Abstract
The current classification of ductal carcinoma in situ (DCIS) is based on nuclear grade, architectural differentiation and the presence of necrosis that does not adequately predict the likelihood of recurrence after breast conserving therapy; therefore, there is a critical need to identify novel predictors of DCIS progression. Ninety seven cases of DCIS were included in the study. CD10 and SP ARC expression in tumor stroma was assessed by standard immunoperoxidase method with ani-CD 10 and anti-SP ARC antibodies. The staining was scored semi-quantitatively as negative, weak or strong. Statistical analysis was performed using the Fisher's exact test. Multivariable analysis was conducted utilizing Exact Logistic Regression software (SAS 9.1 and LogExact). A significant association was observed between the recurrence status and time to recurrence with expression of CD10 (p < 0.001) and SP ARC (p < 0.001). When combining both SP ARC and CD10 expression there was a strong correlation with the shortest time to recurrence. Stromal CD10 and SP ARC expression are new markers of an increased risk for DCIS recurrence, independent of commonly assessed clinical parameters. Thus, stromal CD10 and SP ARC expression levels are promising markers of DCIS recurrence and warrant evaluation in larger prospective studies.
Key words: DCIS, cancer stroma, SPARC, CD10
Introduction
The diagnosis of ductal carcinoma in situ (DCIS) has increased since the early 1980s mainly due to increased mammographic screening.1 Currently DCIS accounts for 20–30% of newly diagnosed breast cancers in screened populations.2 It is estimated that approximately 58,000 new DCIS cases were diagnosed in US in 2008.3
Treatments for DCIS include breast-conserving surgery (BCS) alone, BCS with radiotherapy, and BCS with radiotherapy and hormonal therapy.4,5 Currently there is no consensus on which women should be treated with the different regimens. Local recurrence rates for all patients range from 10–40% with half of all patients with recurrence developing invasive carcinoma.6 The current classification of DCIS based on nuclear grade, architectural differentiation and the presence of necrosis does not adequately predict the likelihood of DCIS recurrence. Therefore, there is a critical need to identify novel predictors of DCIS recurrence.
Tumor cells grow in a complex microenvironment composed of (i) non-epithelial cells (including fibroblasts, pericytes, endothelial and inflammatory cells), (ii) extracellular matrix and (iii) secreted diffusible growth factors/cytokines.7,8 The tumor microenvironment has been postulated to play a key role in cancer initiation, progression and metastasis.9 Recently, proteins associated with invasive breast carcinoma stroma have been shown to correlate with prognosis; expression of both stromal CD10 and SPARC (secreted protein acid rich in cysteine) have been associated with decreased survival.10,11 CD10 functionally is a cell surface zinc-dependent metalloproteinase.10 In general, metalloproteinases can extensively remodel the extracellular matrix (ECM). SPARC is a multi-functional glycoprotein that acts through many signaling pathways and can regulate ECM interactions as well as tissue remodeling.12 Based on this functional work, CD10 and SPARC may likely play an integral and early role in tumor invasion.
To date, there have been no studies addressing the clinical significance of stromal CD10 or SPARC expression in DCIS. Here, we evaluated the predictive value of stromal CD10 and SPARC proteins in DCIS and examined their association with clinicopathological variables and DCIS recurrence.
Results
Of 97 patients, 21 underwent some form of recurrence. Thirteen patients recurred as DCIS and eight as invasive carcinoma. The overall recurrence rate in this study population and percentage of patients progressing to invasive disease are consistent with published rates.13,14 CD10 marker could be measured in stroma of 96 patients and SPARC was measured in stroma of 92 patients. One patient with recurrence had only SPARC but not CD10 marker measured.
Expression of stromal CD10 and SPARC in association with clinicopathologic data.
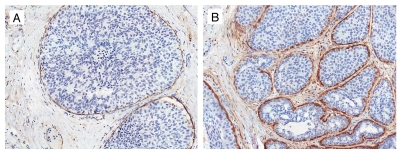
CD10. In normal breast tissue, strong CD10 expression was present in the myoepithelial cells. While majority of DCIS cases maintained myoepithelial cell CD10 expression, a small number of cases had focally absent or attenuated expression. Intralobular stroma in normal breast showed weak CD10 expression, while extralobular stroma was negative. No CD10 staining was present in the luminal epithelial cells. The 96 DCIS cases had stromal CD10 expression. Of those 96 cases, 78 patients had absent or weak expression and 18 patients had high CD10 expression. Representative examples are shown in Figure 1.
Figure 1.
Expression of CD10 in stroma in ductal carcinoma in situ. (A) Weak stromal CD 10 expression with myoepithelial cells showing strong staining. (B) Strong stromal CD 10 expression (CD 10 immunohistochemical stain ×200).
The presence of necrosis, estrogen and progesterone receptors positivity, and HER2 expression were not significantly associated with CD10. However, there was a trend toward CD10 expression and high nuclear grade (Table 1). Interestingly, there was a statistically significant association between younger patient age and higher stromal CD10 expression.
Table 1.
Univariate association between CD10 and recurrence or various clinicopathologic factors
| CD10 category: no (%) | ||||
| 0 | 1 | 2 | p value | |
| Recurrence | <0.001 | |||
| no | 46 (61%) | 24 (32%) | 6 (8%) | |
| yes | 2 (10%) | 6 (30%) | 12 (60%) | |
| Age Group | 0.021 | |||
| <=40 | 3 (43%) | 1 (14%) | 3 (43%) | |
| 40–60 | 19 (38%) | 19 (38%) | 12 (24%) | |
| >60 | 26 (67%) | 10 (26%) | 3 (8%) | |
| Nuclear grade | 0.075 | |||
| 1 | 13 (59%) | 9 (41%) | 0 (0%) | |
| 2 | 20 (48%) | 11 (26%) | 11 (26%) | |
| 3 | 15 (47%) | 10 (31%) | 7 (22%) | |
| Comedo type | 0.524 | |||
| No | 35 (51%) | 23 (33%) | 11 (16%) | |
| yes | 13 (48%) | 7 (26%) | 7 (26%) | |
| ER | 0.496 | |||
| neg | 0.00 | 2 (20%) | 1 (10%) | |
| pos | 41 (48%) | 28 (33%) | 17 (20%) | |
| PR | 0.768 | |||
| neg | 8 (50%) | 6 (38%) | 2 (13%) | |
| pos | 40 (50%) | 24 (30%) | 16 (20%) | |
| Her2 | 1.000 | |||
| neg | 32 (50%) | 20 (31%) | 12 (19%) | |
| pos | 16 (50%) | 10 (31%) | 6 (19%) | |
| Subtype | 0.504 | |||
| ER-Her2+ | 6 (86%) | 1 (14%) | 0 (0%) | |
| ER-PR-Her2− | 1 (33%) | 1 (33%) | 1 (33%) | |
| ER+Her2− | 31 (51%) | 19 (31%) | 11 (18%) | |
| ER+Her2+ | 10 (40%) | 9 (36%) | 6 (24%) | |
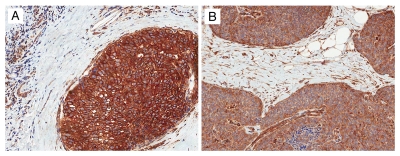
SPARC. The expression of SPARC was most prominent in fibroblasts adjacent to the DCIS lesions. Strong staining was present in 24 cases and weak in 73 cases, respectively. Stroma distant to the DCIS lesion had weak or absent SPARC expression. Endothelial cells and myoepithelial cells showed SPARC staining. Luminal epithelial cells showed weak positivity in majority of cases. Representative examples of SPARC staining are shown in Figure 2.
Figure 2.
Expression of SPARC in stroma in ductal carcinoma in situ. (A) Weak stromal SPARC expression with myoepithelial cells, luminal cells and endothelial cells showing strong staining. (B) Strong stromal SPARC expression (SPARC immunohistochemical stain ×200).
None of the standard potential prognostic factors (nuclear grade, necrosis, comedo type, ER, PR and HER2) with significantly associated with stromal SPARC expression (Table 2). As with CD10 expression levels, there was a statistically significant association between younger patient age at diagnosis and high stromal SPARC expression.
Table 2.
Univariate association between SPARC and recurrence or various clinicopathologic factors
| SPARC category: no (%) | ||||
| 0 | 1 | 2 | p value | |
| Recurrence | <0.001 | |||
| no | 27 (38%) | 33 (46%) | 11 (15%) | |
| yes | 2 (10%) | 6 (29%) | 13 (62%) | |
| Age Group | 0.004 | |||
| <=40 | 3 (43%) | 1 (14%) | 3 (43%) | |
| 40–60 | 10 (20%) | 21 (43%) | 18 (37%) | |
| >60 | 16 (44%) | 17 (47%) | 3 (8%) | |
| Nuclear grade | 0.345 | |||
| 1 | 9 (43%) | 10 (48%) | 2 (10%) | |
| 2 | 12 (29%) | 17 (41%) | 12 (29%) | |
| 3 | 8 (27%) | 12 (40%) | 10 (33%) | |
| Comedo type | 0.762 | |||
| No | 22 (33%) | 29 (43%) | 16 (24%) | |
| Yes | 7 (28%) | 10 (40%) | 8 (32%) | |
| ER | 0.273 | |||
| neg | 5 (50%) | 2 (20%) | 3 (30%) | |
| pos | 24 (29%) | 37 (45%) | 21 (26%) | |
| PR | 0.771 | |||
| neg | 6 (40%) | 6 (40%) | 3 (20%) | |
| pos | 23 (30%) | 33 (43%) | 21 (27%) | |
| Her2 | 0.109 | |||
| neg | 17 (27%) | 31 (50%) | 14 (23%) | |
| pos | 12 (40%) | 8 (27%) | 10 (33%) | |
| Subtype | 0.064 | |||
| ER-Her2+ | 5 (71%) | 0 (0%) | 2 (29%) | |
| ER-PR-Her2− | 0 (0%) | 2 (67%) | 1 (33%) | |
| ER+Her2− | 17 (27%) | 32 (52%) | 13 (21%) | |
| ER+Her2+ | 7 (28%) | 10 (40%) | 8 (32%) | |
Prognostic significance of stromal CD10 and SPARC expression.
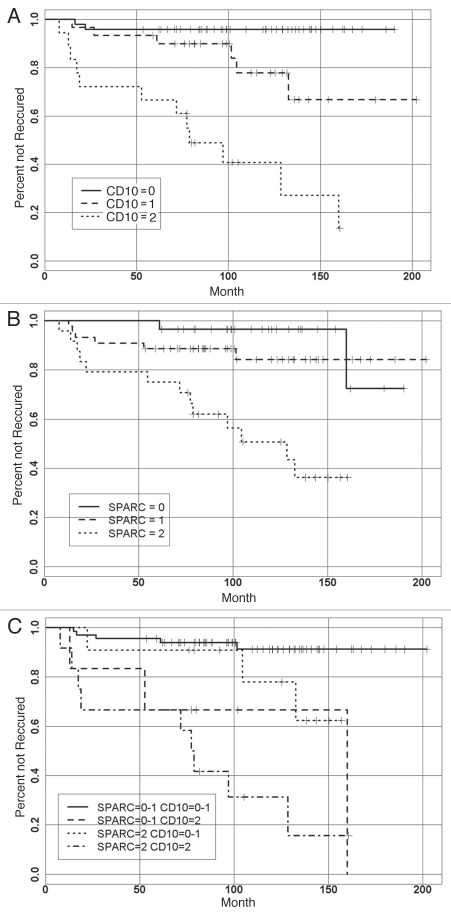
A significant association was observed between the recurrence status and time to recurrence (Fig. 3A and B) with expression of CD10 (p < 0.001) and SPARC (p < 0.001) (Tables 1 and 2). Recurrence was observed in 2 of 48 (4%) patients with CD10 = 0; in 6 of 30 (20%) patients with CD10 = 1; and in 12 of 18 (66%) patients with CD10 = 2. Also, recurrence was observed in 2 of 27 (7%) patients with SPARC = 0; in 6 of 44 (13%) patients with SPARC = 1; and in 13 of 24 (54%) patients with SPARC = 2. When combining both SPARC and CD10 expression there was a strong correlation with the shortest time to recurrence (Fig. 3C). None of the standard potential prognostic factors (nuclear grade, necrosis, comedo type, ER, PR and HER2) were significantly associated with the recurrence status or with the time to recurrence.
Figure 3.
Kaplan-Meier curves for stromal SPARC and CD 10 expression and time to recurrence among DCIS patients. (A) Strong stromal SPARC expression is associated with shorter time to recurrence. (B) Strong stromal CD10 expression is associated with shorter time to recurrence. (C) Strong expressions of both SPARC and CD10 are associated with the shortest time to recurrence.
In the multivariate logistic model incorporating dichotomized new markers (0–1 vs. 2), both CD10 and SPARC were significant predictors of recurrence (Table 3). The odds of recurrence were estimated to be 10.0 times higher (odds ratio = 10.2, 95% CI: 2.7, 37.7) for patients with CD10 = 2 than for patients with CD10 = 0 or 1. Similarly, the odds of recurrence were 3.9 times higher (odds ratio = 3.9, 95% CI: 1.1, 14.3) for patients with SPARC = 2, than for patients with SPARC = 0 or 1.
Table 3.
Results from logistic and Cox models predicting DCIS recurrence
| Logistic model for the rate of recurrence | |||
| Odds Ratio | 95% CI | p value | |
| SPARC 2 vs. 0–1 | 3.94 | 1.09–14.26 | 0.037 |
| CD10 2 vs. 0–1 | 10.02 | 2.66–37.71 | 0.001 |
| Cox model for the time to recurrence | |||
| Hazard Ratio | 95% CI | p value | |
| SPARC 2 vs. 0–1 | 2.35 | 0.82–6.72 | 0.112 |
| CD10 2 vs. 0–1 | 5.93 | 2.05–17.13 | 0.001 |
In the multivariate Cox proportional hazards model, only dichotomized CD10 (0–1 vs. 2) was a significant predictors of time to recurrence (hazard ratio HR = 5.9, 95% CI: 2.1, 17.3; p < 0.001), while dichotomized SPARC was not significant (HR = 2.6, 95% CI: 0.8, 6.7; p = 0.112).
Table 4 compares the SPARC and CD10 profiles of patients who recurred with DCIS and with invasive cancer. However, the difference in either marker expression was not significantly different between DCIS and invasive recurrences. CD10 expression patterns were very similar in patients that recurred with DCIS or with invasive carcinoma. Meanwhile, the majority of invasive recurrences (75%) had strong expression of SPARC (SPARC = 2). Since the number of invasive recurrences is small, there is not enough power to detect this trend as statistically significant.
Table 4.
Association of stromal CD10 and SPARC with recurrence type
| CD10 in stroma score | ||||
| Recurrence | 0 | 1 | 2 | p value |
| DCIS | 1 (8%) | 4 (33%) | 7 (58%) | 1.000 |
| Invasive | 1 (13%) | 2 (25%) | 5 (63%) | |
| SPARC in stroma score | ||||
| Recurrence | 0 | 1 | 2 | p value |
| DCIS | 1 (8%) | 5 (38%) | 7 (54%) | 0.4167 |
| Invasive | 1 (13%) | 1 (13%) | 6 (75%) |
Discussion
In this study we have shown that stromal CD10 and SPARC expression are predictors of DCIS recurrence. Patients included in this study were treated by wide excision only and carefully followed allowing us to eliminate the bias of hormone or radiation treatment in evaluating these markers. In the multivariate analysis strong CD10 expression was a significant predictors of time to recurrence (hazard ratio HR = 5.9, 95% CI: 2.1, 17.3; p < 0.001), while dichotomized SPARC was not significant.
Several studies have investigated stromal CD10 and SPARC expression in human malignancies. Stromal CD10 expression was associated with decreased disease specific and overall survival in invasive breast cancer10,11 and was better predictor of disease outcome than lymph-node status, tumor size, histologic grade, or clinical stage. Stromal CD10 expression also correlated with a higher tumor grade and estrogen receptor negative status. To date only one study has addressed the expression of CD10 in DCIS. This study, evaluating 15 DCIS cases, showed a trend towards stromal CD10 expression in high grade DCIS. Our study was first to asses prognostic significance of stromal CD10 expression in DCIS cases with long term follow up data and to examine its expression with clinico-pathologic data. Our study also showed a trend toward higher nuclear grade with CD10 expression and further defined CD10 as a marker of DCIS recurrence.
This study also provides further evidence that stromal expression of CD10 and SPARC may have a functional role in the early events of breast tumorigenesis and DCIS biology. Functionally, CD10, also known as common acute lymphoblastic leukemia antigen (CALLA), is a zinc-dependent membrane metalloproteinase. Matrix metalloproteinases play key roles in controlling cell growth, differentiation and signal transduction by modulating the activity of peptide factors and regulating their access to the receptors.15 In tumor associated stroma, CD10 and other matrix metalloproteinases facilitate invasion and metastasis by degrading extracellular matrix proteins. Our results suggest that CD10 is expressed in a subset of DCIS cases and confers higher risk of DCIS recurrence.
SPARC, also known as osteonectin and is a 32 kDa secreted glycoprotein that interacts with ECM proteins to promote the disassociation of cells from the matrix, thereby promoting cell motility. SPARC plays an important role in wound healing, embryonic development and tumorigenesis.16 In addition to interacting with ECM components SPARC interacts with growth factors such as VEGF and FGF.
Increased SPARC expression has been identified in multiple tumors and is associated with poor prognosis.17–19 In breast carcinomas SPARC was identified as a member of a cluster of genes associated with increased invasive capacity.20 In addition, mRNA levels of SPARC inversely correlate with estrogen receptor status, indicating that SPARC expression is associated with more aggressive breast cancers.18 The role of SPARC in tumorigenesis is complex since it is expressed in epithelial and stromal compartments. Interestingly, epithelial SPARC expression did not confer worse prognosis in lung and pancreatic cancers whereas stromal SPARC expression has been associated with poor clinical outcomes independent of common clincopathologic parameters.17,21 The mechanism by which stromal SPARC expression confers a worse prognosis is not known. One of the potential mechanisms by which SPARC promotes tumorigenesis is angiogenesis. SPARC was initially identified as a protein secreated by endothelial cells in vitro.22 Increased SPARC expression was detected in newly formed vessels in malignant melanoma xenografts and during neovascularization of aortic stenosis.23,24 SPARC was also shown to mediate several stages of epithelial to mesenchymal transition and its expression is a feature of metaplastic breast carcinomas.25
The prognostic significance of stromal CD10 and SPARC expression seen in our study suggests there may be a need for therapeutic efforts that not only target tumor cells, but that also target the juxta-tumoral stroma that provides the supportive microenvironment. Given the success of compounds that target the tumor microenvironment such as bevacizumab, sunitinib and hedgehog pathway inhibitors it is not unreasonable to hope that agents that target the activated fibroblast component of the tumor microenvironment may improve patient outcomes.26–28
In summary, stromal CD10 and SPARC expression are new markers of an increased risk for DCIS recurrence, independent of commonly assessed clinical parameters, including nuclear grade, comedo necrosis, hormone receptor expression and HER2. Future work will decipher if SPARC and CD10 may be used with a panel of other proteins and parameters (including age) to successfully predict the correct clinical management of women diagnosed with DCIS. It is of note that we found a statistically significant connection between SPARC and CD10 stromal expression and age. Perhaps early-age onset DCIS may be facilitated in a setting of enhanced CD10 and SPARC expression and later-age onset patients (with additional or overlapping molecular events) may not require these proteins to progress to a disease state. Importantly, the mechanisms that confer this more aggressive tumor stromal phenotype clearly warrant additional study.
Material and Methods
Patient population.
Ninety seven DCIS cases were included in this study. Their follow up ranged from 53.4 to 202.1 months (median = 110.8, mean = 114.1). Medical charts were reviewed to obtain treatment and follow up information. Local recurrence was our primary endpoint of interest and was defined as DCIS or invasive breast cancer in the ipsilateral breast at least six months after excision of primary DCIS with negative margins. DCIS or invasive disease identified during the first six months after the index DCIS was considered part of the initial disease. Therefore, women with invasive breast cancers identified during this period were excluded from the cohort as they were considered to have invasive disease, and not pure DCIS, at presentation.
Slides from primary DCIS cases were reviewed for histopathologic features. DCIS architectural type (solid, cribriform, micropapillary and comedo), nuclear grade (low, intermediate, high) and presence or absence of necrosis were recorded. Estrogen receptor, progesterone receptor and HER2 status were obtained from pathology reports.
Immunohistochemistry.
Paraffin-embedded blocks were fixed in 10% neutral-buffered formalin and processed for immunohistochemical analysis. Tissue sections were de-paraffinized in xylene, re-hydrated in ethanol, re-hydrated with water, and washed in 1% phosphate-buffered saline. Prior to primary antibody application, slides underwent antigen retrieval. Slides were steamed for 20 minutes in a Dako Target Retrival Solution, pH 6. After HIER treatment, the slides were allowed to cool for 20 minutes and then were washed 3 times in 1% PBS and then sections were then treated with peroxidaze block for 10 min. For CD10 staining, primary antibody (Vector, cat# VP-C328, Burlingame, CA) was applied to slides and incubated for 60-min, using a 1:200-dilution. Secondary antibody used in the process was Mouse Mach3 from Biocare Medical cat#M3M530l. For SPARC staining, a primary antibody (Ab-Cam, cat# ab14174, Cambridge, MA) was applied to slides and incubated for 60-min, using a 1:200-dilution. The secondary antibody used for immuno-staining was Rabbit Mach3 from Biocare Medical (cat # M3M530l). Immune complexes were visualized with the chromogenic substrate Dako Liquid DAB+ Substrate-Chromogen Solution (DAKO K3468) (diaminobenzidine tetrahydrochloride) for 5 minutes.
Staining was scored semi-quantitatively as negative (0; no staining), weak (1; either diffuse weak staining or strong staining in less than 30% of stromal cells) and strong (2; defined as strong staining of 30% of stromal cells).
Statistical methods.
Association between recurrence status and stromal SPARC and CD10, as well as prognostic factors (nuclear grade, necrosis, hormone receptor and HER2 status) was performed using the Fisher's exact test.
Association between the time to recurrence and stromal SPARC and CD10, as well as between the time to recurrence and prognostic factors (nuclear grade, necrosis, comedo, ER, PR and Her2) was analyzed using log-rank test. Time to recurrence was evaluated using the Kaplan-Meier estimate of the survival curves.
For 21 patients who recurred, association between recurrence type (DCIS or invasive) and each new marker was tested using Fisher's exact test.
For multivariate analyses, SPARC and CD10 scores were dichotomized as 2 vs. 0–1. Exact version of logistic regression was used for multivariate analysis of recurrence rates. Cox proportional hazards model was used for the multivariate analysis of time to recurrence. No violations of the proportional hazards assumptions were detected. Data were analyzed in SAS 9.1 (SAS Institute Inc., Cary, NC) and R (free software environment for statistical computing and graphics).
Acknowledgements
A.K.W. was supported by a Young Investigator Award from Breast Cancer Alliance, Inc. and a Susan G. Komen Career Catalyst Grant. M.P.L. and his laboratory were supported by grants from the NIH/NCI (R01-CA-080250; R01-CA-098779; R01-CA-120876; R01-AR-055660), and the Susan G. Komen Breast Cancer Foundation. Funds were also contributed by the Margaret Q. Landenberger Research Foundation (to M.P.L.). This project is funded, in part, under a grant with the Pennsylvania Department of Health (to M.P.L.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/12449
References
- 1.Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer. 2006;106:2104–2112. doi: 10.1002/cncr.21864. [DOI] [PubMed] [Google Scholar]
- 2.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society, author. Cancer Facts & Figures 2008. 2008. http://wwwcancerorg/downloads/STT/50080.
- 4.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, et al. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GF, Solin LJ, Olivotto IA, Ernster VL, Pressman PI. Consensus conference on the treatment of in situ ductal carcinoma of the breast, April 22–25, 1999. Cancer. 2000;88:946–954. [PubMed] [Google Scholar]
- 6.Silverstein MJ, Lagios MD, Martino S, Lewinsky BS, Craig PH, Beron PJ, et al. Outcome after invasive local recurrence in patients with ductal carcinoma in situ of the breast. J Clin Oncol. 1998;16:1367–1373. doi: 10.1200/JCO.1998.16.4.1367. [DOI] [PubMed] [Google Scholar]
- 7.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 10.Iwaya K, Ogawa H, Izumi M, Kuroda M, Mukai K. Stromal expression of CD10 in invasive breast carcinoma: a new predictor of clinical outcome. Virchows Arch. 2002;440:589–593. doi: 10.1007/s00428-002-0639-4. [DOI] [PubMed] [Google Scholar]
- 11.Makretsov NA, Hayes M, Carter BA, Dabiri S, Gilks CB, Huntsman DG. Stromal CD10 expression in invasive breast carcinoma correlates with poor prognosis, estrogen receptor negativity, and high grade. Mod Pathol. 2007;20:84–89. doi: 10.1038/modpathol.3800713. [DOI] [PubMed] [Google Scholar]
- 12.Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16:260–268. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]
- 13.Bijker N, Peterse JL, Duchateau L, Julien JP, Fentiman IS, Duval C, et al. Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in-situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol. 2001;19:2263–2271. doi: 10.1200/JCO.2001.19.8.2263. [DOI] [PubMed] [Google Scholar]
- 14.Boyages J, Delaney G, Taylor R. Predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Cancer. 1999;85:616–628. [PubMed] [Google Scholar]
- 15.Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1993;82:1052–1070. [PubMed] [Google Scholar]
- 16.Funk SE, Sage EH. The Ca(2+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:2648–2652. doi: 10.1073/pnas.88.7.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 18.Watkins G, Douglas-Jones A, Bryce R, Mansel RE, Jiang WG. Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids. 2005;72:267–272. doi: 10.1016/j.plefa.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka M, Kanda K, Li NC, Fukumori T, Oka N, Kanayama HO, Kagawa S. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol. 2001;166:2495–2499. [PubMed] [Google Scholar]
- 20.Iacobuzio-Donahue CA, Argani P, Hempen PM, Jones J, Kern SE. The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res. 2002;62:5351–5357. [PubMed] [Google Scholar]
- 21.Koukourakis MI, Giatromanolaki A, Brekken RA, Sivridis E, Gatter KC, Harris AL, Sage EH. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 22.Sage H, Johnson C, Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984;259:3993–4007. [PubMed] [Google Scholar]
- 23.Prada F, Benedetti LG, Bravo AI, Alvarez MJ, Carbone C, Podhajcer OL. SPARC endogenous level, rather than fibroblast-produced SPARC or stroma reorganization induced by SPARC, is responsible for melanoma cell growth. J Invest Dermatol. 2007;127:2618–2628. doi: 10.1038/sj.jid.5700962. [DOI] [PubMed] [Google Scholar]
- 24.Charest A, Pepin A, Shetty R, Cote C, Voisine P, Dagenais F, et al. Distribution of SPARC during neo-vascularisation of degenerative aortic stenosis. Heart. 2006;92:1844–1849. doi: 10.1136/hrt.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien HC, Hsiao YH, Lin YS, Yao YT, Juan HF, Kuo WH, et al. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: identification of genes potentially related to epithelial-mesenchymal transition. Oncogene. 2007;26:7859–7871. doi: 10.1038/sj.onc.1210593. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 27.Sweeney P, Karashima T, Kim SJ, Kedar D, Mian B, Huang S, et al. Anti-vascular endothelial growth factor receptor 2 antibody reduces tumorigenicity and metastasis in orthotopic prostate cancer xenografts via induction of endothelial cell apoptosis and reduction of endothelial cell matrix metalloproteinase type 9 production. Clin Cancer Res. 2002;8:2714–2724. [PubMed] [Google Scholar]
- 28.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]