Abstract
The 2,6,9-trisubstituted purine group of cyclin dependent kinase inhibitors have the potential to be clinically relevant inhibitors of cancer cell proliferation. We have recently designed and synthesized a novel dansylated analog of purvalanol B, termed VMY-1-103, that inhibited cell cycle progression in breast cancer cell lines more effectively than did purvalanol B and allowed for uptake analyses by fluorescence microscopy.
ErbB-2 plays an important role in the regulation of signal transduction cascades in a number of epithelial tumors, including prostate cancer (PCa). Our previous studies demonstrated that transgenic expression of activated ErbB-2 in the mouse prostate initiated PCa and either the overexpression of ErbB-2 or the addition of the ErbB-2/ErbB-3 ligand, heregulin (HRG), induced cell cycle progression in the androgen-responsive prostate cancer cell line, LNCaP.
In the present study, we tested the efficacy of VMY-1-103 in inhibiting HRG-induced cell proliferation in LNCaP prostate cancer cells. At concentrations as low as 1 µM, VMY-1-103 increased both the proportion of cells in G1 and p21CIP1 protein levels. At higher concentrations (5 µM or 10 µM), VMY-1-103 induced apoptosis via decreased mitochondrial membrane polarity and induction of p53 phosphorylation, caspase-3 activity and PARP cleavage. Treatment with 10 µM Purvalanol B failed to either influence proliferation or induce apoptosis.
Our results demonstrate that VMY-1-103 was more effective in inducing apoptosis in PCa cells than its parent compound, purvalanol B, and support the testing of VMY-1-103 as a potential small molecule inhibitor of prostate cancer in vivo.
Key words: prostate cancer, apoptosis, cell cycle, CDK inhibitor, p53
Introduction
The cyclins are regulatory proteins that modulate the activity of the cyclin-dependent kinases (Cdks), thereby directing the orderly progression of eukaryotic cells through the cell cycle, ultimately resulting in normal cellular division.1 The deregulation of various multi-protein cyclin/Cdk complexes has been described in many types of human tumors, including prostate cancer.2
The development of small molecules that inhibit the activity of cyclin/Cdk complexes has gained attention recently as technological advances in drug design have lead to improved target selectivity. The 2,6,9-trisubstituted purine group of cyclin dependent kinase inhibitors, which includes roscovitine and the purvalanols,3 act by selectively competing with ATP at its binding site in targeted CDK's. We have recently described the design and synthesis of a novel dansylated-fluorescent analog of purvalanol B, termed VMY-1-103.4 The design of this compound took advantage of both the known crystal structure of the purvalanol B/Cdk2 complex, as well as the fact that the carboxylic acid of the 6-anilino group of purvalanol can be modified without negatively affecting the chemicals ability to compete at the CDK ATP binding site. By synthetically coupling the fluorescent compound dansyl-ethylenediamine to purvalanol B, we found that VMY-1-103 inhibited cell cycle progression and increased apoptosis in breast cancer cell lines more effectively than purvalanol B. Since the dansyl group rendered the compound more lipophilic, we hypothesize that VMY-1-103 has an enhanced ability to diffuse through the cell membrane, increasing its cytoplasmic bioavailability.4
Our previous work has established that increased ErbB-2 signaling combined with the loss of one pten allele can initiate epithelial neoplasia in the mouse prostate that progresses to PCa commensurate with an induction of PI3-kinase/PDK1 signaling.2,5,6 We have also shown that ErbB-2 and the ErbB-2/ErbB-3 ligand heregulin (HRG) induced cell cycle progression in PCa cells in part through induction of cyclin D1.2 Since we have data establishing that VMY-1-103 inhibited cell cycle progression in MCF7 cells,4 we tested the ability of VMY-1-103 to inhibit HRG-induced proliferation in the androgen-sensitive prostate cancer cell line, LNCaP. In this report, we present evidence that VMY-1-103 at concentrations as low was 1 µM increased the proportion of HRG-stimulated LNCaP cells arresting in the G1 phase of the cell cycle. Additionally, VMY-1-103 at 5 µM and 10 µM significantly induced p53 phosphorylation and apoptosis and decreased cell viability in a manner that was caspase-dependent.
Results
VMY-1-103 inhibits heregulin-induced cell cycle progression in LNCaP cells.
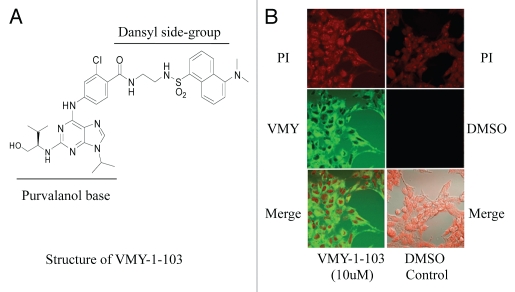
We have recently described the design and synthesis of a novel dansylated-analog of purvalanol B, termed VMY-1-103 (Fig. 1A and reviewed in ref. 4). Incubation of prostate cancer LnCAP cells with 10 µM VMY-1-103 for 12 h resulted in more than 95% of percent of the cells containing cytoplasmic VMY-1-103 (Fig. 1B).
Figure 1.
(A) Structure of VMY-1-103. (B) Uptake and subcellular localization of VMY-1-103 in LNCaP cells. PI, propidium iodide.
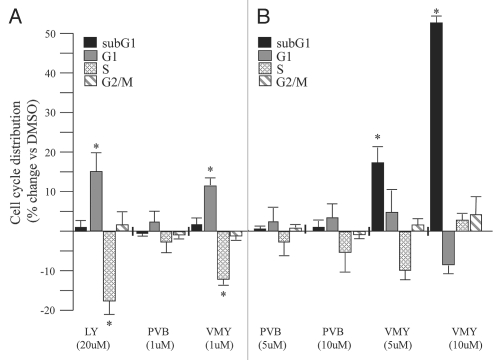
Since HRG induced cell cycle progression in LNCaP cells,2 we next tested the ability of VMY-1-103 to inhibit HRG-induced proliferation in LNCaP cells. Treatment with 1 ng/ml heregulin and either 20 µM LY294002 or 1 µM VMY-1-103 for 18 hours resulted in a significant increase in the number of cells in G1 and a concomitant decrease in the number of cells in S-phase (Fig. 2A). Purvalanol B (1 µM) had no effect (Fig. 2A). Similar results were seen in the presence of serum (Suppl. Fig. 1).
Figure 2.
VMY-1-13 regulation of cell cycle progression and apoptosis in the human prostate cancer cell line LNCaP. Effect of LY294002 (LY), purvalanol B (PVB) or VMY-1-103 (VMY) on HRG-induced cell cycle progression and subG1-phase associated apoptosis at (A) low and (B) high concentrations (5–10 µM). Values are percent change (average ± SD, N = 3) versus cells treated with the vehicle, DMSO. Asterisk, p ≤ 0.05.
VMY-1-103 induces apoptosis in LNCaP cells.
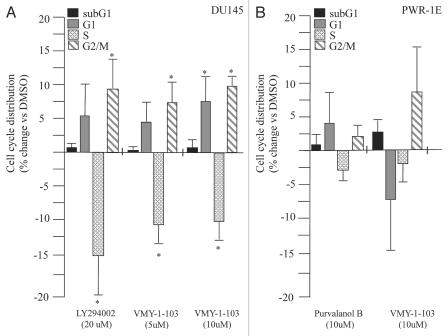
We next tested if higher concentrations of either purvalanol B or VMY-1-103 had any differential effects on cell cycle progression. Treatment of LNCaP cells with either 5 µM or 10 µM purvalanol B failed to significantly alter cell cycle progression or increase the sub-G1 population of apoptotic cells (Fig. 2B). In contrast, 5 and 10 µM VMY-1-103 produced significant dose-dependent increases in the sub-G1 population of apoptotic cells, however the G1 arrest observed at 1 µM was lost (Fig. 2B). Experiments performed on DU145 cells demonstrated that while VMY-1-103 at 10 µM increased the proportion of cells in G1 and G2/M, and decreased the S-phase fraction, there was no significant change in the sub-G1 population of apoptotic cells (Fig. 3A). Likewise, 10 µM VMY-1-103 did not significantly increase the sub-G1 population of cells in the immortalized prostate epithelial cell line PWR-1E (Fig. 3B).
Figure 3.
(A) Effect of VMY-1-103 on cell cycle progression and subG1-phase associated apoptosis in (A) DU145 cells treated with HRG, (B) PWR-1E cells treated with HRG. Values are percent change (average ± SD, N = 3) versus cells treated with the vehicle, DMSO. Asterisk, p ≤ 0.05.
VMY-1-103 induces apoptosis via induction of p53, caspase-3 activity and PARP cleavage.
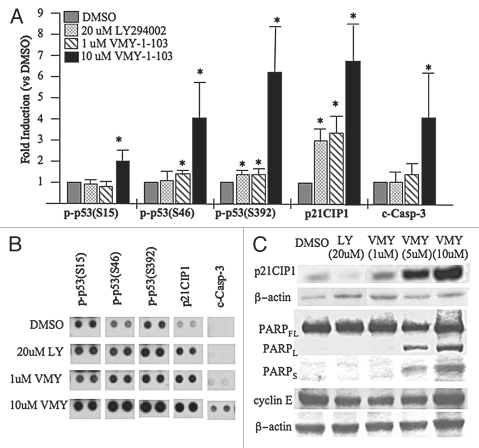
In order to begin to investigate the mechanisms by which VMY-1-103 regulated apoptosis, antibody proteomic arrays were performed. Treatment of HRG-stimulated LNCaP cells with 10 µM VMY-1-103 significantly increased phosphorylation of p53 at serine residues 15, 46 and 392 (Fig. 4A and B), while LY294002 or 1 µM VMY-1-103 had only modest effects versus DMSO control. In addition, 10 µM VMY-1-103 significantly increased the levels of cleaved caspase-3 (“c-casp-3”), the active form generated by proteolytic maturation (Fig. 4). Western blotting further revealed dose-dependent increases in PARP-cleavage and levels of the Cdk inhibitor, p21CIP1 (Fig. 4C). The levels of the late-G1-phase cell cycle regulatory protein cyclin E were unaffected (Fig. 4C), suggesting the cells had undergone a switch from a VMY-1-103-induced cell cycle arrest to apoptosis.
Figure 4.
Activation of pro-apoptotic signaling by VMY-1-103. (A) Regulation of serine phosphorylation of the tumor suppressor protein, p53 (p-p53) as well as levels of p21CIP1 and cleaved caspase-3 (c-casp-3) in LNCaP cells treated with HRG and either 20 µM LY294002, 1 µM VMY-1-103 or 10 µM VMY-1-103. Data are fold induction ± SE (N = 4 measurements). (B) Representative data from the antibody microarrays. (C) Western blot showing induction of p21CIP1 levels and PARP cleavage by VMY-1-103. The FL, L and S subscripts stand for full-length PARP or the long and short cleaved fragments, respectively. Asterisk, p ≤ 0.05.
Apoptosis induced by VMY-1-103 is associated with early mitochondrial membrane depolarization.
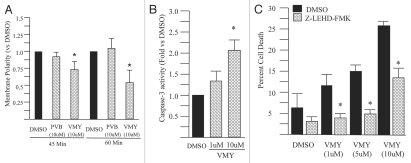
During apoptosis, mitochondria undergo changes in membrane permeability that result in its depolarization. To further explore the mechanisms by which VMY-1-103 affected cell viability, mitochondrial polarity measurements were performed using Mitotracker Red CMXRos and flow cytometry. Addition of 10 µM VMY-1-103, but not 10 µM purvalanol B, resulted in statistically significant decreases in mitochondrial membrane polarity by 45 minutes of treatment (Fig. 5A). These results indicated that mitochondrial depolarization is an early apoptosis-inducing event following VMY-1-103 treatment. Caspase-3 activity, as measured by Z-DEVD-AMC catalysis, was also significantly induced by 10 µM VMY-1-103 (Fig. 5B).
Figure 5.
Capsase activity in HRG stimulated LNCaP cells treated with VMY-1-103. (A) Effects of 10 µM purvalanol B (PVB) or VMY-1-103 (VMY) on mitochondrial membrane polarity. Data are percent change vs. DMSO control. (B) VMY-1-103 induced caspase-3 activity was measured fluorometrically using the caspase-3 substrate, Z-DEVD-AMC. Data are fold increase (±SE) versus DMSO treated cells (N = 4). (C) Abrogation of VMY-1-103 induced apoptosis through inhibition of capsase-9 via Z-LEH D-FMK. Data are average percent change in trypan blue positive cells (±SD, N = 3) of cells treated with increasing amounts of VMY-1-103 and either DMSO (black bars) or 20 µM Z-LEH D-FMK (hatched bars). Asterisk, p ≤ 0.05.
VMY-1-103 modulates components the intrinsic apoptotic pathway in LNCaP cells.
To assess the role that the intrinsic pathway plays in VMY-1-103-induced apoptosis, the caspase-9 inhibitor Z-LEHD-FMK was used. LNCaP cells were treated with Z-LEHD-FMK or DMSO for 1 hour, followed by 12 hrs of HRG and either DMSO or VMY-1-103. Z-LEHD-FMK significantly reduced the VMY-1-103-induced cell death as measured by trypan blue dye exclusion (Fig. 5C).
Mechanistically, these data establish that 1 µM VMY-1-103-induced G1 arrest in LNCaP cells, perhaps in part through an induction of p21CIP.1 At 10 µM, VMY-1-103 induced components of the intrinsic apoptotic pathway, linking mitochondrial membrane depolarization and alterations in p53 phosphorylation at multiple residues to increased caspase-3 activity and PARP cleavage.
Discussion
Accumulating evidence has demonstrated that the purine-substituted family of CDK-inhibitors, such as purvalanol B, have the potential to be useful cancer therapeutics, however the mechanisms by which they regulate cell function has not been entirely elucidated.
In the present report, we examined the effects of a danslylated purvalanol B analog, VMY-1-103, on cell cycle progression and apoptosis in LNCaP prostate cancer cells. VMY-1-103 inhibited HRG-induced cell cycle progression at the G1 phase at 1 µM, and severely reduced cell viability at 10 µM, resulting in a greater than 25% increase in death as measured by trypan blue dye exclusion. VMY-1-103 at 10 µM also induced mitochondrial membrane depolarization, and pathway analyses established that VMY-1-103 increased levels of cleaved caspase-3 (Fig. 4) and reduced the levels of cIAP-1 and XIAP (Ringer L and Albanese C, unpublished). The loss of cell viability was significantly attenuated by the caspase-9 inhibitor, implicating activation the intrinsic apoptotic pathway by VMY-1-103, resulting in the eventual activation of caspase-3 and PARP cleavage.
Increased nuclear p53 levels, resulting from increased protein levels or somatic mutations, correlate with increased clinical PCa grade and Gleason scores. Our studies revealed that VMY-1-103 at 10 µM induced the phosphorylation levels at serine (Ser) residues 15, 46 and 392 on p53. Activation of p53 can lead to cell cycle arrest, apoptosis or senescence. p53-Ser15 is a target of ATR7 and phosphorylation of p53 on Ser15 stabilizes the protein by preventing interactions with Mdm2 and subsequent ubiquitination. Phosphorylation of p53-Ser392 increases the stability of the p53 tetramerization domain.8 Ck2 and SSRP1,9 target Ser392 for phosphorylation and p38/MAPK can also phosphorylate p53 on Ser392, promoting G2 arrest and/or p53-mediated apoptosis. p38/MAPK and HIPK2 phosphorylate p53 on Ser46, thereby mediating apoptosis through p53 apoptosis-inducing protein 1 (p53AIP1).9 Phosphorylation of p53 at Ser15 is associated with increased levels of p21CIP.1,10 Interestingly, however, induction of p21CIP1 was observed at both low and high levels of VMY-1-103, while p53 Ser15-phosphorylation was only observed at higher doses of VMY-1-103. Our results indicate that Ser15 phosphorylation may not be required for transcriptional induction of p21CIP.1 The p21CIP1 protein has been shown to function either as a pro- or anti-apoptotic factor in numerous other studies,11 whether the increased p21CIP1 levels and the altered phosphorylation status of p53 contribute individually or in combination to activating the intrinsic apoptotic pathway remains to be determined.
We also found that VMY-1-103 was far less effective in inducing apoptosis in prostate cells with compromised p53 function (Fig. 3). DU145 PCa cells express a mutant p53 and the non-cancerous PWR-1E cell line was immortalized with SV40, which inhibits p53 function and these exhibit predominantly nuclear p53.12 In addition, VMY-1-103 failed to increase apoptosis in the p53-null cell line, PC3 (Ringer L and Albanese C, unpublished). Further experiments will be required to establish whether knockdown of p53 desensitizes LNCaP cells to VMY-103, or conversely whether the re-expression of wildtype-p53 restores sensitivity in to VMY-1-103 in DU145 or PC3 cells.
We have shown that VMY-1-103 is an effective anti-tumor compound, especially given its rapid pro-apoptotic effect in LNCaP cells. The addition of a dansyl group both rendered the chemical more biologically active than purvalanol B in vitro and enabled the imaging of VMY-1-103 uptake by confocal microscopy. Our data indicate that VMY-1-103 therefore may be useful for the treatment of at least a large subset of early prostate cancers that express wildtype p53, and preclinical studies will be performed to verify its in vivo delivery and biological efficacy in treating PCa. Importantly the dansylation will allow for the quantification of VMY-1-103's in vivo pharmacodymanics and tissue distribution by mass spectroscopy.
Methods and Materials
Cell lines and cell culture.
The human prostate cancer cell lines LNCaP and DU145 were maintained in RPMI and DMEM, respectively, with 10% FCS, 0.1 mM non-essential amino acids, 100 U/ml Penicillin-Streptomycin and 1 mM sodium pyruvate at 37°C in 5% CO2 as previously described.2,5,13 The non-tumorigenic, immortalized cell line, PWR-1E, was maintained in Keratinocyte Serum Free Medium (K-SFM, Life Technologies) supplemented with 0.05 mg/ml bovine pituitary extract and 5 ng/ml EGF. For heregulin 1β (HRG) stimulation studies, subconfluent (approximately 50%) LNCaP, DU145 or PWR-1E cells were treated with 1 ng/ml HRG (R&D Systems. Minneapolis, MN), as previously described.2 The PI3K inhibitor LY294002 (20 µM), the parent CDK inhibitor purvalanol B (Sigma), or VMY-1-103 (Fig. 1A) at 1, 5 and 10 µM were added to the culture medium for up to 18 hours. DMSO was used as vehicle control.
VMY-1-103.
The drug discovery program at Georgetown University Medical Center designed and synthesized (R)-2-chloro-N-(2-(5-(dimethylamino)naphthalene-1-sulfonamido) ethyl)-4-(2-(1-hydroxy-3-methylbutan-2-ylamino)-9-isopropyl-9H-purin-6-ylamino)benzamide or VMY-1-103.4 Briefly, 2-chloro-N-(2-(5-(dimethylamino)naphthalene-1-sulfonamido) ethyl)-4-(2-f luoro-9-isopropyl-9H-purin-6-ylamino)benzamide (0.47 g, 0.75 mmol), R-(-)-2-amino-3-methyl-1-butanol (0.38 g, 3.76 mmol), and diisopropylethylamine (0.65 mL, 3.76 mmol) were dissolved in n-butanol and placed in a sealed tube. The reaction mixture was heated to 120°C for 24 h. After 24 h R-(-)-2-amino-3-methyl-1-butanol (1.88 mmol) and diisopropylethylamine (1.88 mmol) were added and the reaction was allowed to stir for an additional 48 h. The solvent was removed in vacuo and the crude product dissolved in CH2Cl2. The organic layer was washed with water (25 ml) three times followed by brine (50 mL). The organic layer was separated and dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give 0.300 g of crude product. The crude product was purified by column chromatography.
Flow-cytometry.
The prostate cells were collected by trypsinization, fixed in 10% ethanol and resuspended in PBS containing 20 µg/ml propidium iodide (PI) and 5U RNase A. DNA content was measured using a FACStar Plus dual laser FACSort system (Becton-Dickinson) as previously described.14,15
Immunoblotting.
Protein extracts were separated on 4–20% Tris-glycine gels and electro-blotted onto nitrocellulose.2 Protein levels were assessed using antibodies against P21CIP1 (Santa Cruz, sc481) PARP (Cell Signaling, 9542), or cyclin E (DCS60, Cell Signaling, 2946). Anti-β-actin (Cell Signaling, 4967) was used as loading control.
Cell viability and growth.
Following inhibitor treatment, cell viability was determined by trypan blue exclusion. For the apoptosis assays, Proteome Profiler human apoptosis arrays (R&D Systems) were performed as described in the manufacture's protocol. Briefly, nitrocellulose membranes (spotted with 35 antibodies, in duplicate) were blocked with the array buffer (supplied by the manufacturer) for 1 hour at room temperature, the membranes were washed, and incubated with 500 µg of protein overnight at 4°C. Biotinylated primary antibody (supplied by the manufacturer) was added for 1 hour, followed by a 30-minute incubation with Streptavidin-HRP, and finally ECL reagent (1:3,000; Pierce) solution. Array data was developed on x-ray film and spot areas and intensities were analyzed using ImageJ (NIH) software.
Caspase-3 assays.
LNCaP cells were treated for 12 hours with VMY-1-103 and HRG after which protein was extracted. Protein extracts (25 µg) were adjusted to a final volume of 50 µl using lysis buffer. 50 µl of the caspase-3 substrate Z-DEVD-AMC (7-amino 4-methylcoumarin) was added to each sample in a 96 well plate. AMC accumulation, and thus caspase-3 activity, was measured every 3 minutes for 18 cycles using a Victor 3V cytofluorometer (Perkin Elmer) using 360/40 nm excitation and 460/40 nm emission filters. All caspase activity slopes were plotted graphically to ensure that reactions were proceeding at a linear rate for the time interval measured.
Caspase-9 inhibition.
The irreversible caspase-9 inhibitor, Z-LEHD-FMK (Sigma; St. Louis, MO), was added to cells at a final concentration of 20 µM, 10 minutes prior to exposure to HRG and VMY-1-103. The cells were harvested at 12 hours and stained with trypan blue to determine cell viability.
Mitochondrial membrane polarity.
1 × 106 LNCaP cells, treated with HRG and either DMSO or inhibitors, were incubated with 100 nM of Mitotracker Red CMXRos (Molecular Probes, Eugene, OR) for 45 or 60 minutes at 37°C. Mitochondrial membrane polarity was measured by flow cytometry using a FACStar Plus dual laser FACSort system (excitation at 579 nm, emission 599 nm).
Fluorescent imaging.
LNCap cells were seeded on glass coverslips and treated with HRG and either DMSO or 10 µM VMY-1-103 for 18 h. Cells were washed with PBS and fixed in 10% formalin for 10 minutes. Cells were washed with PBS, stained with 0.1 µg/ml propidium iodide (BD Pharmingen) for 5 minutes, and washed with PBS an additional 3 times. The coverslips were mounted onto glass slides with Tris-buffered fluoro-gel (Electron Microscopy Sciences, Hatfield, PA). Multiphoton confocal microscopy was performed (Zeiss510LSM/META/NLO, 63X oil immersion) at 720 nm/480–520 nm (excitation/emission) for VMY-1-103 and 535 nm/617 nm (excitation/emission) for propidium iodide.
Acknowledgements
Cell cycle analyses were performed in the Lombardi Cancer Center's Flow Cytometry shared resource. Fluorescence microscopy was performed in the Lombardi Cancer Center's Microscopy and Imaging Shared Resource.
Grant support, P30 CA51008, R01CA129003 (CA).
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/12208
Supplementary Material
References
- 1.Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocrine Rev. 1999;20:501–534. doi: 10.1210/edrv.20.4.0373. [DOI] [PubMed] [Google Scholar]
- 2.Casimiro M, Rodriguez O, Pootrakul L, Aventian M, Lushina N, Cromelin C, et al. ErbB-2 induces the cyclin D1 gene in prostate epithelial cells in vitro and in vivo. Cancer Res. 2007;67:4364–4372. doi: 10.1158/0008-5472.CAN-06-1898. [DOI] [PubMed] [Google Scholar]
- 3.Chang YT, Gray NS, Rosania GR, Sutherlin DP, Kwon S, Norman TC, et al. Synthesis and application of functionally diverse 2,6,9-trisubstituted purine libraries as CDK inhibitors. Chem Biol. 1999;6:361–375. doi: 10.1016/S1074-5521(99)80048-9. [DOI] [PubMed] [Google Scholar]
- 4.Yenugonda VM, Deb TB, Grindrod SC, Dakshanamurthy S, Yang Y, Paige M, et al. Design, synthesis and evaluation of fluorescent cyclin-dependent kinase inhibitors in human breast cancer cells. doi: 10.1016/j.bmc.2011.02.052. Submitted 2010. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez OC, Lai EW, Vissapragada S, Cromelin C, Avetian M, Salinas P, et al. A reduction in Pten tumor suppressor activity promotes ErbB-2-induced mouse prostate adenocarcinoma formation through the activation of signaling cascades downstream of PDK1. Am J Pathol. 2009;174:2051–2060. doi: 10.2353/ajpath.2009.080859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vissapragada S, Ghosh A, Ringer L, Salinas P, Brophy A, Peaceman D, et al. Dietary n-3 polyunsaturated fatty acids fail to reduce prostate tumorigenesis in the PB-ErbB-2 × Pten(+/−) preclinical mouse model. Cell Cycle. 2010;9:1824–1829. doi: 10.4161/cc.9.9.11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya S, Ray RM, Johnson LR. Role of polyamines in p53-dependent apoptosis of intestinal epithelial cells. Cell Signal. 2009;21:509–522. doi: 10.1016/j.cellsig.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Oren M, Rotter V. Introduction: p53—the first twenty years. Cell Mol Life Sci. 1999;55:9–11. doi: 10.1007/s000180050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller DM, Lu H. p53 serine 392 phosphorylation increases after UV through induction of the assembly of the CK2.hSPT16.SSRP1 complex. J Biol Chem. 2002;277:50206–50213. doi: 10.1074/jbc.M209820200. [DOI] [PubMed] [Google Scholar]
- 10.Yagi A, Hasegawa Y, Xiao H, Haneda M, Kojima E, Nishikimi A, et al. GADD34 induces p53 phosphorylation and p21/WAF1 transcription. J Cell Biochem. 2003;90:1242–1249. doi: 10.1002/jcb.10711. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Bishop WR, Liu M. Differential effects of cell cycle regulatory protein p21(WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug Resist Updat. 2003;6:183–195. doi: 10.1016/s1368-7646(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 12.Webber MM, Bello D, Quader S. Immortalized and tumorigenic adult human prostatic epithelial cell lines: characteristics and applications. Part I. Cell markers and immortalized nontumorigenic cell lines. Prostate. 1996;29:386–394. doi: 10.1002/(SICI)1097-0045(199612)29:6<386::AID-PROS7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez O, Fricke S, Chien C, Dettin L, Vanmeter J, Shapiro E, et al. Contrast-enhanced in vivo imaging of breast and prostate cancer cells by MRI. Cell Cycle. 2006;5:113–119. doi: 10.4161/cc.5.1.2295. [DOI] [PubMed] [Google Scholar]
- 14.Albanese C, D'Amico M, Reutens AT, Fu M, Watanabe G, Lee RJ, et al. Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J Biol Chem. 1999;274:34186–34195. doi: 10.1074/jbc.274.48.34186. [DOI] [PubMed] [Google Scholar]
- 15.Albanese C, Wu K, D'Amico M, Jarrett C, Joyce D, Hughes J, et al. IKKalpha Regulates Mitogenic Signaling through Transcriptional Induction of Cyclin D1 via Tcf. Mol Biol Cell. 2003;14:585–599. doi: 10.1091/mbc.02-06-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.