Abstract
BZL101 is an aqueous extract from the Scutellaria barbata plant shown to have anticancer properties in a variety of human cancers. In order to determine its efficacy on human reproductive cancers, we assessed the responses of two human breast cancer cell lines, estrogen sensitive MCF7 and estrogen insensitive MDA-MB-231, and of two human prostate cancer cell lines, androgen sensitive LNCaP and androgen insensitive PC3 which are human cell lines that represent early and late stage reproductive cancers. BZL101 inhibited reproductive cancer growth in all cell lines by regulating expression levels of key cell cycle components that differ with respect to the cancer cell phenotypes. In early stage estrogen sensitive MCF7 cells, BZL101 induced a G1 cell cycle arrest and ablated expression of key G1 cell cycle regulators Cyclin D1, CDK2 and CDK4, as well as growth factor stimulatory pathways and estrogen receptor-α expression. Transfection of luciferase reporter plasmids revealed that the loss of CDK2, CDK4 and estrogen receptor-α transcript expression resulted from the BZL-dependent ablation of promoter activities. BZL101 growth arrests early stage androgen sensitive LNCaP cells in the G2/M phase with corresponding decreases in Cyclin B1, CDK1 and androgen receptor expression. In late stage hormone insensitive breast (MDA-MB-231) and prostate (PC3) cancer cells, BZL101 induced an S phase arrest with corresponding ablations in Cyclin A2 and CDK2 expression. Our results demonstrate that BZL101 exerts phenotype specific anti-proliferative gene expression responses in human breast and prostate cancer cells, which will be valuable in the potential development of BZL-based therapeutic strategies for human reproductive cancers.
Key words: Scutellaria barbata, BZL101, cell cycle, CDK gene expression, breast cancer, prostate cancer, steroid receptors
Introduction
Reproductive cancer is the second leading cause of cancer death and affects 219,000 people in the United States each year, with 27,000 succumbing to the disease.1 Reproductive cancers are classified by their ability to proliferate in response to steroid hormones; estrogens in the case of breast cancer and androgens in the case of prostate cancer.2 Steroid binding to cognate receptors induce the active conformation of steroid receptors, allowing them to interact with DNA and/or transcription factors within their target gene promoters and associate with appropriate nuclear cofactors to modulate downstream target gene transcription.3,4 At the time of diagnosis, over two-thirds of all breast cancers are estrogen sensitive and 80% of prostate cancers are androgen sensitive. However, both breast and prostate cancers can transition beyond their initial hormone dependent state to a more advanced “hormone insensitive” state.5,6 This change in phenotype is often observed after long term administration of endocrine disruptors used to halt cancer progression.7,8
Several therapies currently exist for selectively targeting hormone-dependent growth of human reproductive cancers. For breast cancer, aromatase inhibitors are the preferred current clinical option, which act by inhibiting estrogen synthesis, thereby decreasing the amount of ligand available for binding to estrogen receptors (ERs). Selective estrogen receptor modulators (SERMs) and selective estrogen receptor downregulators (SERDs) are implemented as an alternative treatment when aromatase inhibitors prove ineffective. SERMs act as antagonists to ER activity, while SERDs ablate ER expression. The primary treatment for patients with prostate cancer is chemical castration,9 aimed at reducing the levels of circulating androgens in the body.
Treatment options that target steroid receptor expression and function are only effective until the cancer transitions beyond hormone sensitivity, which occurs in 80% of prostate cancers within 3 years of diagnosis10 and 20% of breast cancers within 5 years of diagnosis.11,12 Therapies targeting the hormone-insensitive forms of reproductive cancer include radiation and chemotherapy, aimed at inducing enough DNA damage to halt replication of actively dividing cells, including cancer cells. Unfortunately, the off-target effects of radiation and chemotherapy induce harsh and sometimes fatal side effects. Therefore, there exists a critical need for alternative therapies, which could ideally target both hormone sensitive and insensitive reproductive cancers with minimal side effects.
Phytochemicals are a natural source of pharmaceuticals with long histories of human interaction in traditional medicines, which has led to over 60% of anticancer drugs developed in the last 20 years being derived from natural products.13 One such herb, Scutellaria barbata, known colloquially as Ban Zhi Lian (BZL101), has been shown to have anticancer properties in hepatocarcinoma and leukemia. More specifically, BZL101 is able to induce G1 cell cycle arrest followed by apoptosis of these cancers14,15 with effective concentrations of 2 mg/mL and 1 mg/mL, respectively. Aqueous extracts of BZL101 contain several active anticancer compounds,16 which are able to block glycolysis and induce cell death in a number of cancer cell lines,17 and is well tolerated by metastatic breast cancer patients.18 While the effect of BZL101 on the metabolic functioning of cancer cells has been studied, relatively little is known about how BZL101 regulates proliferative signaling and cell cycle control within human reproductive cancer cells. Virtually nothing is known about the BZL101 control of steroid and growth factor signaling pathways, which are both critical to the promotion and progression of reproductive cancers. In addition, to date no studies have systematically compared the effects BZL101 can exert on hormone sensitive and insensitive breast and prostate cancers cells representative of early and late stage cancer phenotypes, even though this preclinical approach could help in the development of BZL101 as a treatment option for reproductive cancers with increased efficacy and reduced side effects. In this report, we show that BZL101 exerts phenotype specific anti-proliferative gene expression responses in human breast and prostate cancer cells that represent early state hormone sensitive cancers as well as later state hormone insensitive cancers.
Results
Anti-proliferative effects of BZL101 in early stage hormone-sensitive and late stage hormone-insensitive human breast and prostate cancer cell lines.
Different classes of human breast and prostate cancer cell lines are available that represent a range of distinct cancer phenotypes that, for example, display properties consistent with early or later stage cancers. Human MCF7 breast cancers and human LNCaP prostate cancer cells are steroid sensitive and are considered cell line models for early stage cancers, whereas, human MDA-MB-231 breast cancer cells and human PC3 prostate cancer cells are steroid independent and are representative of later stage cancers. These four cell lines were employed to assess whether the cell phenotype influences the anticancer properties of BZL101. To initially determine the effects of BZL101 on overall cell proliferation, each of the four human reproductive cancer cell lines were treated with varying concentrations of BZL101 for 48 hrs and the DNA content was monitored by flow cytometry of propidium iodide stained nuclei. The intensity of DNA staining was used to assess the ability of BZL101 to segregate each cell population into apoptotic cells represented by those displaying a sub-G1 DNA content due to DNA fragmentation as well as cells in G1, S and G2/M phase of the cell cycle.
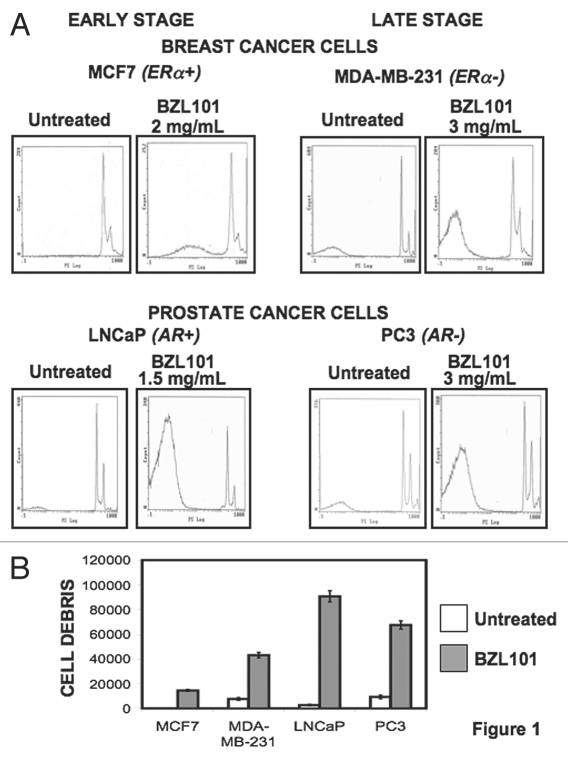
Flow cytometry analysis revealed that BZL101 induced a significant increase in the sub-G1 fraction of cellular staining of each of the cell lines. Figure 1 shows the data from only the optimal BZL101 concentrations for each cell line at 48 hrs of treatment, which represents the near maximal conditions of the response. A significant increase in the sub-G1 peaks of MCF7 and MDA-MB-231 breast cancer cell lines was observed at 2.0 mg/mL and 3.0 mg/mL BZL101, respectively (Fig. 1A). Additionally, LNCaP prostate cancer cells had a maximum sub-G1 peak at a concentration of 1.5 mg/mL BZL101, and PC3 prostate cancer cells exhibited similar effects at 3.0 mg/mL (Fig. 1A). These doses were employed in the subsequent experiments in this study. Quantification of the BZL101-induced increase in DNA fragmentation for each reproductive cancer cell line is shown in Figure 1B.
Figure 1.
Flow cytometry analysis of the anti-proliferative effects of BZL101 in early stage hormone sensitive and late stage hormone insensitive breast and prostate cancer cell lines BZL101 or the vehicle control (untreated control) was applied to the following cancer cell lines: MCF7 (2 mg/mL), MDA-MB-231 (3 mg/mL), LNCaP (1.5 mg/mL) and PC3 (3 mg/mL) for 48 hr. Cells were hypotonically lysed and stained with propidium iodide prior to analysis by coulter cell counter. Cell count versus PI staining is displayed (n = 10,000) per treatment. All analysis was performed in triplicate.
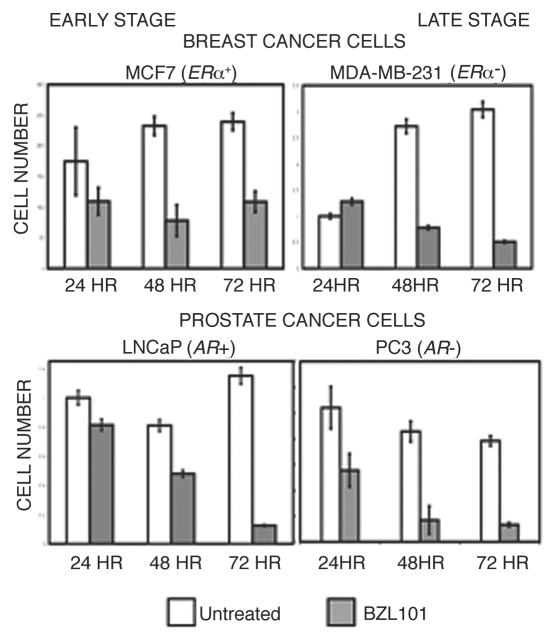
As a complementary approach to assess the anti-proliferative effects of BZL101, cell number was monitored throughout a time course of BZL101 treatment at the optimal concentrations for each reproductive cancer cell line. Although each of the cell lines showed some differences in the kinetics of the response, treatment with BZL101 caused a loss of cell number that was clearly observed after 48 hrs of exposure (Fig. 2). This result is consistent with the observed induction of sub-G1 DNA content, and confirms that BZL101 has a potent overall anti-proliferative response.
Figure 2.
BZL101 inhibits proliferation of human breast and prostate cancer cells MCF7, MDA-MB-231, LNCaP and PC3 human reproductive cancer cells were treated with BZL101 or untreated control over a 72 hr time course. Cells were stained with Trypan blue and cell number accessed with a hemocytometer. Results shown are representative of three independent experiments.
BZL101 induces distinct cell cycle arrests in early and late stage breast and prostate cancer cell lines.
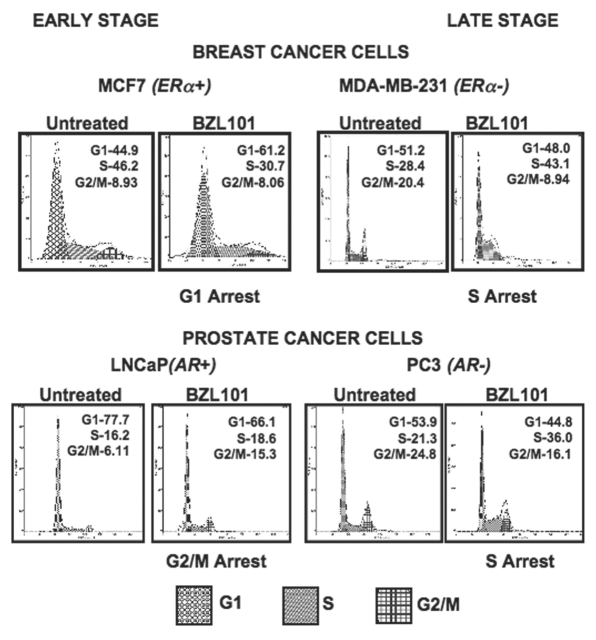
Along with an increase in cells with a sub-G1 DNA content, flow cytometry profiles revealed that treatment of the reproductive cancer cell lines with the corresponding optimal doses of BZL101 induced distinct cell cycle arrests. As shown in Figure 3, an S-phase arrest occurred in both of the tested late stage, hormone insensitive cell lines, MDA-MB-231 breast cancer cells and PC3 prostate cancer cells, as well as in another late stage prostate cancer cell line DU145 (data not shown). BZL101 treatment caused these reproductive cancer cell lines to exhibit a 15% or more increase in percentage of cells in S-phase. The hormone sensitive cell lines MCF7 breast cancer cells and LNCaP prostate cancer cells displayed a BZL101 induced G1 phase arrest (15% increase) and G2/M phase arrest (9%), respectively. These results suggest that BZL101 has specific cell cycle effects that differ depending on the reproductive target cell phenotype.
Figure 3.
BZL101 induction of cell cycle arrest in hormone sensitive and insensitive breast and prostate cancer cell lines. BZL101 was applied to cancer cell lines and compared to untreated control: MCF7 (2 mg/mL), MDA-MB-231 (3 mg/mL), LNCaP (1.5 mg/mL) and PC3 (3 mg/mL) for 48 hr. Cells were hypotonically lysed and stained with propidium iodide prior to analysis by coulter cell counter. Cell count versus PI staining is displayed (n = 10,000) per treatment. All analysis was performed in triplicate.
Selective BZL101-regulated protein production of cell cycle components in human reproductive cancer cells.
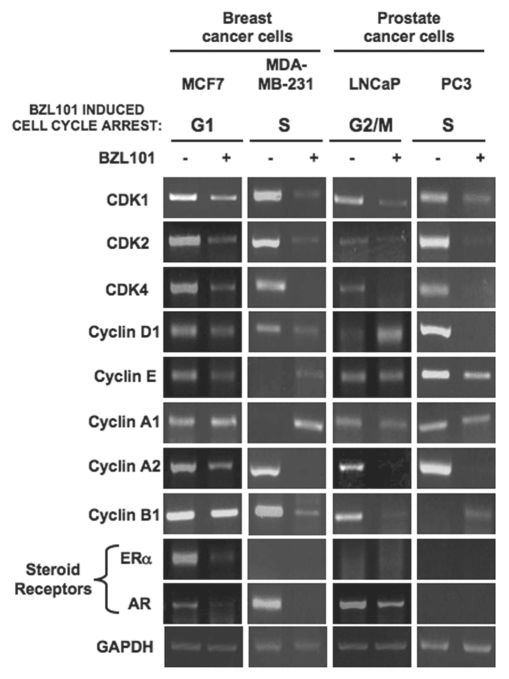
To further characterize the cell cycle arrests, the early stage hormone sensitive and the late stage hormone insensitive breast and prostate cancer cell lines were treated with the optimal concentrations of BZL101 for 48 hrs and effects on expression levels of cell cycle proteins (Fig. 4) and transcripts (Fig. 5) were determined by western Blot and RT-PCR analyses, respectively. Actin levels were used as a constitutive control for the protein analysis and GAPDH expression was used as a positive control for the RT-PCR experiments.
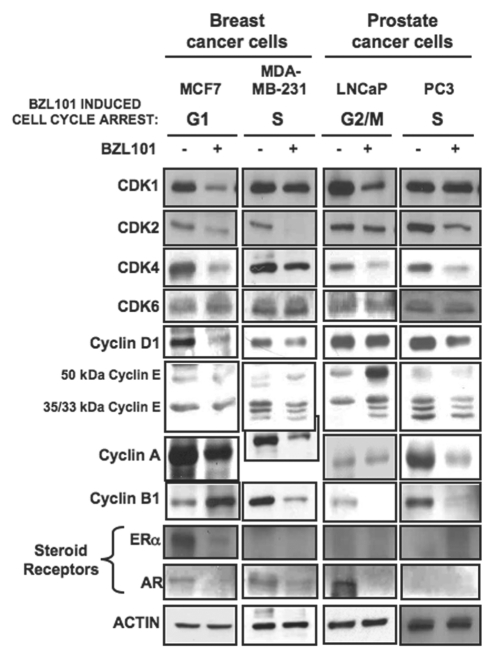
Figure 4.
Effects of BZL101 on the protein levels of cell cycle regulators and steroid receptors BZL101 was applied to cancer cell lines: MCF7 (2 mg/mL), MDA-MB-231 (3 mg/mL), LNCaP (1.5 mg/mL) and PC3 (3 mg/mL) for 48 hr and compared to untreated control. Total cell lysates were subjected to SDS-PAGE and the levels of cell cycle regulators and HSP 90 (loading control) protein was monitored by western blot analysis.
Figure 5.
Effects of BZL101 on the transcripts levels of cell cycle regulatory components and steroid receptors. BZL101 was applied to cancer cell lines: MCF7 (2 mg/mL), MDA-MB-231 (3 mg/mL), LNCaP (1.5 mg/mL) and PC3 (3 mg/mL) for 48 hr and compared to untreated control. RNA levels of cell cycle regulatory components were determined by RT-PCR. The PCR products were visualized on a 1.2% agarose gel stained with ethidium bromide. GAPDH provided a gel loading control for the RT-PCR. Representative gels of three independent experiments are shown.
Consistent with G1 cell cycle arrest observed in early stage MCF7 breast cancer cells, treatment with BZL101 strongly downregulated protein levels of the G1 acting cyclin dependent kinases CDK2 and CDK4 and the G1-specific cyclin D1, with only a modest effect on cyclin E protein levels and no significant change in the level of the G1-acting CDK6 protein (Fig. 4). In MCF7 cells, BZL101 also altered expression of two G2/M cell cycle components; inducing an increase in cyclin B1 protein levels and downregulating CDK1 protein levels (Fig. 4). The BZL101-mediated downregulation of CDK2, CDK4, cyclin D1 and CDK1 were accounted for by the loss of their corresponding transcripts (Fig. 5). The modest downregulation of cyclin A protein results from a specific loss of cyclin A1 transcripts (Figs. 4 and 5).
The overall pattern of regulated cell cycle proteins levels by BZL101 in the early stage prostate cancer cell line, LNCaP cells, which undergoes a G2/M cell cycle arrest, had a noticeable overlap with the pattern observed in MCF7 breast cancer cells. As shown in Figure 4, 48 hrs of BZL101 treatment decreased CDK1 and cyclin B1 levels, both critical to progression through G2/M, as well as downregulating the G1-acting CDK2 and to a lesser extent CDK4. In LNCaP cells, BZL101 also induced the level of the unprocessed 50 kDa cyclin E, which is associated with reduced CDK2 enzymatic activity. Analysis of transcript levels revealed a strong downregulation of the G2/M-acting Cyclin A1, Cyclin A2 and Cyclin B1, as well as of the G1-acting CDK4 transcripts (Fig. 5).
In both late stage hormone insensitive reproductive cancer cells, MDA-MB-231 breast cancer cells and PC3 prostate cancer cells, the optimal 3.0 mg/mL BZL101 induced S-phase arrests. Protein expression analysis of cell cycle components in both cell lines revealed a nearly identical response pattern to BZL101, as CDK2, cyclin D, cyclin A, cyclin B protein levels significantly decreased and CDK4 levels show a modest decrease (Fig. 4). Because the CDK2/cyclin A complex is important for S-phase progression, the reduced expression of these two components by BZL101 effectively blocks the ability of late stage breast and prostate cancer cell lines to progress through the cell cycle. The downregulation of these cell cycle proteins could account for the selective loss of their transcript levels (Fig. 5).
Effects of BZL101 on estrogen receptor and androgen receptor expression.
In hormone responsive human reproductive cancers, steroid receptors play important roles in regulating progression through specific phases of the cell cycle. For example, estrogen receptor-alpha (ERα) is a critical regulator of G1-S phase cell cycle progression in estrogen responsive breast cancer cells with both cyclin D1 and cyclin A2 being known transcriptional targets for ERα.19,20 Therefore, we assessed whether BZL could alter the expression and/or function of this steroid receptor as part of its cell cycle effects. A combination of western blots (Fig. 4) and RT-PCR analysis (Fig. 5) revealed that ERα protein and transcript levels significantly decrease in MCF7 human breast cancer cells in response to BZL101 treatment, indicating that BZL101 possesses intrinsic selective estrogen receptor downregulator (SERD) activity. As expected, the other three tested reproductive cancer cell lines do not produce ERα transcripts. Analysis of the effects of BZL101 on ERα promoter activity in MCF7 cells is described in a following section.
The potential effects of BZL101 on androgen receptor (AR) expression is more complicated because this nuclear receptor exists in both 110 kDa and an 87 kDa isoforms that differ in the number of CAG poly-glutamine repeats in the first exon. Treatment with BZL101 strongly downregulated the protein and transcript expression of the 110 kDa AR in hormone sensitive LNCaP prostate cancer cells, whereas, the hormone insensitive PC3 prostate cancer cell do not express AR (Figs. 4 and 5). Both the MCF7 and the MDA-MB-231 human breast cancer cell lines express the 87 kDa AR, and BZL101 reduced expression of this receptor in both cell lines (Figs. 4 and 5). Taken together, our results suggest that BZL101 can disrupt steroid receptor signaling in human reproductive cancer cells.
BZL101 regulated CDK2, CDK4 and ERα gene expression through downregulation of promoter activities in MCF7 breast cancer cells.
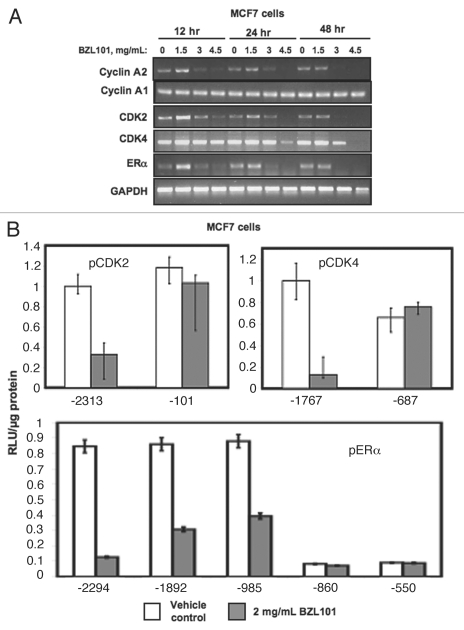
The ability of BZL101 to induce a G1 cell cycle arrest and strongly downregulate ERα expression necessitated the determination of kinetics of cell cycle arrest to compare the loss of CDK2, CDK4 and ERα transcript expression in MCF7 cells treated with multiple concentrations of BZL101 over a 48 hr time course. As shown in Figure 6A, BZL101 strongly downregulated CDK2 and ERα transcript expression within 12 hrs of treatment, which occurs prior to the observed cell cycle arrest at 24 hrs. In contrast, the BZL101 downregulation of CDK4 transcripts occurred over a longer duration of exposure that correlated with the kinetics of the growth arrest (Fig. 6A compared to Fig. 1).
Figure 6.
Kinetics of BZL101 downregulation and loss of promoter activities of cell cycle components and estrogen receptor-alpha in MCF7 cells. (Upper) MCF7 cells were treated with 2 mg/mL of BZL101 over a 48 hr time course. RNA levels of cyclin A1, cyclin A2, CDK2, CDK4 and ERα were determined by RT-PCR. The PCR products were visualized on a 1.2% agarose gel stained with ethidium bromide. GAPDH provided a gel loading control for the RT-PCR. Representative gels of three independent experiments are shown. (Lower) MCF-7 cells were transfected with the indicated CDK2, CDK4 or ERα promoter 5′ deletion constructs linked to a luciferase reporter gene, and 24 hr post-transfection cells were treated for 24 hr with 2 mg/mL BZL101 and compared to untreated control. Relative luciferase activity was evaluated in lysed cells using the Promega Luciferase Assay Kit and normalized to the reporter plasmid activity of the longest promoter fragment for each gene in cells treated with Untreated controls. Two transfection controls (data not shown) included CMV-luciferase to validate transfection efficiency (positive control) and pgl2 to measure background fluorescence (negative control). Bar graphs indicate relative luciferase activity normalized to the protein input and error bars were derived from the results of two independent experiments.
In order to determine if the loss of RNA expression observed for CDK2, CDK4 and ERα in MCF7 cells were due to a corresponding downregulation of promoter activity, MCF7 cells were transfected with individual promoter constructs containing the upstream promoter fragments linked to luciferase reporter genes. As shown in Figure 6B, BZL101 strongly decreased promoter activity of the CDK2 promoter fragment containing −2,313 bp of upstream regulatory sequence, however BZL101 was unable to affect the -101 bp core promoter fragment. Similarly, in MCF7 cells BZL101 strongly downregulated CDK4 promoter activity of a −1,767 promoter-luciferase reporter plasmid but not of luciferase construct containing a −687 promoter fragment. Although the precise boundaries of the BZL101 regulated regions within both the CDK4 and CDK2 promoters was not defined, the unusual feature of this observation is that with the vast majority of anticancer agents, the promoter activities of CDK2 and CDK4 are not regulated. These results suggest that BZL101 has one or more components that alter CDK2 and CDK4 targeted transcription factors that accounts for the loss of gene expression.
BZL101 also strongly downregulates ERα promoter activity, and a more detailed deletion analysis uncovered a BZL101 regulated region in a relatively narrow region of the promoter between −985 bp and −860 bp upstream of the promoter A transcription start site. Within this BZL101 regulated region of the ERα promoter are consensus DNA binding sites for a number of transcription factors involved in developmental pathways and implicated in tumorigenesis, including HNF3-beta (FoxA2),21,22 PRRX2,23 GATA24–26 and NKX-2.27
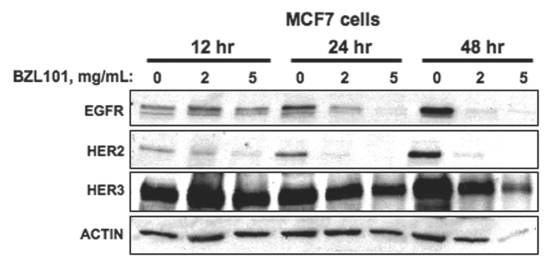
BZL101 alters growth factor receptor expression and intracellular signaling in hormone sensitive MCF7 breast cancer cells.
Growth factor signaling pathways are implicated in the stimulation of cell cycle progression through G1 into S phase of human reproductive cancer cells. Because BZL101 strongly downregulated the transcription of critical G1 acting cell cycle genes and of ERα under conditions in which MCF7 cells undergo a G1 cell cycle arrest, we tested whether critical growth factor receptors may be similarly regulated by this herbal mixture. Therefore, the effects of BZL101 on expression of members of the Epidermal Growth Factor Receptor (EGFR) tyrosine kinase gene family were examined in MCF7 cells treated with or without 2 mg/mL or 5 mg/mL BZL101 over a 48 hr time course. The production of EGFR, ErbB2/HER2 and ErbB3/HER3 protein levels were analyzed by western blots of total cell extracts. As shown in Figure 7, BZL101 treatment strongly downregulated EGFR and ErbB2/HER2 with the most rapid effects on ErbB2/HER2, which showed a significant decrease in expression by 12 hours treatment in cells at both the low and high BZL101 concentrations. EGFR levels was noticeably downregulated by 24 hrs, whereas, the level of ErbB3/HER3 was only modestly altered at the highest BZL101 concentration compared to actin levels (Fig. 7). Taken together, these results suggest that the BZL101-mediated downregulation of two members of the EGFR gene family could conceivably contribute to the overall G1 cell cycle arrest observed in early stage MCF7 breast cancer cells.
Figure 7.
BZL101 alters growth factor receptor expression and intracellular signaling in hormone sensitive MCF7 breast cancer cells. MCF7 cells were treated with either 2 mg/mL or 5 mg/mL of BZL101 over a 48 hr time course. Total cell lysates were subjected to SDS-PAGE and the protein levels of growth factor signaling pathway components and ACTIN (loading control) was monitored by western blot analysis.
Discussion
We have determined that BZL101, an herbal mixture extracted from the Scutellaria barbata plant, is able to arrest the proliferation of hormone sensitive and insensitive human breast and prostate cancer cells that are considered representative models for early and late stage cancers. Our results demonstrate that BZL101 exerts phenotype specific molecular changes of cell cycle and steroid receptor gene expression in these human reproductive cancer cells. As summarized in Figure 8, the specific phase of cell cycle arrest varied based on the individual characteristics of each cell line. In the less metastatic early stage hormone sensitive cell lines tested, MCF7 breast cancer cells and LNCaP prostate cancer cells, BZL101 arrested cell growth in the regulatory phases of the cell cycle; MCF7 cells arresting in the G1 phase of the cell cycle and LNCaP cells arresting in the G2/M phase of the cell cycle. In contrast, in both of the hormone insensitive cell lines that represent late stage cancers, MDA-MB-231 breast cancer cells and PC3 prostate cancer cells, BZL101 induced an S-phase arrest of the cell cycle. Furthermore, BZL101 was effective at slightly lower concentrations in the cell lines representing early stage cancers, 2 mg/mL for MCF7 cells and 1.5 mg/mL for LNCaP cells, as compared to the slightly higher concentration of 3 mg/mL necessary for arrest in the highly metastatic cancer cells MDA-MB-231 and PC3. In all cell lines tested, BZL101 increased the sub-G1 DNA content, which is indicative of cell death.
Figure 8.
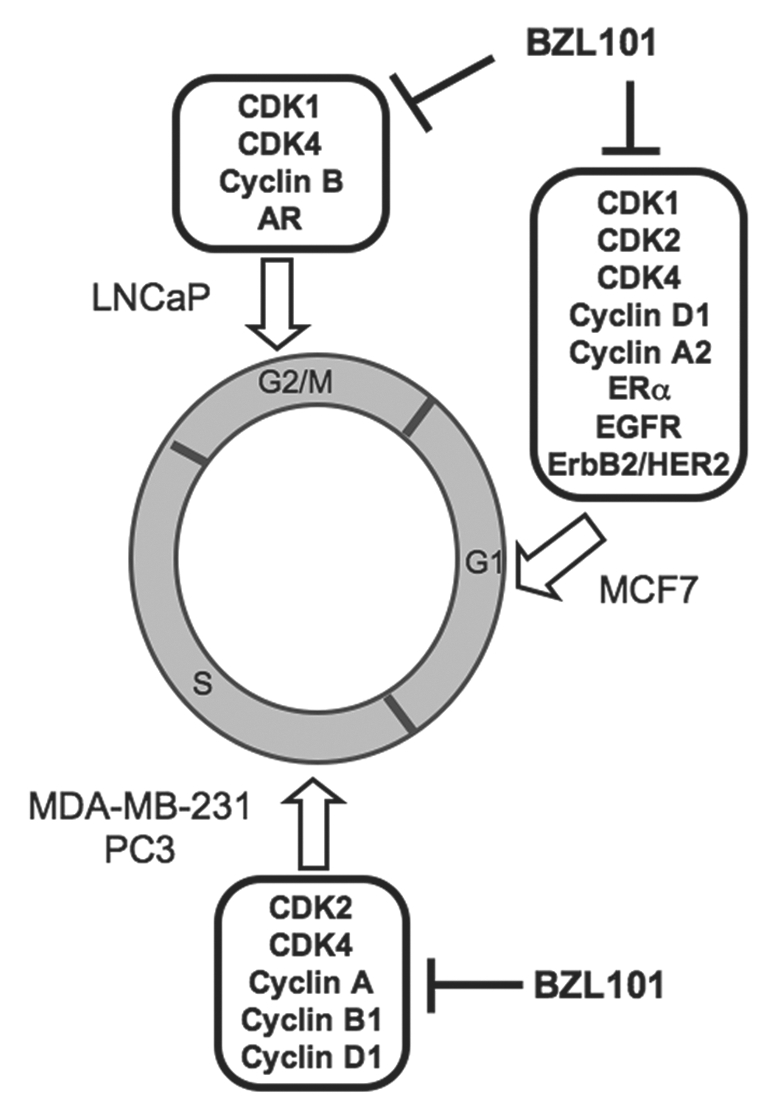
Proposed Model for the BZL101 disruption of cell cycle progression in hormone sensitive and insensitive breast and prostate cancer cell lines. BZL101 blocks cell cycle progression at different phases of the cell cycle that differ depending on the reproductive cancer cell phenotype. A G1 cell cycle arrest was observed in hormone sensitive MCF7 breast cancer cells along with the decreased expression of G1 cell cycle regulators Cyclin D1, CDK2 and at later time points CDK4. BZL101 also strongly inhibited expression of ERα and the EGFR and ErbB2/HER2 member of the EGFR gene family. In hormone sensitive LNCaP prostate cancer cells BZL101 induces a G2/M cell cycle arrest with decreased expression of Cyclin B1 and CDK1 expression as well as downregulate expression of AR. In metastatic, hormone insensitive cell lines MDA-MB-231 and PC3 cells, BZL101 induces a S phase arrest of the cell cycle along with a corresponding decreased expression of Cyclin A, Cyclin B1 and CDK2.
Consistent with the nature of the observed cell cycle arrests, BZL101 regulated the expression of cell cycle regulatory components that function in the corresponding phases of the cell cycle in each of the tested reproductive cancer cell lines (illustrated in Fig. 8). For example, BZL101 strongly downregulated the protein production, transcript expression and/or promoter activities of the G1-acting CDK2, CDK4 and cyclin D1 in MCF7 cells, which underwent a G1 cell cycle arrest. Cyclin B1 initiates G2/M progression by promoting spindle assembly in mitosis,28 and BZL101 treatment of LNCaP cells induces a G2/M cell cycle arrest with a corresponding loss of cyclin B1 expression. Cyclin A and CDK1 protein levels and transcript expression were strongly downregulated by BZL101 in both of the late stage cancer cell lines that undergo an S-phase cell cycle arrest. In each of the cell lines, BZL101 also altered the expression of one or more cell cycle components not directly associated with the phase of the cell cycle arrest. For example, all four cell lines showed a loss of CDK1 transcripts, while MDA-MB-231 and PC3 that undergo a S phase arrest display a significant downregulated expression of the G1-acting CDK2 and CDK4. These results suggest that BZL101 activates transcriptional pathways common to each of the reproductive cancer cell lines. One unusual feature of our observations is that BZL101 signaling selectively regulates the expression of cell cycle components, such the CDKs, which in most instances do not display significant changes in their expression in response to anticancer compounds. We proposed that individual molecules within BZL101 disrupt the function of transcription factors that ultimately target the gene expression of the regulated cell cycle components.
BZL101 ablated ERα and AR expression in estrogen sensitive MCF7 breast cancer cells, and androgen sensitive LNCaP prostate cancer cells, respectively. This disruption of steroid receptor expression implicates BZL101 as a potential therapeutic in the treatment of relatively early stage hormone sensitive breast and prostate cancers. For ERα, deletion analysis of the promoter in MCF7 cells narrowed the functional BZL101 regulated region to a relatively small segment of the promoter that contains consensus DNA elements for the Prx2 transcription factor homolog S8 as well as for HNF3b (FoxA2), GATA and NKX-2 transcription factors. These transcription factors are through to have a roles in breast cancers cell proliferation and/or developmental processes.21–27 Regardless of the nature of the precise BZL101-regulated transcription factor(s), our results suggest that within the BZL101 mixture, specific compounds act as selective estrogen receptor disruptors. We further observed that treatment of MCF7 cells strongly downregulated expression of both EGFR and the ErbB2/HER2 member of the EGF receptor gene family. BZL101 was previously demonstrated to be effective in inhibiting proliferation of the HER2+ hormone insensitive SKBR3 breast cancer cell line,17 and is well tolerated in metastatic breast cancer patients with prior chemotherapeutic exposure.18 We now demonstrate that BZL101 can affect expression levels of HER2 in human breast cancer cells in an early stage cancer cell phenotype.
We have established that BZL101, an aqueous extract from the Scutellaria barbata plant, is an effective anticancer agent capable of inducing cell cycle arrest and increasing cell death of hormone sensitive and insensitive, early and late stage, breast and prostate cancer cell lines.
The chemical composition of BZL101 is complex29–31 and has been shown to contain several anticancer phytochemicals such as apigenin, luteolin and resveratrol,29,32 that can disrupt multiple proliferative pathways. In addition, this herbal mixture was recently shown to contain several anticancer flavones, such as Wogonin, Baicalein and Baicalin, which have both cytostatic and cytotoxic activities in certain cultured cells and can inhibit tumor growth in vivo.33 Intriguingly these flavones have no significant toxic effects on non-cancerous cells. It is tempting to consider that one more of these compounds mediate the ability of BZL101 to inhibit proliferation of a wide range of human reproductive cancer cells representing both early and late stage cancers.
Our studies suggest that individually, exposure of the early and late stage reproductive cancer cell line models used in our study to apigenin and luteolin, which are both found in BZL101, have only very modest to no effects on cell cycle control (data not shown). Conceivably, individual cell lines react with differing sensitivity to combinations of components of BZL101, leading to an overall decrease in proliferation and increased cellular death. We are in the process of determining which combinations of BZL101 containing molecules mediate the selective effects on cell cycle control that are phenotype dependent. The cell cycle arrest and steroid receptor disrupting properties of BZL101 shows the value of this plant extract as a promising therapeutic strategy, which, when combined with conventional treatments for reproductive cancer, could increase efficacy of treatment with reduced toxicity to patients.
Materials and Methods
Reagents.
BZL101 dried powder (Bionovo, Emeryville, CA) was diluted in nano-H2O to 1.5, 2 or 3 g/mL dependent on cell line. Stock concentrations were then added to media and the mixture sterile filtered through a 0.45 µM filter. BZL101 was diluted 1:1,000 in media prior to administration on cells. All other reagents were purchased from Sigma (St. Louis, MO) and were of the highest quality available.
Cell culture.
MCF7 and MDA-MB-231 human breast cancer cells were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in Dulbecco's Modified Eagles Medium (DMEM) from BioWhittaker (Walkersville, MD), supplemented with 10% fetal bovine serum (FBS) from Mediatech (Manassas, VA), 10 µg/ml insulin, 50 U/ml penicillin, 50 U/ml streptomycin and 2 mM L-glutamine from Sigma (St. Louis, MO). MDA-MB-231 cells were grown in Isacoves Modified Eagles Medium (IMDM) from Lonza (Basel, Switzerland), supplemented with 10% FBS from Mediatech, 50 U/ml penicillin, 50 U/ml streptomycin and 2 mM L-glutamine from Sigma (St. Louis, MO). LNCaP and PC3 human prostate cancer cells were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in DMEM supplemented with HEPES buffer solution 1 M, and 2 mM L-glutamine. All cells were grown to subconfluency in a humidified chamber at 37°C containing 5% CO2 prior to treatment.
Flow cytometry.
Cells were plated onto Corning six-well tissue culture dishes (Corning, NY) and were treated with the indicated concentrations of BZL or sterile-filtered nH2O. The medium was changed every 24 hrs. Cells were hypotonically lysed in 500 µl of DNA staining solution 0.5 mg/ml propidium iodide (PI), 0.1% sodium citrate, 0.05% Triton X-100 (Sigma). Lysates were filtered using 60 µm Nitex flow mesh (Sefar America, Kansas City, MO) to remove cell membrane and debris. PI-stained nuclei were detected using a PL-2 detector with a 575 nm band pass filter on a Beckman-Coulter (Fullerton, CA) fluorescence-activated cell sorter analyzer with laser output adjusted to deliver 15 MW at 488 nm. 10,000 nuclei from the total population were analyzed per sample at a rate of 100–200 nuclei/s. The percentages of cells within the G1, S and G2/M phases of the cell cycle were determined by analyzing the histographic output with the multicycle computer program MPLUS, provided by Phoenix Flow Systems (San Diego, CA), in the Cancer Research Laboratory Microchemical Facility at the University of California at Berkeley.
Western blotting.
After the indicated treatments, MCF7 cells were washed with PBS and harvested in 1 ml PBS and pelleted by centrifugation at 2,000 rpm for 10 min. PBS was aspirated and the pellet was resuspended in radio immunoprecipitation buffer (150 mM sodium chloride, 0.5% deoxycholate, 0.1% NP-40, 0.1% SDS and 50 mM Tris) containing protease inhibitors (50 µg/ml phenylmethylsulfonyl fluoride, 10 µg/ml aprotinin, 5 µg/ml leupeptin, 0.1 µg/ml NaF and 10 µg/ml β-glycerophosphate). These extracts were then quantified using the Lowry method from Bio-Rad laboratories (Hercules, CA). Normalized amounts of protein were mixed with Gel Loading Buffer (GLB) composed of 25% glycerol, 0.075% SDS, 1.25 ml B-mercaptoethanol, 10% bromophenol blue, 3.13% 0.5 M SDS and 0.4% SDS at pH 6.8 and fractionated on 10% polyacrylamide/0.1% SDS resolving gels using electrophoresis. Prestained Rainbow Protein ladder (Amersham, Arlington Heights, IL) was used as a reference for size. Proteins were transferred to nitrocellulose membranes where equal loading was verified by Ponceau S staining. Blots were blocked with 5% Nonfat Dry Milk (NFDM) dissolved in TBST (10 mM Tris-HCl at pH 8, 150 mM NaCl and 0.05% Tween 20) at room temperature. The blots were then rinsed briefly with TBST and incubated overnight at 4°C in antibodies diluted in TBST. Antibodies were diluted in TBST as follows: CDK1 (sc-53, 1:500), CDK2 (sc-163, 1:1,000), CDK4 (sc-749, 1:500), CDK6 (sc-7180, 1:200), Cyclin E (sc-20684, 1:200), Cyclin A (sc-596, 1:500), Cyclin B (sc-752, 1:500) were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Cyclin D1 (#CC11) from Calbiochem (San Diego, CA) was diluted 1:200 in TBST. AR (ab47570) from Abcam (Cambridge, UK) was diluted 1:200 in TBST. Hsp90 and actin were used as loading controls. Hsp90 from BD Transduction laboratories #610419 (Franklin Lakes, NJ) was diluted 1:2,000 in TBST. Actin from Cytoskeleton, Incorporated #AAN01 (Denver, CO) was diluted 1:1,000 in TBST. Immunoreactive proteins were detected after incubation with horseradish peroxidase-conjugated secondary antibodies diluted 3 × 10−4 in 1% NFDM in TBST. Blots were then treated with enhanced chemiluminescence reagents (Eastman Kodak, Rochester NY).
RT-PCR.
Total RNA from MCF7 cells treated with indicated treatment was isolated with TRI Reagent according to manufacturer's protocol from Sigma (St. Louis, MO). Total RNA (4 µg) was used to synthesize cDNA using Moloney murine leukemia virus-reverse transcriptase (Promega Corp., Madison, WI) with random hexamers as primers. The cDNA reaction product (200 ng) was used with 10 µM primers. Primers were as follows: CDK1 Forward: 5′-AAG CCG GGA TCT ACC ATA CCC-3′; CDK1 Reverse: 5′-CCT GGA ATC CTG CAT AAG CAC-3′; CDK2 Forward: 5′-CCA GTA CTG CCA TCC GAG AG-3′; CDK2 Reverse: 5′-CGG CGA GTC ACC ATC TCA GC-3′; CDK4 Forward: 5′-CTG AGA ATG GCT ACC TCT CGA-3′; CDK4 Reverse: 5′-AGA GTG TAA CAA CCA CGG GTG-3′; CDK6 Forward: 5′-CTT TGC CTA GTT CAT CGA TAT C-3′; CDK6 Reverse: 5′-CCG AGT AGT GCA TCG CGA TCT-3′; Cyclin A1 Forward: 5′-TTC CCG CAA TCA TGT ACC CTG-3′; Cyclin A1 Reverse: 5′-TAG CCA GCA CAA CTC CAC TCT T-3′; Cyclin A2 Forward: 5′-TCC ATG TCA GTG CTG AGA GGC-3′; Cyclin A2 Reverse: 5′-GAA GGT CCA TGA GAC AAG GC-3′; Cyclin D1 Forward: 5′-TCC TCC TCT TCC TCC TCC TC-3′; Cyclin D1 Reverse: 5′-TCA AGT GTG ACC CAG ACT GC-3′; Cyclin B1 Forward: 5′-AGG AAG AGC AAG CAG TCA GAC-3′; Cyclin B1 Reverse: 5′-GCA GCA TCT TCT TGG GCA CAC-3′; AR Forward: 5′-CCT GAT CTG TGG AGA TGA AGC TTC-3′; AR Reverse: 5′-TGT CGT GTC CAG CAC ACA CTA CAC-3′; ERα Forward: 5′-AGC ACC CAG TGA AGC TAC-3′; ERα Reverse: 5′-TGA GGC ACA CAA ACT CCT-3′; GAPDH Forward: 5′-TGA AGG TCG GAG TCA ACG GAT TTG-3′; GAPDH Reverse: 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′. PCR products were analyzed on 1.2% agarose along with 1-kb Plus DNA ladder from Invitrogen (Carlsbad, CA) and the products visualized with GelRed from Biotium (Hayward, CA).
Luciferase assays.
MCF7 cells grown to 70% confluency in six-well Nunc plates and were transfected with 2 µg/well of the indicated plasmid construct. Transfections were performed in serum free media using Superfect (Qiagen) transfection reagent as per manufacturers instructions. Cells were treated 24 hrs post transfection with DMSO or 2 mg/mL BZL for 24 hrs. Cells were then lysed and relative luciferase activity was evaluated using the Promega Luciferase Assay kit (Promega Corp., Madison, WI). Relative luciferase activities were normalized to the protein input and pCDK2-2313 or pCDK4-1767 construct with standard error. Experiments were performed in triplicate to verify accuracy.
Acknowledgements
The authors would like to thank Gretchen Arnason and Kevin Poindexter in the Firestone laboratory for their technical assistance during the early stages of the work, as well as Emma Shtivelman and Isaac Cohen (Bionovo Inc., Emeryville, CA) for their helpful discussions during the entire study. This study was supported by NIH Public Service grant CA102360 awarded from the National Cancer Institute. C.N. Marconett was supported by a dissertation fellowship from the California Breast Cancer Research Program (#13GB-1801).
Abbreviations
- CDK
cyclin-dependent kinase
- BZL101
ban zhi lian
- LNCaP
lymph node carcinoma of the prostate
- SERM
selective estrogen receptor modulator
- SERD
selective estrogen receptor downregulator
- ER
estrogen receptor
- AR
androgen receptor
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/12424
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Mohla S, Stearns V, Sathyamoorthy N, Rosenfeld MG, Nelson P. The biology of hormone refractory breast and prostate cancer: An NCI workshop report. Cancer Biol Ther. 2009;8:1975–1985. doi: 10.4161/cbt.8.21.9918. [DOI] [PubMed] [Google Scholar]
- 3.Adeyemo O, Kallio PJ, Palvimo JJ, Kontula K, Janne OA. A single-base substitution in exon 6 of the androgen receptor gene causing complete androgen insensitivity: the mutated receptor fails to transactivate but binds to DNA in vitro. Hum Mol Genet. 1993;2:1809–1812. doi: 10.1093/hmg/2.11.1809. [DOI] [PubMed] [Google Scholar]
- 4.Kallio PJ, Palvimo JJ, Mehto M, Janne OA. Analysis of androgen receptor-DNA interactions with receptor proteins produced in insect cells. J Biol Chem. 1994;269:11514–11522. [PubMed] [Google Scholar]
- 5.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000;27:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 6.Aneja R, Zhou J, Zhou B, Chandra R, Joshi HC. Treatment of hormone-refractory breast cancer: apoptosis and regression of human tumors implanted in mice. Mol Cancer Ther. 2006;5:2366–2377. doi: 10.1158/1535-7163.MCT-06-0205. [DOI] [PubMed] [Google Scholar]
- 7.Traish AM, Morgentaler A. Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: a potential molecular switch for tumour growth. Br J Cancer. 2009;101:1949–1956. doi: 10.1038/sj.bjc.6605376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui Y, Parra I, Zhang M, Hilsenbeck SG, Tsimelzon A, Furukawa T, et al. Elevated expression of mitogen-activated protein kinase phosphatase 3 in breast tumors: a mechanism of tamoxifen resistance. Cancer Res. 2006;66:5950–5959. doi: 10.1158/0008-5472.CAN-05-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch JH, Batuello JT, Crawford ED, Gomella LG, Kaufman J, Petrylak DP, Joel AB. Therapeutic strategies for localized prostate cancer. Rev Urol. 2001;2:39–48. [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 11.Reddel RR, Alexander IE, Koga M, Shine J, Sutherland RL. Genetic instability and the development of steroid hormone insensitivity in cultured T 47D human breast cancer cells. Cancer Res. 1988;48:4340–4347. [PubMed] [Google Scholar]
- 12.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 13.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 14.Dai ZJ, Wang XJ, Li ZF, Ji ZZ, Ren HT, Tang W, et al. Scutellaria barbate extract induces apoptosis of hepatoma H22 cells via the mitochondrial pathway involving caspase-3. World J Gastroenterol. 2008;14:7321–7328. doi: 10.3748/wjg.14.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EK, Kwon KB, Han MJ, Song MY, Lee JH, Ko YS, et al. Induction of G1 arrest and apoptosis by Scutellaria barbata in the human promyelocytic leukemia HL-60 cell line. Int J Mol Med. 2007;20:123–128. [PubMed] [Google Scholar]
- 16.Liu JY, Wang YR, Liu Y. Optimizing extraction process of Scutellaria barbata by golden section method. Zhongguo Zhong Yao Za Zhi. 2008;33:896–899. [PubMed] [Google Scholar]
- 17.Fong S, Shoemaker M, Cadaoas J, Lo A, Liao W, Tagliaferri M, et al. Molecular mechanisms underlying selective cytotoxic activity of BZL101, an extract of Scutellaria barbata, towards breast cancer cells. Cancer Biol Ther. 2008;7:577–586. doi: 10.4161/cbt.7.4.5535. [DOI] [PubMed] [Google Scholar]
- 18.Perez AT, Arun B, Tripathy D, Tagliaferri MA, Shaw HS, Kimmick GG, et al. A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Res Treat. 2009;120:111–118. doi: 10.1007/s10549-009-0678-5. [DOI] [PubMed] [Google Scholar]
- 19.Wiese TE, Kral LG, Dennis KE, Butler WB, Brooks SC. Optimization of estrogen growth response in MCF-7 cells. In Vitro Cell Dev Biol. 1992;28:595–602. doi: 10.1007/BF02631033. [DOI] [PubMed] [Google Scholar]
- 20.Sundar SN, Marconett CN, Doan VB, Willoughby JA, Sr, Firestone GL. Artemisinin selectively decreases functional levels of estrogen receptor-alpha and ablates estrogen-induced proliferation in human breast cancer cells. Carcinogenesis. 2008;29:2252–2258. doi: 10.1093/carcin/bgn214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espina AG, Mendez-Vidal C, Moreno-Mateos MA, Saez C, Romero-Franco A, Japon MA, Pintor-Toro JA. Induction of Dlk1 by PTTG1 inhibits adipocyte differentiation and correlates with malignant transformation. Mol Biol Cell. 2009;20:3353–3362. doi: 10.1091/mbc.E08-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. Prostate. 2006;66:1013–1028. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- 23.Jones FS, McKean DM, Meech R, Edelman DB, Oakey RJ, Jones PL. Regulation of vascular smooth muscle cell growth and adhesion by paired-related homeobox genes. Chest. 2002;121:89–90. [PubMed] [Google Scholar]
- 24.Marconett CN, Sundar SN, Poindexter KM, Stueve TR, Bjeldanes LF, Firestone GL. Indole-3-Carbinol Triggers AhR-dependent ERalpha Protein Degradation in Breast Cancer Cells Disrupting an ERalpha-GATA3 Transcriptional Cross-regulatory Loop. Mol Biol Cell. 2010;21:1166–1177. doi: 10.1091/mbc.E09-08-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang SH, Chen Y, Weigel RJ. GATA-3 as a marker of hormone response in breast cancer. J Surg Res. 2009;157:290–295. doi: 10.1016/j.jss.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Kouros-Mehr H, Kim JW, Bechis SK, Werb Z. GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol. 2008;20:164–170. doi: 10.1016/j.ceb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendall J, Liu Q, Bakleh A, Krasnitz A, Nguyen KC, Lakshmi B, et al. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc Natl Acad Sci USA. 2007;104:16663–16668. doi: 10.1073/pnas.0708286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, Zhang X, Jiang Q, Clarke PR, Zhang C. Cyclin B1 is localized to unattached kinetochores and contributes to efficient microtubule attachment and proper chromosome alignment during mitosis. Cell Research. 2008;18:268–280. doi: 10.1038/cr.2008.11. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Xue X, Xiao Y, Zhang F, Xu Q, Liang X. Purification and preparation of compounds from an extract of Scutellaria barbata D. Don using preparative parallel high performance liquid chromatography. J Sep Sci. 2008;31:1669–1676. doi: 10.1002/jssc.200700609. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Liu H, Lei J, Tan W, Hu X, Zou G. Antitumor activity of chloroform fraction of Scutellaria barbata and its active constituents. Phytother Res. 2007;21:817–822. doi: 10.1002/ptr.2062. [DOI] [PubMed] [Google Scholar]
- 31.Dai SJ, Wang GF, Chen M, Liu K, Shen L. Five new neo-clerodane diterpenoid alkaloids from Scutellaria barbata with cytotoxic activities. Chem Pharm Bull (Tokyo) 2007;55:1218–1221. doi: 10.1248/cpb.55.1218. [DOI] [PubMed] [Google Scholar]
- 32.Kim DI, Lee TK, Lim IS, Kim H, Lee YC, Kim CH. Regulation of IGF-I production and proliferation of human leiomyomal smooth muscle cells by Scutellaria barbata D. Don in vitro: isolation of flavonoids of apigenin and luteolin as acting compounds. Toxicol Appl Pharmacol. 2005;205:213–224. doi: 10.1016/j.taap.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its manin active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35:57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]