Abstract
Ethyl naphtho[2,1-b]furan-2-carboxylate (2) on reaction with hydrazine hydrate in presence of acid catalyst in ethanol medium affords naphtho[2,1-b]furan-2-carbohydrazide (3). The reaction of substituted acetophenones (4a-c) with aromatic aldehydes (5a-e) produces chalcones (6a-o) via the Claisen condensation. The reaction of naphtho[2,1-b]furan-2-carbohydrazide (3) with chalcones (6a-6o) in presence of acetic acid as catalyst in dioxane produces 1-(naphtho[2,1-b]furan-2-yl-carbonyl)-3,5-disubstituted-2,3-dihydro-1H-pyrazoles (7a-o). The structures of newly synthesized compounds have been established by elemental analysis and spectral studies. The compounds 7a-o have been evaluated for their antimicrobial activity and some selected compounds evaluated for antiinflammatory, analgesic, anthelmintic, diuretic and antipyretic activities.
Keywords: Naphtho[2,1-b]furan; naphthofuropyrazoles; pyrazoles; pharmacological activities
The pyrazole-based derivatives have shown several biological activities, many of them are currently being tested and/or clinically evaluated for new drug discovery1. Various pyrazole and pyrazoline derivatives have been reported to possess antinociceptive effect in mice2, antimicrobial3,4, insecticidal5 and local anesthetic6 activities. Some of the pyrazole derivatives also serve as intermediates in dye industry7 and act as growth inhibitors of phytopathogenic fungi8. The biheterocyclic compounds in which pyrazole moiety is coupled with furan or benzofuran nucleus exhibit antimicrobial and antiinflammatory activities9–10. However there are no reports in literature concerning coupling of pyrazole ring with another biologically active naphtho[2,1-b]furan nucleus, either directly or through carbon bridge.
Receiving impetus from these reports, guided by the principle that combination of two or more biologically active heterocyclic systems enhances the biological profile of molecules many folds11 and in continuation of our research for more potent derivatives of naphtho[2,1-b]furan derivatives12–17, we report in this paper synthesis and pharmacological investigation of novel biheterocyclics, 1-(naphtho[2,1-b]furan-2-yl-carbonyl-3,5-disubstituted-2,3-dihydro-1H-pyrazoles (7a-7o) bridged via carbonyl group.
MATERIALS AND METHODS
Melting points were determined with open capillary and are uncorrected. IR spectra were recorded in KBr pellets by using Shimadzu FT-IR 8000 Spectrometer. 1H NMR and 13C NMR were recorded in DMSO-d6 on Bruker-400 MHz Spectrometer. Chemical shifts are recorded in δ relative to TMS as internal standard. Mass spectral data were obtained on a Brucker Apex-II Mass Spectrometer. Elemental analyses were performed using a Vario-EL elemental analyzer. Purity of the compounds was checked by TLC. All the animals were maintained under standard conditions and had access to pelleted animal feed and water. The study protocols were approved by the institutional animal ethics committee (CPCSEA Regd.No.157/1999).
Chemical synthesis:
To a solution of 2-hydroxy-1-naphthaldehyde (1) (5.16 g, 0.03 mol) in dry N,N-dimethylformamide (25 ml), ethylchloroacetate (3.66 g, 0.03 mol) and anhydrous potassium carbonate (12.4 g, 0.9 mol) were added and the reaction mixture was refluxed on water bath for 24 h. The reaction mixture was then poured into ice cold water, to obtain the product ethyl naphtho-[2,1-b]furan-2-carboxylate (2) as solid, which was collected by filtration, dried and recrystallised from ethanol.
A mixture of ethyl naphtho-[2,1-b]furan-2-carboxylate (2) (2.40 g, 0.01 mol), catalytic amount of concentrated hydrochloric acid and hydrazine hydrate (1 g, 0.02 mol) were refluxed in absolute ethanol (25 ml) for 2 h on water bath. Then the reaction mixture was cooled to room temperature, the solid thus obtained was filtered and dried. The product, naphtho-[2,1-b]furan-2-carbohydrazide (3) obtained was recrystallised from ethanol.
Freshly distilled acetophenone (4) (2.6 g, 0.0215 mol) was added to a cooled mixture of sodium hydroxide (1.1 g, 0.0275 mol), water (10 ml) and rectified spirit (6 ml). To this mixture 4-methoxybenzaldehyde (5b) (2.9 g, 0.0215 mol) was added drop wise maintaining the temperature at < 20°. After the addition was over, the reaction mixture was stirred vigorously until the reaction mixture became thick. It was cooled overnight in ice chest. The solid obtained was filtered and washed with cold water and rectified spirit. The crude chalcone, 3-(4-methoxyphenyl)-1-phenylprop-2-en-1-one (6b) was dried and recrystallised from rectified spirit. The compounds 6a, 6c-o were synthesized by the same method described above using 4-chloroacetophenone, 4-hydroxyacetophenone and different aromatic aldehydes.
To a solution of 3-(4-methoxyphenyl)-1-phenylprop-2-en-1-one (6b) (1.38 g, 0.005 mol) in dioxane (25 ml), acetic acid (0.5 ml) was added and the mixture was kept for stirring for 30 minutes. To this mixture naphtho-[2,1-b]furan-2-carbohydrazide (3) (1.01g, 0.005 mol) was added and refluxed for 24 h. After completion of the reaction, the reaction mixture was poured to ice cold water, solid separated was filtered and dried. The crude product was recrystallised from ethanol. The compounds 7a, 7c-o were synthesized similarly from 6a, 6c-o. The characterization data of the synthesized compounds are reported in Table 1. The structures of newly synthesized compounds were elucidated by IR, NMR spectral studies, which are reported in Table 2.
TABLE 1.
CHARACTERIZATION DATA OF THE COMPOUNDS 7a-O
| Compd. | R1 | R2 | Mol. formula | Yield (%) | mp(0) | Found (Calcd.) % N |
|---|---|---|---|---|---|---|
| 7a | H | H | C28H20 N2O2 | 68 | 235 | 6.64 (6.73) |
| 7b | H | 4-OCH3 | C29H22 N2O3 | 65 | 241 | 6.18 (6.27) |
| 7c | H | 4-OH | C28H20 N2O3 | 63 | 248 | 6.39 (6.48) |
| 7d | H | 4-Cl | C28H19N2O2Cl | 70 | >250 | 6.14 (6.21) |
| 7e | H | 4-NO2 | C28H19 N3O4 | 62 | >250 | 9.00 (9.11) |
| 7f | 4-Cl | H | C28H19N2O2Cl | 71 | >250 | 6.16 (6.21) |
| 7g | 4-Cl | 4-OCH3 | C29H21N2O3Cl | 67 | >250 | 5.70 (5.82) |
| 7h | 4-Cl | 4-OH | C28H19N2O3Cl | 68 | >250 | 5.91 (6.00) |
| 7i | 4-Cl | 4-Cl | C28H18N2O2Cl2 | 70 | >250 | 5.68 (5.77) |
| 7j | 4-Cl | 4-NO2 | C28H18N3O4Cl | 65 | >250 | 8.38 (8.47) |
| 7k | 4-OH | H | C28H20N2O3 | 66 | >250 | 6.39 (6.48) |
| 7l | 4-OH | 4-OCH3 | C29H22N2O4 | 71 | >250 | 5.99 (6.06) |
| 7m | 4-OH | 4-OH | C28H20N2O4 | 65 | >250 | 6.13 (6.25) |
| 7n | 4-OH | 4-Cl | C28H19N2O3Cl | 68 | >250 | 5.91 (6.00) |
| 7o | 4-OH | 4-NO2 | C28H19N3O5 | 67 | >250 | 8.70. (8.80) |
Satisfactory C and H analysis was obtained for all the compounds.
TABLE 2.
IR AND NMR SPECTRAL DATA OF 2-(1-NAPHTHO[2,1-b]FURAN-2-YL-CARBONYL)-3,5-DISUBSTITUTED-2,3- DIHYDRO-1H-PYRAZOLES
| Comp. | R1 | R2 | IR (KBr) (C=O) | 1H NMR |
|---|---|---|---|---|
| 7a | H | H | 1694 | δ 6.1 (s, 1H, NH), δ 7.3-8.5 (m, NCHPh +CHCPh +17ArH) |
| 7b | H | 4-OCH3 | 1663 | δ 3.8 (s, 3H, OCH3), δ 6.0 (s, 1H, NH), d 7.0-8.5 (m, NCHPh+CHCPh +16ArH) |
| 7c | H | 4-OH | 1675 | δ 4.7 (b, 1H, OH), δ 6.1 (s, 1H, NH), δ 7.3-8.6, (m, NCHPh+CHCPh +16ArH) |
| 7d | H | 4-Cl | 1683 | δ 5.8 (s, 1H, NH), δ 7.4-8.6 (m, NCHPh+ CHCPh +16ArH) |
| 7e | H | 4-NO2 | 1678 | δ 6.2 (s, 1H, NH), δ 7.2-8.8 (m, NCHPh+ CHCPh +16ArH) |
IR streching frequencies are measured in cm−1 and 1H NMR chemical shift are expressed in δ ppm, values in comparison with standard TMS.
Antimicrobial activity:
The in vitro antimicrobial activity was carried out against 24 h old cultures of two bacteria and two fungi by cup-plate method18. The compounds (7a-o) have been tested for their antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus and antifungal activity against Aspergillus niger and Curvularia lunata. Chloramphenicol and fluconazole were used as standards for antibacterial and antifungal activity respectively. The compounds were tested at a concentration in of 0.001 mol/ml in DMF against all the organisms. The zone of inhibition was compared with the standard drug after 24 h of incubation at 25° for antibacterial activity and 48 h at 30° for antifungal activity. The results are reported in Table 3.
TABLE 3.
ANTIMICROBIAL ACTIVITY OF THE COMPOUNDS 7a-o
| Compd. | Zone of inhibition in mm | |||
|---|---|---|---|---|
| P. aeruginosa | S. aureus | Aspergillus niger | Curvularia lunata | |
| 7a | 17 | 17 | 17 | 18 |
| 7b | 15 | 16 | 14 | 15 |
| 7c | 15 | 15 | 16 | 17 |
| 7d | 17 | 16 | 15 | 17 |
| 7e | 16 | 16 | 16 | 15 |
| 7f | 17 | 15 | 16 | 16 |
| 7g | 15 | 16 | 16 | 17 |
| 7h | 16 | 17 | 19 | 18 |
| 7i | 19 | 19 | 18 | 18 |
| 7j | 17 | 16 | 18 | 17 |
| 7k | 19 | 18 | 18 | 19 |
| 7l | 20 | 19 | 19 | 18 |
| 7m | 19 | 18 | 19 | 18 |
| 7n | 18 | 20 | 19 | 19 |
| 7o | 19 | 18 | 20 | 19 |
| Standard | 24 | 26 | 24 | 22 |
| DMF | + ve | + ve | + ve | + ve |
Zone of inhibition expressed in mm. The Cup-plate method was followed and chloramphenicol and flucanazole were used as standards. The concentration of the drug used is 0.001 mol/ml in DMF
Antiinflammatory activity:
The antiinflammatory activity was evaluated by a rat paw edema method, which is based on plethysmographic measurement of carrageenan-induced acute rat paw edema19–20. For this study, wistar rats of either sex, weighing between 100-200 g, were used and were divided into 7 groups, of 4 animals each. The group I served as control, the group II received ibuprofen and served as standard, and the groups III-VII received orally the test compounds. These drugs were administered 1 h before the injection of carrageenan. After 1 h all the animals were injected subcutaneously with a suspension of carrageenan in Tween-80 (0.1%, 0.05 ml) solution to the left hind paw in the subplantar region and the paw volume was measured immediately. After 3 h the paw volume was measured in control, in standard and in test groups. Percent inhibition of paw volume was calculated using the formula, % inhibition= (1-Vt/Vc)×100, where, Vt is the mean increase in the paw volume in test animals group and Vc is the mean increase in the paw volume in control group. The results are reported in Table 4.
TABLE 4.
ANTIINFLAMMATORY ACTIVITY OF COMPOUNDS 7a-e
| Compd. | Group | Paw volume ±SEM after 3 h | % Inhibition of edema after 3 h |
|---|---|---|---|
| Control | I | 0.98±0.02 | ---- |
| Ibuprofen | II | 0.20±0.01 | 79.59 |
| 7a | III | 0.57±0.02 | 41.83 |
| 7b | IV | 0.42±0.01 | 55.14 |
| 7c | V | 0.48±0.02 | 51.02 |
| 7d | VI | 0.51±0.01 | 52.04 |
| 7e | VII | 0.53±0.01 | 45.91 |
The control used Tween-80 (0.1%, 1 ml), and standard used ibuprofen and the concentration of drug used 30 mg/kg body weight in Tween-80 (0.1%) solution. Number of animals used in each group is 4
Analgesic activity:
Analgesic activity was determined by the method based on acetic acid- induced writhing in mice21–22. For this experiment, colony bred swiss mice of either sex weighing 25-35 g mice were divided into control, standard and different test groups containing 6 animals each. The results are reported in Table 5. Percent inhibition of writhing was calculated using the formula, % Inhibition= (1−Nt/Nc)×100, where, Nt is the mean number of writhing in test animals and Nc represented mean number of writhing in control.
TABLE 5.
ANALGESIC ACTIVITY OF COMPOUNDS 7a-e
| Comp. | Group | R1 | R2 | Mean number of writhings ±SEM | % Protection |
|---|---|---|---|---|---|
| Control | I | ---- | ---- | 42.15±3.16 | ---- |
| Aspirin | II | ---- | ---- | 12.20±1.50 | 71.00 |
| 7a | III | H | H | 18.23±2.18 | 56.74 |
| 7b | IV | H | 4-OCH3 | 15.31±1.96 | 63.67 |
| 7c | V | H | 4-OH | 17.84±2.14 | 57.67 |
| 7d | VI | H | 4-Cl | 14.78±1.83 | 64.93 |
| 7e | VII | H | 4-NO2 | 16.43±2.02 | 61.07 |
Acetic acid-induced writhing method, Tween-80 (0.1%, 0.5 ml) used as control, aspirin used as standard, concentration of drug used 100 mg/kg in 0.1% Tween-80 suspension, Number of animals used in each group 6
Anthelmintic activity:
Anthelmintic activity was evaluated using Pheritima posthuma (class-Annelida and order-Oligichaeta). The technique adopted was that described by Giand et al.23 with slight modification24. The worms with normal motility were selected for the experiment and albendazole is used as standard for comparison of the activity. The results are tabulated in Table 6.
TABLE 6.
ANTHELMINTIC ACTIVITY OF COMPOUNDS 7a-o
| Comp. | R1 | R2 | Mean paralyzing time (min) | Mean death time (min) |
|---|---|---|---|---|
| Standard | ---- | ----- | 35 | 44 |
| 7a | H | H | 71 | 144 |
| 7b | H | 4-OCH3 | 100 | 150 |
| 7c | H | 4-OH | 70 | 119 |
| 7d | H | 4-Cl | 71 | 144 |
| 7e | H | 4-NO2 | 177 | 281 |
| 7f | 4-Cl | H | 218 | 259 |
| 7g | 4-Cl | 4-OCH3 | 105 | 119 |
| 7h | 4-Cl | 4-OH | 101 | 143 |
| 7i | 4-Cl | 4-Cl | 106 | 173 |
| 7j | 4-Cl | 4-NO2 | 113 | 164 |
| 7k | 4-OH | H | 121 | 192 |
| 7l | 4-OH | 4-OCH3 | 134 | 175 |
| 7m | 4-OH | 4-OH | 72 | 113 |
| 7n | 4-OH | 4-Cl | 83 | 125 |
| 7o | 4-OH | 4-NO2 | 96 | 134 |
Giand et al. method was used for the study. 0.1% Tween-80 prepared in 25 ml of 6% dextrose solution used as control, albendazole used as standard, concentration of drug used is 25 mg in 0.1% Tween-80 in 25 ml of 6% dextrose solution, number of animals in each group 4 worms
Diuretic activity:
The diuretic activity was evaluated on wistar rats using the method reported by Lipschitz25. Rats of either sex, weighing between 100-200 g were divided into 7 groups, each containing 6 animals. Group I served as control, group II served as standard. The groups III-VII received orally the test compounds at the dose of 30 mg/kg body weight in Tween-80 (0.1%, 5 ml). Each group of animals was kept in different metabolic cages provided with a wire mesh at the bottom and a funnel to collect urine. Urine excreted was collected after 5 h and the values are tabulated in Table 7.
TABLE 7.
RESULTS OF DIURETIC ACTIVITY OF COMPOUNDS 7a-e
| Comp. | Group | R1 | R2 | Volume of urine collected in ml after 5 h. | T/S (Lipschitz value) |
|---|---|---|---|---|---|
| Control | I | --- | ---- | 8 | 0.27 |
| Frusemide | II | --- | ---- | 29 | 1.00 |
| 7a | III | H | H | 14 | 0.48 |
| 7b | IV | H | 4-OCH3 | 15 | 0.52 |
| 7c | V | H | 4-OH | 16 | 0.55 |
| 7d | VI | H | 4-Cl | 15 | 0.52 |
| 7e | VII | H | 4-NO2 | 19 | 0.65 |
Control, concentration of drug used is 30 mg/kg in Tween-80 (0.1%, 5 ml) solution. Number of animals in each group 6
Antipyretic activity:
The antipyretic activity was carried out on wistar rats as described by the method based on yeast-induced hyperpyrexia method26. The rats weighing 150-170 g were selected and divided into 6 groups each having 6 animals. The rectal temperature and its hourly variation were recorded at the beginning of the experiment using a digital Telethermometer. The rats showing rise in rectal temperature of 0.5° or more were distributed in to different group of 6 each and test drugs, Group I received tween–80 as control and group-II received paracetamol as standard drug and groups III-VI received test compounds. The decrease in rectal temperature was noted using telethermometer at 1 h intervals up to 3 h. All values are expressed as mean±SEM. The results are reported in Table 8.
TABLE 8.
RESULTS OF ANTIPYRETIC ACTIVITY OF COMPOUNDS 7b-e
| Comp. | Group | R1 | R2 | Mean rectal temperature | |||
|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | ||||
| Control | I | ---- | ---- | 38.7 | 38.6±0.16 | 38.5±0.16 | 38.5±0.04 |
| Paracetamol | II | ---- | ---- | 38.4 | 37.9±0.19 | 37.8±0.17 | 37.7±0.17 |
| 7b | III | H | 4-OCH3 | 38.2 | 38.1±0.17 | 38.1±0.11 | 38.0±0.09 |
| 7c | IV | H | 4-OH | 37.9 | 37.8±0.22 | 37.9±0.18 | 37.8±0.11 |
| 7d | V | H | 4-Cl | 37.8 | 37.7±0.11 | 37.8±0.16 | 37.7±0.14 |
| 7e | VI | H | 4-NO2 | 38.5 | 38.4±0.12 | 38.2±0.08 | 38.1±0.15 |
Yeast-induced hyperpyrexia method, Tween-80 is used as control, paracetamol is used as standard, concentration of drug used is 100 mg in Tween-80. Number of animals used in each group is 6
RESULTS AND DISCUSSION
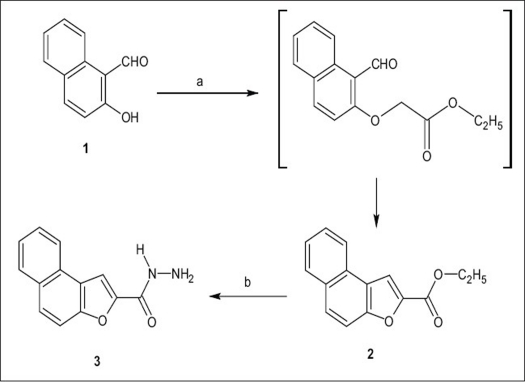
The required starting material to accomplish the synthesis of title compounds, ethyl naphtho[2,1-b]furan-2-carboxylate (2) was obtained by the reaction of 2-hydroxy-1-naphthaldehyde (1) with ethyl chloroacetate in presence of anhydrous potassium carbonate and in dry DMF at reflux temperature. Both condensation as well as cyclisation occurred in single step and produced ethyl naphtho-[2,1-b]furan-2-carboxylate (2) in good yield. The reaction of ethyl naphtho-[2,1-b]furan-2-carboxylate (2) with hydrazine hydrate in presence of acid catalyst in ethanol produced naphtho-[2,1-b]furan-2-carbohydrazide (3). The synthetic route is shown in Scheme 1.
Scheme 1.
Synthetic route for the synthesis of 3 a-Reaction carried out with ethyl chloroacetate in presence of K2CO3 and acetone; b- reaction carried out with hydrazine hydrate in ethanol.
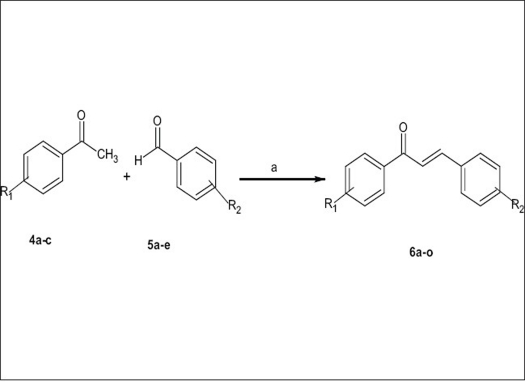
The chalcones (6a-o) were synthesized by Claisen condensation between substituted acetophenones (4a-c) and different aromatic aldehydes27 (5a-e). The selection of substituted acetophenones and substituted aromatic aldehydes was based on presence of electron withdrawing and electron releasing groups which would assist in later studies, on structure activity relationship. The synthetic route shown in Scheme 2.
Scheme 2.
Synthetic route for the synthesis of 6a-o a- Reaction carried out in ethanol solution of sodium hydroxide at 25°
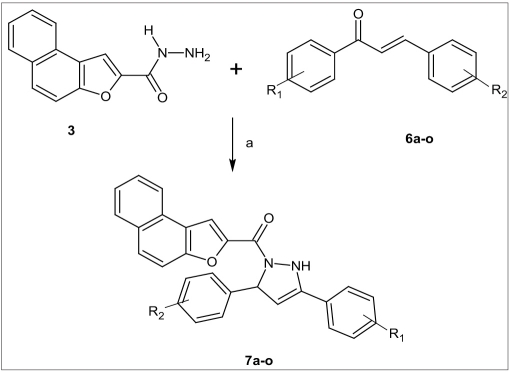
The reaction of naphtho[2,1-b]furan-2-carbohydrazide (3) with chalcones 6a-o to obtain the title compounds (7a-o) was attempted by employing various reagents and reaction conditions. However, the desired condensation was successful only when the reaction was carried out by using acetic acid as catalyst and dioxane as a solvent at reflux temperature. The target compounds 1-(naphtho[2,1-b]furan-2-ylcarbonyl)-3,5-disubstituted-2,3dihydro-1H-pyrazoles (7a-o) were obtained in good yield. The synthetic route is shown in Scheme 3.
Scheme 3.
Synthetic route for the synthesis of 7a-o a- Reaction carried out in acetic acid and dioxane at reflux temperature.
The compounds containing naphthofuran were known to exhibit a wide spectrum of biological and pharmacological activities28–30. Hence, it was intrigued to evaluate newly synthesized compounds for antimicrobial, antiinflammatory, analgesic, anthelmintic, diuretic and antipyretic activities by adopting literature procedure.
The newly synthesized compounds were evaluated for antimicrobial activity by cup-plate method. Antibacterial activity was evaluated against Pseudomonas aerugenosa and Staphylococcus aureus using chloramphenicol as standard drug, The compounds 7i, 7k, 7l, 7m and 7o exhibited activity against P. aerugenosa, while compound 7n showed activity against S. aureus. For antifungal activity Aspergillus niger and Curvularia lunata were used as test organisms and fluconazole as standard drug. The compounds 7a-o shows lesser activity than the standard drugs.
Among the compounds tested for antiinflammatory activity, the compounds 7a-e shows lesser activity than the standard drugs. The results of anthelmintic activity indicate that most of the compounds were found to be less active than the standard drugs. The compounds 7a-e were found to display lesser activity when compared to standard drug. The results of antipyretic activity indicated that compound 7e exhibited equal antipyretic activity of reducing the temperature to the extent of 0.4°. Rest of the compounds was less active than the standard drug. From these results it is possible to conclude that most of the synthesized compounds were pharmacologically active. However, no conclusion could be drawn on the effect of electron donating or electron withdrawing groups on pharmacological activities.
Acknowledgments
The authors thank the Chairman, Department of Chemistry, Kuvempu University and the Principal, SCS College of Pharmacy, Harapanahalli for providing laboratory facilities, the Convener, Sophisticated Instruments Facility, IISc, Bangalore for providing spectral data and to UGC for awarding Senior Research Fellowship (to MNK).
Footnotes
Kumaraswamy, et al.: 2-(1-naphtho[2,1-b]furan-2-yl-carbonyl)-3,5-disubstituted-2,3-dihydro-1H-pyrazoles
REFERENCES
- 1.Park HJ, Lee JC, Kim YJ, Lee KI. Unexpected behavior of 5-phenoxy pyrazoles derivatives. Bull Korean Chem Soc. 2005;26:4. [Google Scholar]
- 2.Tabarelli Z, Rubin MA, Berlese DB, Sauzem PD, Missio TP, Teixeira MV, et al. Antinoceptive effect of novel pyrazolines in mice. Braz J Med Biol Res. 2004;37:1531. doi: 10.1590/s0100-879x2004001000013. [DOI] [PubMed] [Google Scholar]
- 3.Kidwai M, Goel Y, Kumar R. Microwave assisted synthesis and antifungal activity of 1,2,4-triazine, 1,2,4-triazole, tetrazole and pyrazoles derivatives. Indian J Chem. 1998;37B:174. [Google Scholar]
- 4.Greenhill JV. Pyrazoles with fused six-membered heterocyclic ring. In: Katritzky AR, Rees CW, editors. Comprehensive heterocyclic chemistry. Vol. 5. Oxford: Pergamon Press; 1984. p. 302. [Google Scholar]
- 5.Verma RK, Nayal SS. Study of insecticidal activity of some pyrazoles derivatives against American cockroaches. Indian J Chem Tech. 2003;10:347. [Google Scholar]
- 6.Bruno O, Ranise A, Bondavalli F, Schenone P, D'Amico M, Filippelli A, et al. 3,5-diphenyl-1H-pyrazole derivatives XI. N-aryl-5(3)-phenyl-4-(3,5-diphenyl-1-pyrazolyl)-3(5)-pyrazole amines, 5-substituted, 4,5-dihydro-3-phenyl-1-4-(3,5-diphenyl-1-pyrazolyl)-H-pyrazoles and 2,6-disubstituted-1,6-dihydro-4-phenyl-5-(3,5-diphenyl-1-pyrazolyl) pyrimidines with antipyretic, antiinflammatory and other activities. Il Farmaco. 1993;48:949. [PubMed] [Google Scholar]
- 7.Lubs HA. In the chemistry of synthetic dyes and pigments. Washington: American Chem Soc; 1970. [Google Scholar]
- 8.Vicentini CB, Romagnoli C, Andreotti E, Mares D. Synthetic pyrazole derivatives as growth inhibitors of some phytopathogenic fungi. J Agric Food Chem. 2007;55:10331. doi: 10.1021/jf072077d. [DOI] [PubMed] [Google Scholar]
- 9.Manna F, Chimenti F, Fioravanti R, Bolasco A, Secci D, Chimenti P, et al. Synthesis of some pyrazoles derivatives and preliminary investigation of their affinity binding to p-gylcoprotein. Bioorg Med Chem Lett. 2005;15:4632. doi: 10.1016/j.bmcl.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 10.Harinadhababu V, Manna SK, Srinivasan KK, Bhat VG. Synthesis and biological evaluation of 1,3,5-trisubstituted pyrazolines bearing benzofurans. Indian J Heterocycl Chem. 2004;13:253. [Google Scholar]
- 11.Ravindra KC, Vagdevi HM, Vaidya VP. Synthesis and antimicrobial activity of novel naphtho[2,1-b]furo-5H-[3,2-d][1,3,4]thiadiazolo[3,2-a]pyrimidin-5-one. ARKIVOC. 2008;11:1. [Google Scholar]
- 12.Mahadevan KM, Vaidya VP. Synthesis and pharmacological evaluation of some potent naphtho[2,1-b]furo-pyrazolyl, oxadiazolyl and coumaryl derivatives. Indian J Pharm Sci. 2003;65:128. [Google Scholar]
- 13.Kumaraswamy MN, Prathima Mathias DA, Chandrashekhar C, Vaidya VP. Synthesis and pharmacological evaluation of 2-mercapto-4-substituted-naphtho[2,1-b]furo[3,2-d] pyrimidines. Indian J Pharm Sci. 2006;68:731. [Google Scholar]
- 14.Kumaraswamy MN, Vaidya VP. Novel method for the synthesis of symmetrical and asymmetrical azines involving naphtho[2,1-b]furan and their antimicrobial activity. Indian J Heterocycl Chem. 2005;14:193. [Google Scholar]
- 15.Mahadevan KM, Vaidya VP, Vagdevi HM. Synthesis of novel naphtho-[2,1-b]furopyrimidine derivatives. Indian J Chem. 2003;42B:1931. [Google Scholar]
- 16.Nagaraj GK, Kumaraswamy MN, Vaidya VP, Mahadevan KM. Microwave assisted synthesis of naphtho[2,1-b]furan, 1,3,4-benzotriazepines. A potent antimicrobial agent. ARKIVOC. 2006;10:211. [Google Scholar]
- 17.Vagdevi HM, Vaidya VP. Studies in naphthofurans: Part III. Synthesis of 2-substituted naphtho[2,1-b]furans, 2-(2'-aryl-3'-acetyl-1',3',4'-oxadiazolyl)aminonaphtho[2,1-b]furans and their biological activities. Indian J Heterocycl Chem. 2001;10:253. [Google Scholar]
- 18.Seely HW, Van Demark PJ. Microbes in action: A laboratory manual of Microbiology. Mumbai: DB Taraporewala Sons and Co; 1975. p. 55. [Google Scholar]
- 19.Mukhanova TI, Lykova OA, Alekseeva LM, Granik VG. New approach to synthesis of derivatives of 2-(5-hydroxybenzofuryl-3)naphthofurans. Chem Heterocycl Compd. 1998;34:651. [Google Scholar]
- 20.Lambert RJ, Pearson J. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitoryconcentration (NIC) values. J Appl Microbiol. 2000;88:784. doi: 10.1046/j.1365-2672.2000.01017.x. [DOI] [PubMed] [Google Scholar]
- 21.Koster R, Anderson M, Olbur EJ. Acetic-acid for analgesic screening. Fed Proc. 1959;18:412. [Google Scholar]
- 22.Turner RA. Analgesics in: Screning methods in pharmacology. New York: Academic Press; 1965. [Google Scholar]
- 23.Aswar M, Aswar U, Watkar B, Vyas M, Wagh A, Gujar KN. Anthelmintic activity of Ficus benghalensis. Int J Green Pharm. 2008;2:170. [Google Scholar]
- 24.Kalluraya B, Sreenivasa S. Synthesis and pharmacological properties of some quinoline derivatives. Il Farmaco. 1998;53:399. doi: 10.1016/s0014-827x(98)00037-8. [DOI] [PubMed] [Google Scholar]
- 25.Lipschitz WL, Hadidian Z, Kerpscar A. Bioassy of diuretics. J Pharmacol Exp Ther. 1943;79:97–110. [Google Scholar]
- 26.Hukkeri VI, Patil BS, Savadi RV, Nagarathna CV. Analgesic, antipyretic and diuretic activities of Basella rubralinn. Indian Drugs. 2004;41:536. [Google Scholar]
- 27.Furniss BS, Hannaford AJ, Smith PW, Tatchell AR. Vogel's textbook of practical organic chemistry. 5th ed. ELBS Publication; 1989. p. 1034. [Google Scholar]
- 28.Ramesh D, Chandrashekhara C, Vaidya VP. Synthesis of novel American naphtho[2,1-b]fur[3,2-b]pyridine derivatives as a potential antimicrobial agents. Indian J Chem. 2008;47B:753. [Google Scholar]
- 29.Rajashekhara H, Ramesh D, Chandrashekhara C, Mahadevan KM, Vaidya VP. Synthesis of 2-(3-nitronaphtho[2,1-b]furan-2-yl)-5-substituted-1,3,4-oxadiazoles and their biological activities. Indian J Heterocycl Chem. 2007;16:353. [Google Scholar]
- 30.Chandrashekhara C, Kumaraswamy MN, Basavaraj KM, Vaidya VP. Synthesis, antimicrobial and anthelmintic activities of 2,9-disubstituted [2,1-b]furans. Indian J Heterocycl Chem. 2007;16:341. [Google Scholar]