation of Chk1 correlates with activity. The downstream target of Chk1, TLK1 (tousled like kinase 1) was shown to be phosphorylated in a Chk1-dependent manner in response to hypoxia (Fig. 2A and B). These experiments, using pharmacological inhibition of Chk1 (Gö6976 or UNC-01), were supported by data showing the siRNA mediated depletion of Chk1 also reduced the levels of phosphorylated TLK1 in hypoxia (McGurk C and Hammond EM, data not shown).
Figure 2.
TLK1 is phosphorylated by Chk1 in response to hypoxia. (A) RKO cells were treated with the Chk 1 inhibitor UCN-01 (dose) and exposed to hypoxia (0.02% O2) as indicated. HU was used as a positive control. The levels of both phosphorylated and total TLK1 are shown. (B) RKO cells were treated with the Chk 1 inhibitor Gö6976 (100 nM) and exposed to hypoxia (0.02% O2) as indicated. HU was used a appositive control. The levels of both phosphorylated and total TLK1 are shown.
TLK1 is a serine/threonine kinase named for its similarity to the tousled gene in Arabidopsis thaliana, which is essential for proper flower and leaf development. Tousled genes are absent in yeast and other eukaryotic microbes and were thought to be specific to multi cellular organisms until homologues in Trypanosoma brucei were sequenced. Mammalian TLK1 was identified in 1999 and was shown to be maximally active in S-phase leading to the hypothesis that TLK1 has a role in cell cycle progression.34 More recently TLK1 has been reported to be involved in both the processing of newly replicated DNA and chromatin formation. The kinase activity of TLK1 decreases in response to genotoxic stress, such as ionising radiation or treatment with hydroxyurea.35,36 The proposed mechanism of action is that in response to DNA damage ATM phosphorylates Chk1 which in turn phosphorylates and deactivates TLK1.36 The downstream targets of TLK1 include assembly factor anti-silencing function 1 (Asf1), DEAD-box protein p68 and histone H3.37–39 TLK1 is the first reported Chk1 target to be phosphorylated in hypoxic conditions. The phosphorylation of TLK1 persisted for at least 2 hours after reoxygenation (McGurk C and Hammond EM unpublished). A previous report indicates that another known target of Chk1, cdc25A is not phosphorylated in response to hypoxia suggesting that hypoxia-activated Chk1 has a distinct set of targets.30 After phosphorylation by either Chk1 or Chk2, cdc25A is quickly degraded and as a result promotes cell cycle arrest in both the G1 and G2 phases. It is tempting to speculate that during exposure to severe hypoxia neither a G1 or G2 arrest are required as the cells cannot enter S phase and are therefore arrested in G1. It is possible that Chk1 does contribute to the hypoxia induced S phase arrest, through phosphorylation of for example TLK1, but that this effect is masked by the more dramatic loss of nucleotides.
Our data indicate that the Chk1 response to hypoxia and indeed the DNA damage response in general appears to be transient. Chk1 is robustly phosphorylated after 6 hours of hypoxia but this signal is absent by 18 hours. This correlated with falling levels of Chk1 which has been noted previously in response to other replication stresses. This led us to question the hypoxia-induced DNA damage response initiating signal. Regions of single stranded DNA have been shown to accumulate during hypoxia previously and the presence of stalled replication verified by DNA fiber analysis. Our data and the literature suggest a model in which replication stalls leading to an accumulation of RPA (replication protein A) coated single stranded DNA which is the signal for initiating ATR activity.40 Our studies have shown that replication does not resume during hypoxia exposure and so our model assumed a constant level of ssDNA (single stranded DNA). However, in light of the transient response of Chk1 we revisited this and quantified both ssDNA and RPA foci in hypoxia over time. The data demonstrated that both the level of ssDNA and RPA foci decreased after an initial peak in the acute time frame. This suggests a model whereby the DDR (DNA damage response) is induced in response to hypoxia-induced replication arrest and remains so during the time period which cells remain replication competent but, once this is passed the DDR is not sustained. The mechanism by which the levels of ssDNA decrease remains to be investigated.
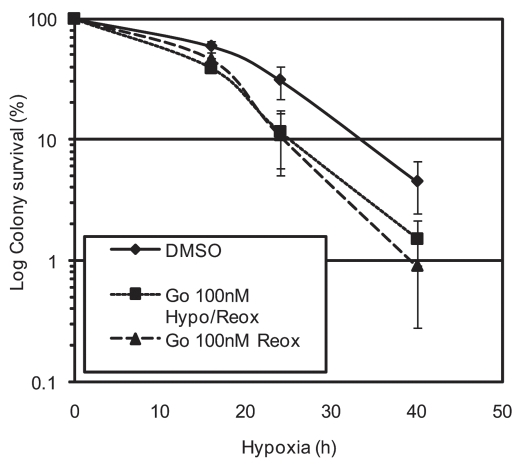
The role of Chk1 in the response to reoxygenation is more clear and correlates with the response to other stresses reported in the literature.41–43 Our DNA fiber analysis demonstrated that in response to inhibition of Chk1 the rate of new origin firing in response to reoxygenation significantly increased.17 This supports the suggested role for Chk1 in delaying origin firing during a DNA damaging stress to ensure accurate repair of the DNA.41,44 Failure to pause replication restart in these situations encourages the incorporation of DNA damage and therefore genomic instability. It is also likely that, like Chk2, Chk1 contributes to the reoxygenation-induced cell cycle arrest.30,45 Taken together these data indicate that Chk1 functions during both hypoxia and reoxygenation. We have carried out a modified form of our colony survival experiment to determine in which phase, hypoxia or reoxygenation, the cells are more sensitive (Fig. 4).46 Asynchronous RKO cells were exposed to hypoxia for the times indicated and the Chk1 inhibitor (Gö6976) was added as indicated, either present during both hypoxia and reoxygenation or just during reoxygenation. The colony survival shown demonstrates that the increased sensitivity to hypoxia/reoxygenation is dependent on inhibition of Chk1 during reoxygenation as the cells are no more sensitive if Chk1 is also inhibited during hypoxia. This suggests that the role of Chk1 in hypoxia has a less significant impact on cell viability than it's role in reoxygenation. This was somewhat surprising as it has been demonstrated that ATR, through Chk1 signalling, acts to protect stalled replication forks collapse and that this is also likely in hypoxic conditions as loss of ATR was shown to lead to accumulation of S phase specific DNA damage.33 Our hypothesis is that loss of Chk1 during hypoxia would lead to replication fork collapse and hence increased sensitivity. It is likely that if synchronized cells were used this would be evident. It must also be considered that it is possible that Chk1 activity is not fully inhibited by the Gö6976 and that there is residual Chk1 activity in hypoxia and equally that the inhibitor may also inhibit Chk2 to some degree (Fig. 4). The data suggest that during acute exposure to levels of hypoxia (<0.1% O2) ATR signalling to Chk1 acts to maintain fork stability. Loss of Chk1 during this period sensitizes S phase cells to hypoxia/reoxygenation, although this is not an obvious effect in asynchronous cell populations. In the absence of Chk1 replication restart rates are affected, presumably as a result of its role in HR, and cells ultimately undergo p53-dependent apoptosis. However, the loss of Chk1 leads to a more significant increase in the sensitivity to hypoxia/reoxygenation if the cells are exposed to hypoxia for longer/chronic periods of time. Our data indicate that after this exposure the S phase cells are unable to resume replication, due to the disassembly of the replisome, and that this is independent of the Chk1 status. Therefore suggesting that the increase in sensitivity is actually due to an effect on the G1 or G2 cells (Fig. 5). Given the overlap between the downstream targets of Chk1 and Chk2 it is perhaps not surprising that Chk1 should contribute to reoxygenation-induced cell cycle arrest. This hypothesis remains to be verified and the use of specific siRNAs to target Chk1 and not Chk2 will be essential.
Figure 4.
Inhibition of Chk1 during reoxygenation is sufficient to sensitise cells to hypoxia/reoxygenation. RKO cells were exposed to hypoxia (0.02% O2) for the time periods indicated either in the presence/absence of 100 nM Gö6976. Cells were then returned to normal incubation conditions and 100 nM Gö6976 added to some. Colonies were allowed to form (>50 cells), stained and counted. All conditions were carried out in triplicate.
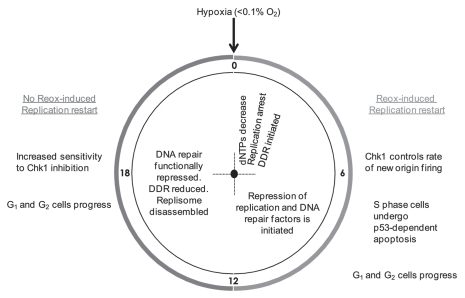
Figure 5.
A schematic representation of how the response to hypoxia alters with increasing exposure time.
Conclusion
Determining the role of Chk1 in hypoxia/reoxygenation is of great interest as potent inhibitors have been generated and are now in clinical trials.47 It seems likely that the future for these therapies is in combination with standard agents which may include those that alter tumor oxygenation levels (for example radiotherapy and anti-angiogenesis agents). Our data suggests caution as we have demonstrated, that if Chk1 is inhibited during the reoxygenation of severely hypoxic cells whilst the replisome is still intact these cells escape the checkpoint and restart replication. Without p53 to act as the guardian these cells potentially accumulate genomic instability and contribute to tumorigenesis.
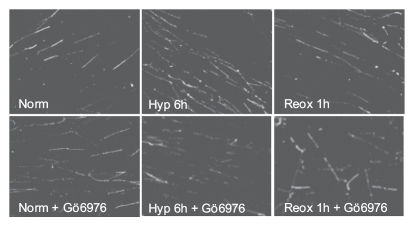
Figure 3.
Inhibition of Chk1 during reoxygenation leads to an increase in new origin firing. RKO cells, in the presence of either vehicle alone or 100 nM of the Chk1 inhibitor Gö6976 were exposed to 6 h hypoxia and reoxygenated for 1 h and DNA fibers were produced. Replication rates were determined from fiber length measurements and the number of replication structures (including new origins) were scored and were reported previously.17,48
Acknowledgements
This work was supported by the Cancer Research UK, grant ref C6515/A9321 awarded to E.M. Hammond.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12059
References
- 1.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]
- 2.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 3.Hockel M, Schlenger K, Hockel S, Vaupel P. Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res. 1999;59:4525–4528. [PubMed] [Google Scholar]
- 4.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and Radiation Response in Human Tumors. Semin Radiat Oncol. 1996;6:3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 5.Hockel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001;28:36–41. [PubMed] [Google Scholar]
- 6.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 7.Brown JM. Tumor microenvironment and the response to anticancer therapy. Cancer Biol Ther. 2002;1:453–458. doi: 10.4161/cbt.1.5.157. [DOI] [PubMed] [Google Scholar]
- 8.Brown JM. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007;435:297–321. doi: 10.1016/S0076-6879(07)35015-5. [DOI] [PubMed] [Google Scholar]
- 9.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 10.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 11.Cardenas-Navia LI, Mace D, Richardson RA, Wilson DF, Shan S, Dewhirst MW. The pervasive presence of fluctuating oxygenation in tumors. Cancer Res. 2008;68:5812–5819. doi: 10.1158/0008-5472.CAN-07-6387. [DOI] [PubMed] [Google Scholar]
- 12.Hammond EM, Kaufmann MR, Giaccia AJ. Oxygen sensing and the DNA-damage response. Current Opinion in Cell Biology. 2007;19:680–684. doi: 10.1016/j.ceb.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 14.Papandreou I, Krishna C, Kaper F, Cai D, Giaccia AJ, Denko NC. Anoxia is necessary for tumor cell toxicity caused by a low-oxygen environment. Cancer Res. 2005;65:3171–3178. doi: 10.1158/0008-5472.CAN-04-3395. [DOI] [PubMed] [Google Scholar]
- 15.Hammond EM, Green SL, Giaccia AJ. Comparison of hypoxia-induced replication arrest with hydroxyurea and aphidicolin-induced arrest. Mutat Res. 2003;532:205–213. doi: 10.1016/j.mrfmmm.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Hammond EM, Giaccia AJ. The role of ATM and ATR in the cellular response to hypoxia and reoxygenation. DNA Repair (Amst) 2004;3:1117–1122. doi: 10.1016/j.dnarep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Pires IM, Bencokova Z, Milani M, Folkes LK, Li JL, Stratford MR, et al. Effects of Acute versus Chronic Hypoxia on DNA Damage Responses and Genomic Instability. Cancer Res. 2010;70:925–935. doi: 10.1158/0008-5472.CAN-09-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Probst H, Schiffer H, Gekeler V, Scheffler K. Oxygen dependent regulation of mammalian ribonucleotide reductase in vivo and possible significance for replicon initiation. Biochem Biophys Res Commun. 1989;163:334–340. doi: 10.1016/0006-291x(89)92140-2. [DOI] [PubMed] [Google Scholar]
- 19.Blow JJ, Dutta A. Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol. 2005;6:476–486. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 21.Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, et al. Hypoxia-induced downregulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 22.Meng AX, Jalali F, Cuddihy A, Chan N, Bindra RS, Glazer PM, et al. Hypoxia downregulates DNA double strand break repair gene expression in prostate cancer cells. Radiother Oncol. 2005;76:168–176. doi: 10.1016/j.radonc.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Huang LE, Bindra RS, Glazer PM, Harris AL. Hypoxia-induced genetic instability—a calculated mechanism underlying tumor progression. J Mol Med. 2007;85:139–148. doi: 10.1007/s00109-006-0133-6. [DOI] [PubMed] [Google Scholar]
- 24.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, et al. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Lara PC, Lloret M, Clavo B, Apolinario RM, Bordon E, Rey A, et al. Hypoxia downregulates Ku70/80 expression in cervical carcinoma tumors. Radiother Oncol. 2008;89:222–226. doi: 10.1016/j.radonc.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Bindra RS, Crosby ME, Glazer PM. Regulation of DNA repair in hypoxic cancer cells. Cancer Metastasis Rev. 2007;26:249–260. doi: 10.1007/s10555-007-9061-3. [DOI] [PubMed] [Google Scholar]
- 27.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–1229. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25:1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan N, Koritzinsky M, Zhao H, Bindra R, Glazer PM, Powell S, et al. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res. 2008;68:605–614. doi: 10.1158/0008-5472.CAN-07-5472. [DOI] [PubMed] [Google Scholar]
- 30.Freiberg RA, Hammond EM, Dorie MJ, Welford SM, Giaccia AJ. DNA damage during reoxygenation elicits a Chk2-dependent checkpoint response. Mol Cell Biol. 2006;26:1598–1609. doi: 10.1128/MCB.26.5.1598-1609.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Junttila MR, Evan GI. p53—a Jack of all trades but master of none. Nat Rev Cancer. 2009;9:821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 32.Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 33.Hammond EM, Dorie MJ, Giaccia AJ. Inhibition of ATR Leads to Increased Sensitivity to Hypoxia/Reoxygenation. Cancer Res. 2004;64:6556–6562. doi: 10.1158/0008-5472.CAN-04-1520. [DOI] [PubMed] [Google Scholar]
- 34.Sillje HH, Takahashi K, Tanaka K, Van Houwe G, Nigg EA. Mammalian homologues of the plant Tousled gene code for cell cycle-regulated kinases with maximal activities linked to ongoing DNA replication. EMBO J. 1999;18:5691–5702. doi: 10.1093/emboj/18.20.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groth A, Lukas J, Nigg EA, Sillje HH, Wernstedt C, Bartek J, et al. Human Tousled like kinases are targeted by an ATM- and Chk1-dependent DNA damage checkpoint. EMBO J. 2003;22:1676–1687. doi: 10.1093/emboj/cdg151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krause DR, Jonnalagadda JC, Gatei MH, Sillje HH, Zhou BB, Nigg EA, et al. Suppression of Tousled-like kinase activity after DNA damage or replication block requires ATM, NBS1 and Chk1. Oncogene. 2003;22:5927–5937. doi: 10.1038/sj.onc.1206691. [DOI] [PubMed] [Google Scholar]
- 37.De Benedetti A. Tousled kinase TLK1B counteracts the effect of Asf1 in inhibition of histone H3-H4 tetramer formation. BMC Res Notes. 2009;2:128. doi: 10.1186/1756-0500-2-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kodym R, Henockl C, Furweger C. Identification of the human DEAD-box protein p68 as a substrate of Tlk1. Biochem Biophys Res Commun. 2005;333:411–417. doi: 10.1016/j.bbrc.2005.05.136. [DOI] [PubMed] [Google Scholar]
- 39.Sillje HH, Nigg EA. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr Biol. 2001;11:1068–1073. doi: 10.1016/s0960-9822(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 40.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, et al. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–258. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 42.Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets and DNA breakage. Mol Cell Biol. 2005;25:3553–3562. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syljuasen RG, Sorensen CS, Nylandsted J, Lukas C, Lukas J, Bartek J. Inhibition of Chk1 by CEP-3891 accelerates mitotic nuclear fragmentation in response to ionizing Radiation. Cancer Res. 2004;64:9035–9040. doi: 10.1158/0008-5472.CAN-04-2434. [DOI] [PubMed] [Google Scholar]
- 44.Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, et al. The cell cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 45.Gibson SL, Bindra RS, Glazer PM. Hypoxia-induced phosphorylation of Chk2 in an ataxia telangiectasia mutated-dependent manner. Cancer Res. 2005;65:10734–10741. doi: 10.1158/0008-5472.CAN-05-1160. [DOI] [PubMed] [Google Scholar]
- 46.Hammond EM, Freiberg RA, Giaccia AJ. The roles of Chk1 and Chk2 in hypoxia and reoxygenation. Cancer Lett. 2005 doi: 10.1016/j.canlet.2005.06.029. E-pub. [DOI] [PubMed] [Google Scholar]
- 47.Janetka JW, Ashwell S. Checkpoint kinase inhibitors: a review of the patent literature. Expert Opin Ther Pat. 2009;19:165–197. doi: 10.1517/13543770802653622. [DOI] [PubMed] [Google Scholar]
- 48.Petermann E, Maya-Mendoza A, Zachos G, Gillespie DA, Jackson DA, Caldecott KW. Chk1 requirement for high global rates of replication fork progression during normal vertebrate S phase. Mol Cell Biol. 2006;26:3319–3326. doi: 10.1128/MCB.26.8.3319-3326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]