Abstract
Many types of cancer cells possess the ability to evade apoptosis, leading to their rapid and uncontrolled proliferation. As major regulators of apoptosis, Bcl-2 proteins serve as emerging targets for novel chemotherapeutic strategies. In this study, we examined the involvement of Bcl-2 proteins in apoptosis induced by the chemotherapeutic agent actinomycin D. A dramatic decrease in anti-apoptotic myeloid leukemia cell differentiation protein (Mcl-1) mRNA and protein expression was detected upon actinomycin D treatment. Further, Mcl-l overexpression caused resistance to cell death upon treatment with actinomycin D, implicating a role for the downregulation of Mcl-1 in actinomycin D-induced apoptosis. We also explored the therapeutic potential of actinomycin D in combination with ABT-737, an experimental agent that inhibits anti-apoptotic Bcl-2 proteins. Actinomycin D sensitized cells to ABT-737 treatment in a Bak- or Bax-dependent manner. Importantly, low concentrations of actinomycin D and ABT-737 were more effective in inducing cell death in transformed cells than their untransformed counterparts. A synergistic effect of actinomycin D and ABT-737 on cell death was observed in several human tumor cell lines. Like actinomycin D treatment, knocking down Mcl-1 expression greatly sensitized tumor cells to ABT-737 and Mcl-1 overexpression abrogated the cytotoxic effect induced by ABT-737 and actinomycin D. These results suggest that the downregulation of Mcl-1 by actinomycin D is likely responsible for the observed synergistic effect between the two drugs. Overall, our studies provide compelling evidence that the combination of actinomycin D and ABT-737 may lead to an effective cancer treatment strategy.
Key words: apoptosis, Bcl-2 proteins, Mcl-1, actinomycin D, ABT-737, NSCLC, pancreatic carcinoma
Introduction
Many types of cancer cells are able to evade apoptosis. Among the major regulators of cellular response to apoptotic stimuli is the Bcl-2 family of proteins.1 Bcl-2 was the founding member of the family found to inhibit cell death,2 but more than 20 Bcl-2-related proteins have been identified.3 All of them share one or more distinct domains of homology and function to promote or inhibit apoptosis. Bak and Bax are key multi-domain pro-apoptotic Bcl-2 proteins and cells deficient in both proteins are unable to undergo apoptosis.4 Upon activation, Bak and Bax function to permeabilize the mitochondrial outer membrane and release apoptogenic factors into the cytosol.5 The BH3-only proteins are a second group of pro-apoptotic Bcl-2 proteins that include BID, BIM, BIK, BAD, PUMA and NOXA. They share a homologous BH3 domain and are normally kept inactive by diverse mechanisms, becoming active in response to death signals to function with other Bcl-2 proteins in promoting apoptotic signaling. How the BH3-only Bcl-2 proteins activate Bak and Bax is still controversial,6 but BH3-only proteins have been shown to bind to and inactivate anti-apoptotic Bcl-2 proteins.7–9
In addition to Bcl-2, the anti-apoptotic Bcl-2 proteins include four other members: Bcl-XL, Bcl-w, Mcl-1 and A1. These proteins function to protect the cell from apoptotic insults, primarily by preventing disruption of mitochondrial outer membrane integrity by pro-apoptotic Bcl-2 proteins.5 Antagonists of antiapoptotic Bcl-2 proteins are an attractive target for new cancer therapeutics because many cancers overexpress these proteins,10 and this overexpression can be correlated with resistance to treatment.11,12 Because tumor cells are under stress and may depend on alterations in their apoptotic signaling pathways for survival, neutralizing the function of anti-apoptotic Bcl-2 proteins represents an attractive strategy for the elimination of cancer cells. One approach involves identifying compounds that specifically bind anti-apoptotic Bcl-2 proteins to neutralize their function.12 This can be achieved by designing small molecules which mimic BH3-only Bcl-2 proteins.
ABT-737 is one such small molecule that binds with high affinity to Bcl-2, Bcl-XL and Bcl-w, but not to Mcl-1 or A1.13 A mimetic of the BH3 Bcl-2 protein BAD, ABT-737 has been shown to specifically induce the apoptotic signaling pathway. As might be expected, cells deficient in Bax and Bak or caspase-9 can not be killed by ABT-737.14 ABT-737 has proven effective as a single agent in killing various tumor cell lines and primary patient-derived cells, including follicular lymphoma, small cell lung carcinoma and chronic lymphocytic leukemia.13 However, other tumor cell lines, such as pancreatic carcinoma and malignant melanoma, are more resistant to treatment with ABT-737 as a single agent.15,16 This resistance may be related to the critical role of overexpressed Mcl-1 in the survival of certain cancers,17–20 since ABT-737 is inefficient at neutralizing Mcl-1. Therefore, additional studies have focused on using ABT-737 in combination with other chemotherapeutic agents, with major interest in compounds that downregulate Mcl-1 or promote its degradation. 21 Ideally, adding ABT-737 to another agent would sensitize cells to a lower dosage of the chemotherapeutic agent, thereby minimizing its toxicity and side effects.
Here, we report a potent synergistic cytotoxic activity of the traditional chemotherapeutic agent actinomycin D in combination with ABT-737. Actinomycin D (Dactinomycin) is a transcriptional inhibitor currently part of the treatment regimen for cancers such as melanoma22 and Wilms tumor.23 We found that actinomycin D rapidly and efficiently downregulated Mcl-1. Based on this finding, we tested actinomycin D in combination with ABT-737 and found that the combination of actinomycin D and ABT-737 synergistically enhanced apoptosis in two pancreatic tumor cell lines and two non-small cell lung cancer (NSCLC) lines, likely due to the comprehensive assault on anti-apoptotic Bcl-2 proteins. Because of the strong synergism observed between actinomycin D and ABT-737, this drug combination may have important therapeutic implications and lead to a novel cancer treatment strategy.
Results
Pro-apoptotic Bak or Bax can mediate actinomycin D-induced cell death.
While the pro-apoptotic Bcl-2 proteins Bak and Bax are redundant promoters of apoptosis in many apoptotic paradigms,4,24 some studies suggest that apoptosis in response to certain death stimuli is mediated exclusively by Bak or Bax.25,26 To determine the ability of Bak and Bax to individually mediate cell death induced by actinomycin D, different mouse cell lines exclusively expressing Bak or Bax were generated. First, Bak or Bax was stably re-expressed by retroviral infection in mouse embryonic fibroblasts (MEFs) and IL-3-dependent hematopoetic cells deficient in both Bak and Bax (BB; Sup. Fig. 1A and C). While the parental cells (BB-parental) and cells expressing the vector only (BB-GFP) displayed minimal cell death in response to actinomycin D, significant cell death was observed in cells expressing either Bak or Bax in a time-dependent fashion (Sup. Fig. 1B and D).
Mcl-1 mRNA and protein are downregulated in response to actinomycin D.
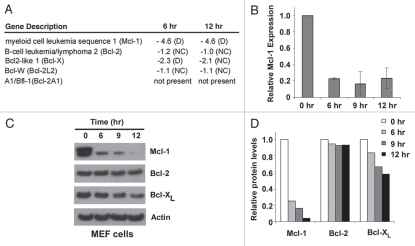
To further explore the involvement of other Bcl-2 proteins in cell death induced by actinomycin D, microarray analysis was carried out to characterize the global changes in Bcl-2 gene expression levels upon actinomycin D treatment. In wild-type MEF cells treated with actinomycin D for 6 and 12 hours, cell death was not yet observed by propidium iodide (PI) staining, making them suitable for studying early gene expression changes in response to actinomycin D. Despite being a transcriptional inhibitor, actinomycin D did not reduce the expression of most Bcl-2 genes examined, as many remained unchanged and some (e.g., Noxa) were upregulated at these time points (Sup. Fig. 2; Olberding and Li, unpublished data). However, the most notable change in gene expression within the Bcl-2 protein family was Mcl-1 (Fig. 1A). While the other anti-apoptotic Bcl-2 proteins showed little or no consistent changes in gene expression in response to actinomycin D, Mcl-1 gene expression was dramatically decreased at both the 6 hour and 12 hour time points. The downregulation of Mcl-1 mRNA was confirmed by realtime PCR (Fig. 1B) and correlated with a remarkable decrease in Mcl-1 protein levels. While Bcl-XL protein levels were reduced to a lesser degree compared with those of Mcl-1, no dramatic change was observed in Bcl-2 protein levels (Fig. 1C and D). These data demonstrate that Mcl-1 was downregulated most dramatically among anti-apoptotic Bcl-2 proteins in actinomycin D-treated cells.
Figure 1.
Actinomycin D treatment leads to a decrease in Mcl-1 expression. (A) Changes in mRNA expression of anti-apoptotic Bcl-2 genes after 6 and 12 hours of actinomycin D treatment (0.2 µg/ml) compared to untreated cells was determined in wild-type MEF cells by microarray analysis (mouse genome 430 2.0 array, Affymetrix). The A1 probe set did not detect A1 mRNA at any time points according to signal intensities and present/absent calls by GCOS1.4 sofware (Affymetrix). Data are represented as fold-changes in mRNA compared to untreated cells. Negative values indicate downregulation. D, decrease; NC, no change. (B) RT-qPCR was used to assess relative mRNA levels of Mcl-1 in wild-type MEF cells treated with actinomycin D. Data are normalized to actin transcript expression and are depicted relative to mRNA levels at the zero hour time point. Data are mean ± standard deviation of two independent experiments. (C) The expression of anti-apoptotic Bcl-2 proteins in wild-type MEF cells upon 0.2 µg/ml actinomycin D treatment was determined by western blot. Actin represents a loading control. 20 µg whole cell lysate per sample was analyzed. (D) The intensities of Mcl-1, Bcl-2, Bcl-XL and actin shown in (A) were quantified using ImageJ software (NIH). The data are normalized to actin protein levels and represented relative to the protein levels at the zero hour time point.
Mcl-1 downregulation is involved in actinomycin D-induced apoptosis.
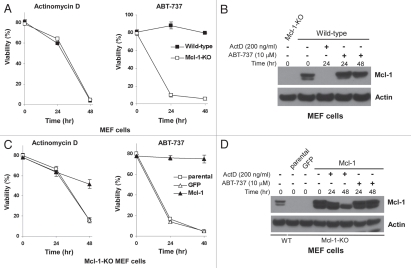
To determine whether the downregulation of Mcl-1 plays a role in actinomycin D-induced apoptosis, the effect of actinomycin D on wild-type and Mcl-1-deficient (Mcl-1Δ/-) MEFs was studied. Comparable levels of cell death were observed in both cell types upon actinomycin D exposure (Fig. 2A). This result is not unexpected since the lack of Mcl-1 expression in Mcl-1Δ/- MEFs mimics the rapidly decreased Mcl-1 levels in wildtype cells in response to actinomycin D exposure (Fig. 2B). To determine whether these results were specific to actinomycin D, Mcl-1Δ/- and wild-type MEFs were treated with ABT-737, since Mcl-1 knockout MEFs have been shown to be sensitive to ABT-737.27 In contrast to actinomycin D, ABT-737 treatment did not decrease Mcl-1 protein levels (Fig. 2B). Furthermore, Mcl-1Δ/- MEFs were highly susceptible to ABT-737 treatment compared to wild-type cells, indicating that sustained Mcl-1 protein levels in wild-type cells play a critical protective role (Fig. 2A). In addition, Mcl-1Δ/- MEFs overexpressing Mcl-1 were more resistant to actinomycin D treatment than parental and the vector control cells (Fig. 2C), which could be attributed to the substantial amount of Mcl-1 in Mcl-1-overexpressing cells after 48 hours of actinomycin D treatment (Fig. 2D). Thus, the overexpression of Mcl-1 allowed enough protein to be present at the later time point to provide a protective effect. Finally, Mcl-1 overexpression protected Mcl-1Δ/- MEFs from ABT-737-induced cell death (Fig. 2C), further demonstrating the importance of Mcl-1 in determining the sensitivity to ABT-737.
Figure 2.
Mcl-1 downregulation is involved in actinomycin D-induced apoptosis. (A) Wild-type (WT) and Mcl-1Δ/- (KO) MEFs were treated with 0.2 µg/ml actinomycin D or 10 µM ABT-737 and cell viability was measured by PI staining. Experiments were repeated independently three times and data represent mean ± standard deviation of triplicate experiments. (B) The expression of Mcl-1 in the indicated MEF cells treated with actinomycin or ABT-737 for 24 hours was determined by western blot (25 µg whole cell lysates). Actin was a loading control. (C) Overexpressing Mcl-1 conferred some resistance to actinomycin D-induced cell death. MEF cells were treated with actinomycin D (0.2 µg/ml) or ABT-737 (10 µM). GFP represents cells containing the empty expression vector (pBabeIresGFP). (D) Levels of Mcl-1 protein expression in the indicated MEF cells treated with actinomycin D or ABT-737 for 24 or 48 hours were characterized by western blot using 25 µg whole cell lysates, with actin as a loading control. All cell viability experiments were repeated independently three times and data represent mean ± standard deviation of triplicate experiments.
Actinomycin D sensitizes ME F cells to ABT-737 treatment in a Bak- or Bax-dependent manner.
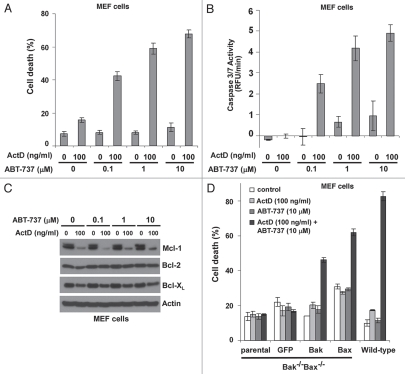
Since actinomycin D causes a dramatic decrease in Mcl-1 expression levels in MEFs and the sensitivity of MEFs to ABT-737 can be greatly enhanced by reducing Mcl-1 levels (Fig. 2), we studied the combinatorial effects of actinomycin D and ABT-737 on cell death. Wild-type MEF cells were treated for 24 hours with different combinations of actinomycin D and ABT-737 (Fig. 3A). While each agent induced minimal cell death individually, the combination of actinomycin D and ABT-737 had a significant cytotoxic effect on cells. This effect was more dramatic with increasing concentrations of ABT-737 (up to 10 µM). Furthermore, caspase 3/7 activity was also greatly enhanced by the combination of actinomycin D and ABT-737, indicating that the activation of caspase signaling pathways was involved in cell death induced by the drug combination. Additionally, after 12 hours of drug treatment, Mcl-1 protein levels were more dramatically decreased than those of Bcl-2 or Bcl-XL, implicating the reduction of Mcl-1 expression in the cytotoxicity of actinomycin D and ABT-737 (Fig. 3C and Sup. Fig. 3).
Figure 3.
ABT-737 and actinomycin D synergistically induce cell death in a Bak- or Bax-dependent manner. (A) MEF cells were treated with the indicated concentrations of actinomycin D or ABT-737 for 24 hours and cell death was measured by PI staining. Data represent mean ± standard deviation of triplicate experiments and are representative of three independent experiments. ActD, actinomycin D. (B) Caspase-3/7 activity under the indicated conditions was measured by fluorometric assay. Values are normalized to untreated cells. Mean ± standard deviation of triplicate experiments are shown and have been repeated independently three times. (C) Characterization of anti-apoptotic Bcl-2 protein expression after 12 hours of treatment under the indicated conditions. 20 µg cell lysates were analyzed by western blot. (D) The indicated MEF cells were treated with actinomycin D or ABT-737. Cell death was measured by PI staining after 24 hours of treatment. Data represent mean ± standard deviation of triplicate experiments. Experiments were performed independently three times.
To further investigate the mechanism of cell death induced by the combination of actinomycin D and ABT-737, MEF cells deficient in Bak and Bax with or without re-expressed Bak or Bax were treated with the drug combination for 24 hours. While the combination of actinomycin D and ABT-737 did not induce cell death in Bak-/-Bax-/- parental and the empty vector control cells, it displayed cytotoxic activities on cells expressing either Bak or Bax (Fig. 3D). Therefore, Bak or Bax can individually mediate the capability of actinomycin D to sensitize cells to ABT-737 treatment.
Actinomycin D and ABT-737 have a synergistic cytotoxic effect on pancreatic carcinoma and non-small cell lung carcinoma cell lines.
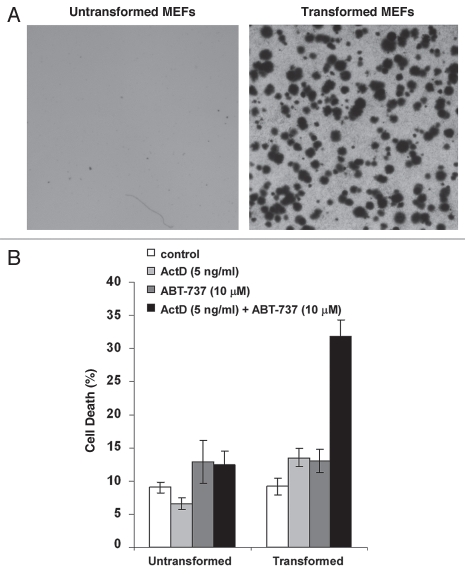
To explore the possibility that combining actinomycin D and ABT-737 could be a therapeutic treatment for tumors, we investigated whether tumor cells are more sensitive to the drug treatment than non-tumor cells. Due to their distinct Bcl-2 protein expression profiles, cells derived from various tissues show different sensitivity to both actinomycin D and ABT-737 treatment. Thus, we studied the response of untransformed MEFs and their isogenic counterparts transformed by expressing the oncogenes K-Ras and E1A to the actinomycin D and ABT-737 treatment.28 At the concentration achievable in plasma of patients over 12 hours following drug administration (5 ng/ml),29 actinomycin D sensitized transformed MEF cells to ABT-737 treatment, but not untransformed MEFs (Fig. 4). These results suggest that the combination of actinomycin D and ABT-737 could specifically induce cell death in tumor cells but not normal cells.
Figure 4.
The low concentrations of actinomycin D in combination with ABT-737 induce cell death specifically in transformed MEFs. (A) The indicated MEF cells were cultured in soft agar and representative images of the plates are shown. (B) The indicated MEF cells were treated with 5 ng/ml actinomycin D or 10 µM ABT-737. Cell death was measured after 24 hours of treatment. Data represent mean ± standard deviation of four independent experiments.
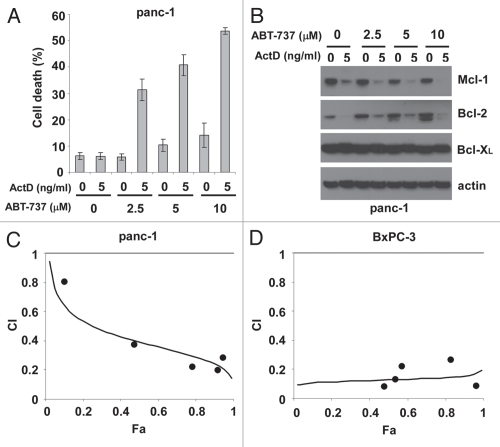
Certain types of tumor cells exhibit strong resistance to ABT-737 treatment.30 Thus, we investigated whether actinomycin D sensitizes ABT-737-resistant tumor cells. We first studied two human pancreatic carcinoma cell lines that display resistance to ABT-737: panc-1 and BxPC-3. While ABT-737 as high as 10 µM failed to induce significant cell death in panc-1 cells, the combination of actinomycin D and ABT-737 exhibited strong cytotoxic activities 72 hours after treatment (Fig. 5A). Mcl-1 protein levels were dramatically reduced by actinomycin D treatment, regardless of the presence of ABT-737 (Fig. 5B), consistent with observations in MEF cells (Fig. 3C). Interestingly, while Bcl-XL protein levels were relatively unchanged, a prominent decrease in Bcl-2 was also observed in cells treated with actinomycin D. To investigate whether the cytotoxic effects of actinomycin D and ABT-737 were synergistic, we carried out median-effect analysis using the Chou-Talalay method.31 After 72 hours of treatment, the cell death levels of panc-1 cells treated with various concentrations of actinomycin D (5–150 ng/ml) and ABT-737 (0.83–25 µM) at a constant dose ratio were measured. The combination index (CI) values were determined. As shown in Figure 5C, in the range of tested drug concentrations, CI values were smaller than 1, indicating synergism of drug combination. Similarly, upon 48 hours of treatment with different combinations of actinomycin D (0.5–20 ng/ml) and ABT-737 (0.25–10 µM) at a fixed concentration ratio, the percentage of BxPC-3 cell death was measured. The resulting combination index (CI) values indicated that actinomycin D and ABT-737 exhibited synergistic cytotoxic effects on BxPC-3 cells (Fig. 5D).
Figure 5.
Actinomycin D and ABT-737 synergistically induce apoptosis in pancreatic tumor cells. (A) Human pancreatic carcinoma panc-1 cells were treated with different combinations of actinomycin D and ABT-737 for 72 hours. Mean ± standard deviation of triplicate experiments is shown. (B) The amounts of anti-apoptotic Bcl-2 proteins in lysates (30 µg) from panc-1 cells treated with actinomycin D or ABT-737 for 72 hours were examined by western blot. Actin represents a control for protein loading. (C) Upon 72 hour-treatment with various combinations of actinomycin D (5–150 ng/ml) and ABT-737 (0.83–25 µM) at a constant concentration ratio, the percentage of panc-1 cell death was measured and combination index (CI) values were calculated as described in Materials and Methods. (D) Human pancreatic tumor cell BxPC-3 was treated with various doses of actinomycin D (0.5–20 ng/ml) and ABT-737 (0.25–10 µM) at a constant dose ratio for 48 hours and cell death was measured. The values of CI were determined as described in Materials and Methods. All cell death data are representative of three independent experiments.
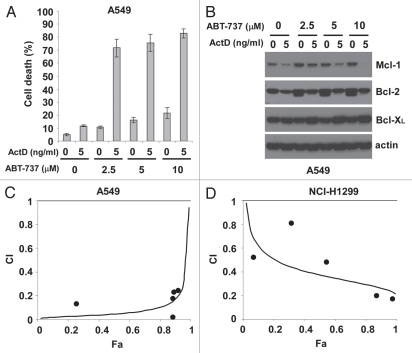
As in panc-1 cells, minimal cell death was observed with actinomycin D or ABT-737 alone in A549 non-small cell lung carcinoma cells, while the combination induced cell death (Fig. 6A). Mcl-1 protein levels were dramatically decreased in A549 cells treated with actinomycin D (Fig. 6B). Protein levels of Bcl-2 were also decreased in response to actinomycin D treatment, while Bcl-XL protein levels increased slightly. Various combinations of actinomycin D (5–300 ng/ml) and ABT-737 (0.83–50 µM) at a constant dose ratio showed synergistic activities of the two drugs on A549 cells (Fig. 6C). Furthermore, different concentrations of actinomycin D (0.64–16 ng/ml) and ABT-737 (2–50 µM) also induced synergistic cell death in another human non-small cell lung carcinoma cell line, NCI-H1299 (Fig. 6D). Overall, actinomycin D and ABT-737 had a strong synergistic effect on cell death in four human tumor cells examined.
Figure 6.
Actinomycin D and ABT-737 synergistically kill NSCLC tumor cells. (A) Upon treatment with different combinations of actinomycin D and ABT-737 for 48 hours, the percentage of cell death of human lung carcinoma A549 cells was measured. The data depict mean ± standard deviation of triplicate experiments. (B) The levels of different anti-apoptotic Bcl-2 proteins in A549 cell lysates (30 µg) under the indicated conditions were determined by western blot. Actin represents a control for protein loading. (C) A549 cells were treated with various combinations of actinomycin D (5–300 ng/ml) and ABT-737 (0.83–50 µM) at a fixed concentration ratio for 48 hours and cell death was measured. The values of combination index (CI) were determined as described in Materials and Methods. (D) Upon 80 hour-treatment with various concentrations of actinomycin D (0.64–16 ng/ml) and ABT-737 (2–50 µM) at a fixed dose ratio, the percentage of NCI-H1299 cell death was determined and CI values were computed as described in Materials and Methods. All experiments were performed independently three times.
Downregulation of Mcl-1 is involved in mediating the synergistic effect of actinomycin D and ABT-737 on cell death.
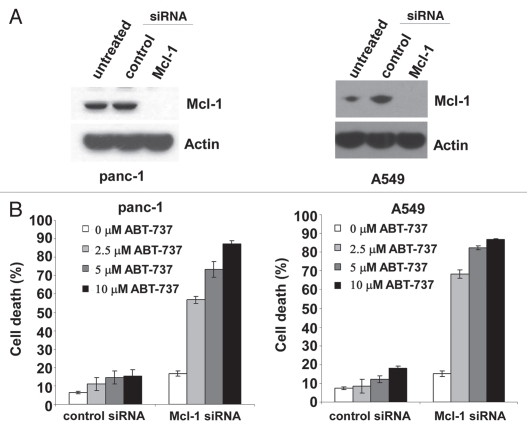
We further investigated the role of Mcl-1 in the synergistic cytotoxic effect of actinomycin D and ABT-737 in tumor cells. Mcl-1 was transiently knocked down in panc-1 and A549 tumor cells to recapitulate Mcl-1 downregulation observed with actinomycin D treatment. Introduction of Mcl-1 siRNA into both panc-1 and A549 cells was effective at reducing Mcl-1 protein expression, whereas the control siRNA had no effect on Mcl-1 expression (Fig. 7A). Both panc-1 and A549 cells with reduced Mcl-1 expression were notably more sensitive to ABT-737 compared to cells treated with the control siRNA (Fig. 7B). Thus, Mcl-1 protein levels play a role in determining ABT-737 sensitivity in both human tumor cell lines.
Figure 7.
Reducing Mcl-1 expression in Panc-1 and A549 cells sensitizes them to ABT-737 treatment. (A) Mcl-1 protein expression was reduced by siRNA in panc-1 and A549 cells. Cell lysates of 1 × 105 panc-1 or A549 cells were examined by western blot. (B) Panc-1 or A549 cells with different expression levels of Mcl-1 were treated with the indicated concentrations of ABT-737 and cell viability was measured by PI staining 72 hours after treatment for panc-1 cells or 48 hours for A549 cells. Data represent mean ± standard deviation of triplicate experiments and have been repeated three times independently.
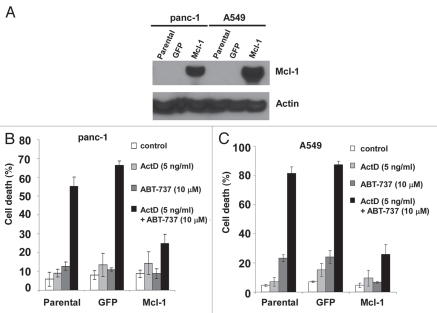
To further elucidate the role of Mcl-1 in the synergistic killing activity of actinomycin D and ABT-737, the effect of Mcl-1 overexpression on cell viability was examined. Mcl-1 was overexpressed in panc-1 and A549 tumor cells and each cell type was treated with actinomycin D and ABT-737 alone or in combination (Fig. 8). While the parental cells and cells containing the empty expression vector (GFP) displayed high levels of cell death in the presence of both actinomycin D and ABT-737, tumor cells overexpressing Mcl-1 were more resistant to cell death induced by the drug combination (Fig. 8). Therefore, Mcl-1 plays a role in preventing cell death induced by the combination of actinomycin D and ABT-737 in the two tumor cell types examined.
Figure 8.
Mcl-1 overexpression in tumor cells reduces cell death induced by ABT-737 and actinomycin D. Panc-1 cells (A) or A549 cells (B) overexpressing Mcl-1 and control cells were treated with the indicated amounts of ABT-737 and actinomycin D for 72 hours (Panc-1) or 48 hours (A549). Cell viability was determined by PI staining and data represent mean ± standard deviation of triplicate experiments. Experiments were performed independently three times.
Discussion
New cancer therapeutic strategies to activate apoptotic pathways directly in tumor cells have recently emerged and one approach aims to either reduce the activity of anti-apoptotic Bcl-2 proteins or enhance the function of pro-apoptotic Bcl-2 proteins. ABT-737 is relatively new experimental agent that only efficiently targets anti-apoptotic Bcl-2, Bcl-XL and Bcl-w, making it less effective in killing tumor cells where Mcl-1 plays a critical survival role.21 Therefore, we investigated whether the combination of actinomycin D, a traditional chemotherapeutic drug that downregulates Mcl-1 expression and ABT-737 effectively kills tumor cells by cooperatively inhibiting activities of anti-apoptotic Bcl-2 proteins. Actinomycin D was shown to downregulate Mcl-1 protein expression and synergistically induce apoptosis in both human lung and pancreatic carcinoma cell lines when combined with ABT-737. Mcl-1 downregulation by actinomycin D was a critical event in mediating this synergism, as Mcl-1 overexpression diminished the cell death induced by the drug combination. Further, Mcl-1 knockdown studies confirmed a critical role of Mcl-1 expression levels in ABT-737 resistance and the importance of downregulating/neutralizing Mcl-1 to achieve effective killing in these tumor cells. Overall, we present a novel chemotherapeutic drug combination that efficiently and systematically targets anti-apoptotic Bcl-2 proteins and induces apoptosis in tumor cells.
Actinomycin D is a chemotherapeutic drug used in the treatment of various tumors, including Wilms tumor,23 Ewing sarcoma,32 melanoma,22 gestational trophoblastic neoplasia33 and rhabdomyosarcoma.34 As an older agent, actinomycin D is often used in combination with other chemotherapeutic drugs to treat cancer patients. Recently, there are efforts to explore actinomycin D as a chemotherapeutic strategy to specifically target critical molecules involved in tumor development and maintenance, such as p53.35,36 Our current studies provide evidence that actinomycin D downregulates Mcl-1 and is very effective at inducing tumor cell death in combination with ABT-737.
As an important anti-apoptotic Bcl-2 protein, Mcl-1 exerts its function upstream in the cellular response to apoptotic stimuli.37 Numerous cancer types have been shown to overexpress Mcl-1.38,39 Mcl-1 overexpression has also been linked to therapeutic resistance in various tumor types.40,41 Thus, abrogating Mcl-1 anti-apoptotic function will render Mcl-1-dependent tumor cells more susceptible to chemotherapeutic agents. Recently, there has been much focus on effective approaches to either reduce Mcl-1 expression levels or neutralize its anti-apoptotic activities. In our current studies, we provide strong evidence that actinomycin D efficiently downregulates Mcl-1 protein levels in all cell lines examined, in accord with a previous study of multiple myeloma cells.42 As a transcriptional inhibitor that binds to DNA at the transcription initiation complex, actinomycin D reduces global mRNA levels. However, at lower concentrations, actinomycin D may specifically decrease levels of mRNA with a short half-life, such as Mcl-1 mRNA.43 This is supported by our microarray analysis demonstrating that Mcl-1 mRNA levels decreased rapidly, as early as 6 hours after actinomycin D treatment in MEF cells (Fig. 2). By downregulating Mcl-1 mRNA levels, actinomycin D was particularly effective at rapidly decreasing Mcl-1 protein levels (Fig. 1), since Mcl-1 is a short half-life protein whose degradation is regulated by caspasemediated and proteasome-dependent pathways.37,42
In addition to Mcl-1, expression of other Bcl-2 proteins could also be affected by actinomycin D. In MEF cells, Noxa mRNA levels were upregulated upon actinomycin D treatment (Sup. Fig. 2). Increased Noxa protein could interact with Mcl-1, leading to subsequent Mcl-1 protein degradation,1 which could be another mechanism for the reduction of Mcl-1 protein levels in actinomycin D-treated cells. Interestingly, Bcl-2 protein level reduction was observed in panc-1 and A549 cells treated with actinomycin D, whereas Bcl-2 levels in untransformed MEF cells were largely unchanged (Figs. 3, 5 and 6). Decreased Bcl-2 levels could also render ABT-737 more effective at inducing cell death. Thus, it is possible that actinomycin D enhances the cytotoxic activities of ABT-737 by different mechanisms. Among them, reducing Mcl-1 expression levels could be a major one, evident by the largely diminished cell death observed in Mcl-1-overexpressing tumor cells treated with the drug combination (Fig. 8).
Our data indicate that the cytotoxic activity of ABT-737 and actinomycin D can be mediated by either Bak or Bax (Fig. 4). Upon the combined drug treatment, the level of cell death in cells individually expressing Bak or Bax was not as high as in wild-type cells, which can be attributed to the additive effect of Bak and Bax in wild-type cells as compared to the cells re-expressing only one of the proteins. Our results are also consistent with previous studies demonstrating a redundant role of Bak and Bax in the regulation of apoptosis during mammalian development and in mediating cell death induced by various death stimuli.4,44 Furthermore, pro-apoptotic activities of both Bak and Bax have been shown to be antagonized by Mcl-1. Bak is kept inactive by associating with both Mcl-1 and Bcl-XL and is sufficient to induce apoptosis individually if both Mcl-1 and Bcl-XL are neutralized.24,45 Similarly, Mcl-1 also antagonizes Bax function, as Mcl-1 inhibits Bax-mediated cytochrome c release following Bax's translocation to the mitochondria.46 Because treating cells with actinomycin D renders Mcl-1 inactive by downregulating its expression levels and the addition of ABT-737 neutralizes Bcl-2 and Bcl-XL, it is plausible that Bak or Bax alone is able to mediate apoptosis when the functions of all anti-apoptotic Bcl-2 proteins are completely abrogated. In contrast to our results, a previous study found that both Bak and Bax were necessary for a synergistic effect on cell death induced by the combination of ABT-737 and the CDK inhibitor roscovitine.27 Although Mcl-1 downregulation by roscovitine was responsible for the enhancement of ABT-737 cytotoxicity in this case, it is possible that Mcl-1 expression was not reduced efficiently enough to allow Bak or Bax alone to mediate the apoptotic signaling cascade. In our studies, Mcl-1 downregulation by actinomycin D was rapid and effective, possibly allowing for more efficient Bax or Bak pro-apoptotic function.
Our studies indicate that tumor cells resistant to ABT-737 as a single agent respond to the combined treatment of actinomycin D and ABT-737 in a synergistic fashion. Pancreatic cancers are typically difficult to treat with few effective treatment strategies available and pancreatic carcinoma cell lines have exhibited resistance to ABT-737 treatment alone.16 This resistance may be due to high levels of Mcl-1 expression, as numerous pancreatic cancers and cell lines exhibit overexpressed Mcl-1.47 The combination of ABT-737 and TRAIL has been shown to synergistically induce apoptosis in pancreatic carcinoma cells lines.16 However, the mechanism of cooperation between the two drugs did not appear to involve Mcl-1, although the study confirms Mcl-1 downregulation as an effective way to sensitize pancreatic carcinoma cells to ABT-737.
Non-small cell lung carcinoma (NSCLC) is another cancer type that might be treated effectively with the combination of actinomycin D and ABT-737. High levels of Mcl-1 expression in NSCLC appear to play a role in resistance to various chemotherapies. In NSCLC cell lines, ABT-737 resistance has been correlated with high levels of Mcl-l,20 making Mcl-1 an attractive target for NSCLC treatment. In our study using the A549 NSCLC cell line, downregulation of Mcl-1 expression levels sensitizes cells to ABT-737 treatment alone, whereas overexpression of Mcl-1 decreases the synergistic effect of ABT-737 and actinomycin D, supporting a role for Mcl-1 in mediating resistance to treatment of NSCLC.
While the clinical use of ABT-737 is limited because it is not orally bioavailable, our data would suggest the use of actinomycin D in combination with ABT-263, another experimental but orally active agent that mechanistically functions like ABT-737.48,49 ABT-263 has been shown to enhance apoptosis induced by chemotherapeutics in hematologic tumors50 and is currently in Phase II clinical trials. We provide evidence that the traditional chemotherapeutic agent actinomycin D, combined with ABT-737, is extremely effective at killing pancreatic and NSCLC tumor cells, probably due to its effective downregulation of Mcl-1 and further studies with ABT-263 in these tumors are warranted. Overall, our studies could potentially lead to a novel therapeutic strategy to treat pancreatic and NSCLC cancer patients.
Materials and Methods
Reagents.
Actinomycin D was obtained from Sigma Aldrich (St. Louis, MO) and diluted in DMSO. ABT-737 was provided by Abbott Laboratories (Abbott Park, IL) and diluted in DMSO. Antibodies used for western blot analysis were anti-β-actin mAb (Sigma), anti-Bak, NT pAb (Upstate; Lake Placid, NY), anti-Bax pAb (Abcam; Cambridge, MA), anti-Mcl-1 mAb (Epitomics; Burlingame, CA), anti-Bcl-XS/L S-18 pAb (Santa Cruz; Santa Cruz, CA), anti-mouse Bcl-2 mAb (Santa Cruz), anti-human Bcl-2 mAb (Santa Cruz).
Cell culture.
Immortalized IL-3-dependent Bak-/-Bax-/-hematopoietic cells were cultured as described previously.51 Wild-type murine Bak and Bax cDNAs were re-expressed in IL-3-dependent Bak-/-Bax-/- cells and Bak-/-Bax-/- MEF cells by retroviral infection and stable clones expressing Bak and Bax were selected as described previously.51,52 Mcl-1Δ/- MEFs and their wild-type counterparts were kindly provided by Dr. J. Opferman and generated as described previously.53 Human non-small cell lung carcinoma A549 and NCI-H1299 cells were obtained from ATCC (Manassas, VA). Human BxPC-3 pancreatic carcinoma cells were obtained from ATCC and panc-1 human pancreatic carcinoma cells were kindly obtained from Dr. Robert Mitchell.
Untransformed and transformed MEF cells were kindly provided by Dr. Wei-Xing Zong.
Mcl-1 overexpression.
Murine Mcl-1 cDNA was kindly provided by Dr. J. Opferman and cloned into the retroviral expression vector pBabeIRESGFP. Murine Mcl-1 cDNA was overexpressed in Mcl-1Δ/- MEFs, A549 and panc-1 cells by retroviral infection with virus generated by transfection of 293T/17 cells with pBabe-mouse Mcl-1-IRESGFP and two packaging plasmids (pUMVC, pMD2.G) using Lipofectamine 2000 (Invitrogen; Carlsbad, CA). Cells that were not ≥90% GFP positive by flow cytometry were either sorted by the University of Louisville Brown Cancer Center Flow Cytometry Laboratory (MoFlo cell sorter; Beckman Coulter; Miami, FL) or an additional round of retroviral infection was performed to maximize the number of cells overexpressing Mcl-1.
Mcl-1 knockdown.
Human Mcl-1 siRNA (sc-35877; Santa Cruz) and Control siRNA-A (sc-37007; Santa Cruz) was transfected into A549 and panc-1 cells using Lipofectamine RNAiMax (Invitrogen) according to the manufacturer's instructions for forward transfection at a final concentration of 10 nM. 24 hours after transfection, Mcl-1 knockdown was verified by western blot and 2.5, 5 and 10 µM ABT-737 or DMSO vehicle control was added. Cell viability by propidium iodide staining and flow cytometry was measured 24 and 48 hours after addition of ABT-737.
Gene expression analysis.
Wild-type MEFs were treated with 0.2 µg/ml actinomycin D for 6 and 12 hours and total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA quality was confirmed using a 2100 Bioanalyzer (Agilent; Santa Clara, CA). cRNA was generated using GeneChip 3′ IVT Express kit (Affymetrix; Santa Clara, CA). Hybridization to mouse genome 430 2.0 array chips (Affymetrix) and the data analysis using the MAS5 algorithm in GCOS 1.4 (Affymetrix) were performed by the University of Louisville Microarray Facility. Fold-change data for each gene was only considered if at least one present call was reported for any time point analyzed.
Real-time PCR.
Wild-type MEFs cells were treated with 0.2 µg/ml actinomycin D for 6, 9 and 12 hours and total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was generated by reverse transcription using random hexamer primers and SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. TaqMan Gene Expression Assays specific for mouse Mcl-1 and actin and TaqMan Universal PCR Master Mix, no AmpErase UNG (Applied Biosystems; Foster City, CA) were used to amplify the cDNA on the 7900HT Fast Real-Time PCR System (Applied Biosystems). CT values were calculated using RQ Manager, version 1.2 (Applied Biosystems) and normalized to actin transcript expression CT values as an internal control. Normalized 2^ CT values were used to calculate fold-changes in gene expression relative to values from untreated (time zero) cells.
Western blot analysis.
Whole cell lysates were generated by resuspending cells in RIPA buffer containing protease inhibitors (Complete, Roche; Indianapolis, IN). Undissolved cell debris was pelleted by centrifugation and protein concentration was determined by Bradford Assay (Protein Assay Reagent; Bio-Rad; Hercules, CA). Upon suspension in RIPA buffer, MEF cells were incubated on ice for 10 minutes and sonicated for 5 seconds at 10% amplitude (Sonic Dismembrator, Model 500, Fisher Scientific; Hampton, NH) before centrifugation. 20–25 µg of total protein was electrophoresed in 4–12% Bis Tris gels (Invitrogen or Bio-Rad) and transferred to PVDF (Millipore; Billerica, MA) for incubation with the appropriate primary and secondary antibodies. Proteins were detected using ECL western blotting substrate (Pierce; Rockford, IL). Where indicated, lysates corresponding to an equivalent number of cells were electrophoresed as above by pelleting a given number of cells and resuspending them in 1X LDS loading dye (Invitrogen).
Cell viability/death assays.
Cells were harvested, propidium iodide (Invitrogen) was added and cell viability was measured by propidium iodide exclusion using flow cytometry (FACSCalibur, Becton Dickinson). Data from experiments indicating the percentage of cell death represent 100 minus the cell viability measurement.
Soft agar colony formation assay.
10,000 exponentially growing cells were added to 2 ml 0.6% agar, which was then poured into a well in a 6-well plate. After agar solidified, 2 ml regular cell culture medium was added on the top of the agar and the plate was incubated under the regular tissue culture condition. Medium was replaced with fresh cell culture medium every 3–5 days. Following incubation for 10–12 days, the liquid cell culture medium was replaced with 1 ml of 12 mM MTT. The images of cell colonies were photographed after 10–20 minutes incubation with MTT.
Caspase 3/7 assays.
Caspase 3/7 activity was detected directly in cells using the SensoLyte® Homogeneous Rh110 Caspase 3/7 Assay Kit (AnaSpec; San Jose, CA). Cells were plated in 96-well white-walled plates and the assay was carried out according to the manufacturer's instructions, except half the volumes for each reagent were used. The fluorescence signal (Ex/Em = 496 nm/520 nm) was measured kinetically over 2 hours at 1 minute intervals using the Gemini EM microplate spectrofluormeter (Molecular Devices; Sunnyvale, CA). Data was plotted as relative fluorescence units (RFUs) versus time and the slope was determined (RFU/min) and normalized to untreated cells as an indicator of caspase 3/7 activity.
Chou-talalay synergism assay.
The synergistic effect of actinomycin D and ABT-737 on cell viability was determined by Chou-Talalay median dose effect analysis.31 IC50 of each drug on a tumor cell line was first determined. Various combinations of actinomycin D and ABT-737 at a constant ratio above or below their IC50 values were administered to cells. Following certain period of incubation, cell viability was determined and Fa was calculated as the ratio between the cell death levels of drugtreated cells and those of untreated control cells. Combination index (CI) was determined using CompuSyn software (Biosoft, Cambridge, UK).
Acknowledgements
We thank Drs. S. Fesik and S. Rosenberg (Abbott laboratories) for providing ABT-737; Dr. J. Opferman for the Mcl-1Δ/- MEFs and mouse Mcl-1 cDNA; Dr. Robert Mitchell for providing the panc-1 cell line; Dr. W.X. Zong for providing untransformed and transformed MEF cells. Dr. John Eaton for critical reading of the manuscript; Colins Eno for cloning assistance; Sabine Waigel and Vennila Arumugam at the University of Louisville Microarray facility and Christopher Worth at the University of Louisville Brown Cancer Center Flow Cytometry Lab for cell sorting. This work was supported by NIH grants CA106599, RR018733, funding from the J.G. Brown Cancer Center (C.L.) and the statistical support was partially funded by NIH grant 3P20RR018733-07S1.
Abbreviations
- MEF
mouse embryonic fibroblasts
- NSCLC
non-small cell lung carcinoma
- PI
propidium iodide
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/13274
Supplementary Material
References
- 1.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 3.Hardwick JM, Youle RJ. SnapShot: BCL-2 proteins. Cell. 2009;138:404. doi: 10.1016/j.cell.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youle RJ. Cell biology. Cellular demolition and the rules of engagement. Science. 2007;315:776–777. doi: 10.1126/science.1138870. [DOI] [PubMed] [Google Scholar]
- 7.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 sub-families. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 9.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 10.Kirkin V, Joos S, Zornig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229–249. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 12.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 14.Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 15.Okumura K, Huang S, Sinicrope FA. Induction of Noxa sensitizes human colorectal cancer cells expressing Mcl-1 to the small-molecule Bcl-2/Bcl-xL inhibitor, ABT-737. Clin Cancer Res. 2008;14:8132–8142. doi: 10.1158/1078-0432.CCR-08-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–2951. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Morgan-Lappe S, Huang X, Li L, Zakula DM, Vernetti LA, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–3979. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 20.Wesarg E, Hoffarth S, Wiewrodt R, Kroll M, Biesterfeld S, Huber C, et al. Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int J Cancer. 2007;121:2387–2394. doi: 10.1002/ijc.22977. [DOI] [PubMed] [Google Scholar]
- 21.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67:2908–2911. doi: 10.1158/0008-5472.CAN-07-0082. [DOI] [PubMed] [Google Scholar]
- 22.Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer. 2009;115:1932–1940. doi: 10.1002/cncr.24220. [DOI] [PubMed] [Google Scholar]
- 23.Jagt CT, Zuckermann M, Ten KF, Taminiau JA, Dijkgraaf MG, Heij H, et al. Veno-occlusive disease as a complication of preoperative chemotherapy for Wilms tumor: A clinico-pathological analysis. Pediatr Blood Cancer. 2009;53:1211–1215. doi: 10.1002/pbc.22202. [DOI] [PubMed] [Google Scholar]
- 24.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kepp O, Rajalingam K, Kimmig S, Rudel T. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. EMBO J. 2007;26:825–834. doi: 10.1038/sj.emboj.7601533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madesh M, Zong WX, Hawkins BJ, Ramasamy S, Venkatachalam T, Mukhopadhyay P, et al. Execution of superoxide-induced cell death by the proapoptotic Bcl-2-related proteins Bid and Bak. Mol Cell Biol. 2009;29:3099–3112. doi: 10.1128/MCB.01845-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 downregulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 28.Guerriero JL, Ditsworth D, Fan Y, Zhao F, Crawford HC, Zong WX. Chemotherapy induces tumor clearance independent of apoptosis. Cancer Res. 2008;68:9595–9600. doi: 10.1158/0008-5472.CAN-08-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veal GJ, Errington J, Sludden J, Griffin MJ, Price L, Parry A, et al. Determination of anti-cancer drug actinomycin D in human plasma by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:237–243. doi: 10.1016/s1570-0232(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 30.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. Bcl-2 inhibitors: small molecules with a big impact on cancer therapy. Cell Death Differ. 2009;16:360–367. doi: 10.1038/cdd.2008.137. [DOI] [PubMed] [Google Scholar]
- 31.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 32.Milano-Bausset E, Gaudart J, Rome A, Coze C, Gentet JC, Padovani L, et al. Retrospective comparison of neutropenia in children with Ewing sarcoma treated with chemotherapy and granulocyte colony-stimulating factor (G-CSF) or pegylated G-CSF. Clin Ther. 2009;31:2388–2395. doi: 10.1016/j.clinthera.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Newlands ES, Mulholland PJ, Holden L, Seckl MJ, Rustin GJ. Etoposide and cisplatin/etoposide, methotrexate and actinomycin D (EMA) chemotherapy for patients with high-risk gestational trophoblastic tumors refractory to EMA/cyclophosphamide and vincristine chemotherapy and patients presenting with metastatic placental site trophoblastic tumors. J Clin Oncol. 2000;18:854–859. doi: 10.1200/JCO.2000.18.4.854. [DOI] [PubMed] [Google Scholar]
- 34.Khatua S, Nair CN, Ghosh K. Immune-mediated thrombocytopenia following dactinomycin therapy in a child with alveolar rhabdomyosarcoma: the unresolved issues. J Pediatr Hematol Oncol. 2004;26:777–779. doi: 10.1097/00043426-200411000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto K, Kitabayashi I, Taya Y. KAP1 dictates p53 response induced by chemotherapeutic agents via Mdm2 interaction. Biochem Biophys Res Commun. 2006;351:216–222. doi: 10.1016/j.bbrc.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Choong ML, Yang H, Lee MA, Lane DP. Specific activation of the p53 pathway by low dose actinomycin D: a new route to p53 based cyclotherapy. Cell Cycle. 2009;8:2810–2818. doi: 10.4161/cc.8.17.9503. [DOI] [PubMed] [Google Scholar]
- 37.Opferman JT. Unraveling MCL-1 degradation. Cell Death Differ. 2006;13:1260–1262. doi: 10.1038/sj.cdd.4401978. [DOI] [PubMed] [Google Scholar]
- 38.Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H, Florian S, Sonneck K, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105:3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 39.Sieghart W, Losert D, Strommer S, Cejka D, Schmid K, Rasoul-Rockenschaub S, et al. Mcl-1 overexpression in hepatocellular carcinoma: a potential target for antisense therapy. J Hepatol. 2006;44:151–157. doi: 10.1016/j.jhep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Hussain SR, Cheney CM, Johnson AJ, Lin TS, Grever MR, Caligiuri MA, et al. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: downregulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin Cancer Res. 2007;13:2144–2150. doi: 10.1158/1078-0432.CCR-06-2294. [DOI] [PubMed] [Google Scholar]
- 41.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–556. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, Gojo I, Fenton RG. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood. 2002;99:1885–1893. doi: 10.1182/blood.v99.6.1885. [DOI] [PubMed] [Google Scholar]
- 43.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–1336. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 45.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Germain M, Milburn J, Duronio V. MCL-1 inhibits BAX in the absence of MCL-1/BAX Interaction. J Biol Chem. 2008;283:6384–6392. doi: 10.1074/jbc.M707762200. [DOI] [PubMed] [Google Scholar]
- 47.Miyamoto Y, Hosotani R, Wada M, Lee JU, Koshiba T, Fujimoto K, et al. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X and Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73–82. doi: 10.1159/000011933. [DOI] [PubMed] [Google Scholar]
- 48.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 49.Shoemaker AR, Mitten MJ, Adickes J, Ackler S, Refici M, Ferguson D, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–3277. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 50.Ackler S, Mitten MJ, Foster K, Oleksijew A, Refici M, Tahir SK, et al. The Bcl-2 inhibitor ABT-263 enhances the response of multiple chemotherapeutic regimens in hematologic tumors in vivo. Cancer Chemother Pharmacol. 2010;66:869–880. doi: 10.1007/s00280-009-1232-1. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Olberding KE, White C, Li C. Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ. 2010 doi: 10.1038/cdd.2010.68. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.