Abstract
Here, we investigated the possible predictive value of stromal caveolin-1 (Cav-1) as a candidate biomarker for clinical outcome in triple negative (TN) breast cancer patients. A cohort of 85 TN breast cancer patients was available, with the necessary annotation and nearly 12 years of follow-up data. Our primary outcome of interest in this study was overall survival. Interestingly, TN patients with high-levels of stromal Cav-1 had a good clinical outcome, with >50% of the patients remaining alive during the follow-up period. In contrast, the median survival for TN patients with moderate stromal Cav-1 staining was 33.5 months. Similarly, the median survival for TN patients with absent stromal Cav-1 staining was 25.7 months. A comparison of 5-year survival rates yields a similar pattern. TN patients with high stromal Cav-1 had a good 5-year survival rate, with 75.5% of the patients remaining alive. In contrast, TN patients with moderate or absent stromal Cav-1 levels had progressively worse 5-year survival rates, with 40 and 9.4% of the patients remaining alive. In contrast, in a parallel analysis, the levels of tumor epithelial Cav-1 had no prognostic significance. As such, the prognostic value of Cav-1 immunostaining in TN breast cancer patients is compartment-specific, and selective for an absence of Cav-1 staining in the stromal fibroblast compartment. A recursive-partitioning algorithm was used to assess which factors are most predictive of overall survival in TN breast cancer patients. In this analysis, we included tumor size, histologic grade, whether the patient received surgery, radiotherapy or chemotherapy, CK5/6, EGFR, p53 and Ki67 status, as well as the stromal Cav-1 score. This analysis indicated that stromal loss of Cav-1 expression was the most important prognostic factor for overall survival in TN breast cancer. Virtually identical results were obtained with CK5/6 (+) and/or EGFR (+) TN breast cancer cases, demonstrating that a loss of stromal Cav-1 is also a strong prognostic factor for basal-like breast cancers. Our current findings may have important implications for the close monitoring and treatment stratification of TN and basal-like breast cancer patients.
Key words: caveolin-1, mammary tumor stroma, stromal biomarkers, cancer survival, cancer-associated fibroblasts
Introduction
We recently identified a loss of stromal caveolin-1 (Cav-1) in tumor-associated fibroblasts as a new prognostic biomarker for clinical outcome in human breast cancers.1
Our previous studies, nearly 15 years ago, demonstrated that loss of Cav-1 in fibroblastic cells (NIH-3T3 cells) occurs upon cellular transformation with various oncogenes [Ras (G12V), v-Abl, Bcr-Abl, v-Crk]2 or deletion of tumor suppressor genes (p53).3 Thus, we speculated that a loss of Cav-1 might serve as a marker for the human breast cancer-associated fibroblast phenotype.
To test this hypothesis directly, we isolated matched normal and cancer-associated fibroblasts from 11 breast cancer patients, and determined their levels of the Cav-1 protein product.4 Indeed, 8 out of 11 patient-derived cancer-associated fibroblasts showed a significant reduction in Cav-1 protein levels.4
To determine the possible clinical relevance of these findings, we used antibodies directed against Cav-1 to stain a well-annotated breast cancer TMA (tumor micro-array) containing a cohort of 160 breast cancer patients, with nearly 20 years of follow-up data.5 Remarkably, our results indicated that a loss of stromal Cav-1 was associated with an increased risk of tumor recurrence, metastasis, tamoxifen-resistance and overall poor clinical outcome.5 Interestingly, the prognostic value of stromal Cav-1 appeared to be independent of epithelial marker status, and was effective in ER+, PR+, HER2+ and even triple-negative patients.5 However, only 16 triplenegative patients were present in this cohort.5
Thus, to more stringently assess the efficacy of stromal Cav-1 as a biomarker in triple-negative patients, we examined a new breast cancer patient cohort consisting solely of triple-negative (TN) patients. This new cohort contains 85 patients with nearly 12 years of follow-up data. Here, we show that TN patients with high-levels of stromal Cav-1 had a good clinical outcome, with >50% of the patients remaining alive during the follow-up period. In contrast, the median survival for TN patients with moderate stromal Cav-1 staining was 33.5 months. Similarly, the median survival for TN patients with absent stromal Cav-1 staining was 25.7 months. Nearly identical results were obtained with CK5/6 (+) and/or EGFR (+) TN breast cancer cases, indicating that a loss of stromal Cav-1 is also a strong prognostic factor for basal-like breast cancers. Our current findings have important implications for the monitoring and possible treatment stratification of TN and basal-like breast cancer patients.
Results
Prognostic value of stromal caveolin-1 immunostaining for predicting clinical outcome in TN breast cancer patients.
Here, we investigate the predictive value of caveolin-1 (Cav-1) as a candidate biomarker for clinical outcome in triple negative breast cancer.
For this purpose, we used Cav-1 antibodies to stain paraffin-embedded tumor tissue sections taken from TN breast cancer patients at surgical resection. This cohort consists of 85 patients seen at Thomas Jefferson University Hospital, and is well annotated, with access to a variety of other markers, and nearly 140 months (∼12 years) of follow-up data. In this series, our primary outcome of interest was overall survival. Expression of Cav-1 both in the epithelial and stroma compartments was scored for comparison purposes.
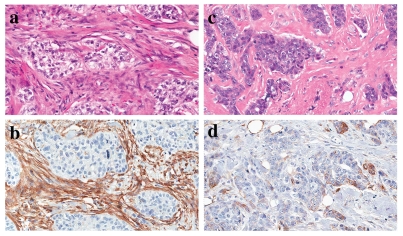
Figure 1 shows representative images of H&E and Cav-1 antibody stained tissue sections, highlighting the differences observed in Cav-1 immunostaining in the fibroblastic stromal compartment.
Figure 1.
Stromal Cav-1 staining in triple-negative breast cancer patients. Tissue sections cut from formalin-fixed paraffin-embedded triple-negative breast cancer samples were either subjected to H&E staining (a and c) to visualize overall cell morphology, or immunostaining with antibodies directed against Cav-1 (b and d). Note the presence of prominent stromal Cav-1 immunostaining in (b), and the absence of stromal Cav-1 immunostaining in (d). Immunostained sections were counter-stained with hematoxylin. Note that (a and b) are paired images from one representative tumor, while (c and d) are paired images from another representative tumor.
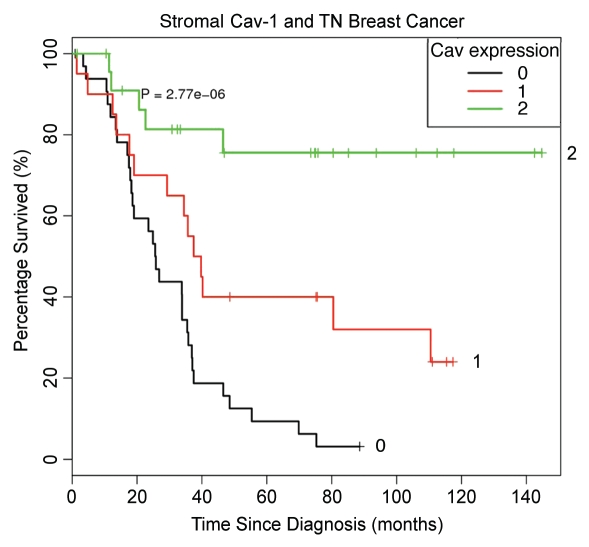
Of the 85 TN breast cancers examined, 83 could be semiquantitatively scored for stromal Cav-1 levels (0, no staining; 1, mild-to-moderate staining; 2, strong staining). Interestingly, 24 patients showed high levels of Cav-1 stromal staining, while 22 showed a lower, intermediate level of staining, and 37 showed an absence of Cav-1 stromal staining. Stromal Cav-1 levels were then combined with follow-up data to generate Kaplan-Meier survival curves, plotting percentage survival (%) versus time since diagnosis (in months). Figure 2 shows the results of this analysis, which were highly statistically significant (p = 2.8 × 10−6).
Importantly, patients with high-levels of stromal Cav-1 (score = 2), had a good clinical outcome, with >50% of the patients remaining alive during the follow-up period (nearly 12 years) (Table 1). In contrast, the median survival for patients with moderate stromal Cav-1 staining (score = 1) was 33.5 months. Similarly, the median survival for patients with absent stromal Cav-1 staining (score = 0) was 25.7 months.
Table 1.
Overall survival (OS) summaries by stromal Cav-1 status
| Survival | Stromal Cav-1 | ||
| Measure | 0 | 1 | 2 |
| Median survival | 25.7 mo | 37.5 mo | >50% alive |
| 5-year survival | 9.4% | 40.0% | 75.5% |
The P value for testing the differences in these three survival curves is 2.7 × 10−6 using the log-rank test.
A comparison of 5-year survival rates yields a similar pattern. Patients with high stromal Cav-1 had a good 5-year survival rate, with 75.5% of the patients remaining alive in this period. In contrast, patients with moderate or absent stromal Cav-1 levels had progressively worse 5-year survival rates, with 40 and 9.4% of the patients remaining alive during this period.
Thus, a reduction or absence of stromal Cav-1 levels is a strong prognostic factor in TN breast cancer patients. Interestingly, a loss of stromal Cav-1 was independent of tumor histologic grade (I–II versus III–IV) and other markers, such as cytokeratin 5/6 (CK5/6) and epidermal growth factor receptor (EGFR) status (Table 2). However, a strong association was observed between a loss of stromal Cav-1 and the presence of lymph node (LN) metastasis (Table 3).
Table 2.
Association of stromal Cav-1 expression with pathological and molecular markers
| Stromal Cav-1 Expression | |||||
| N | 0 (N = 37) | 1 (N = 22) | 2 (N = 24) | p value | |
| Tumor size | 66 | 1.80/2.50/4.00 | 1.40/1.70/2.50 | 1.45/2.00/3.00 | 0.282 |
| p53 status | 69 | 0.705 | |||
| neg | 46% (13) | 38% (6) | 52% (12) | ||
| pos (>0%) | 54% (15) | 62% (10) | 48% (11) | ||
| p53 status | 69 | 0.502 | |||
| neg | 71% (20) | 56% (9) | 74% (17) | ||
| pos (>70%) | 29% (8) | 44% (7) | 26% (6) | ||
| CK 5/6 | 82 | 0.366 | |||
| neg | 35% (13) | 52% (11) | 33% (8) | ||
| pos | 65% (24) | 48% (10) | 67% (16) | ||
| EGFR | 83 | 0.918 | |||
| neg | 62% (23) | 59% (13) | 67% (16) | ||
| pos | 38% (14) | 41% (9) | 33% (8) | ||
| Histologic grade | 85 | 0.411 | |||
| I–II | 19% (7) | 32% (7) | 17% (4) | ||
| III–IV | 81% (30) | 68% (15) | 83% (20) | ||
| Lymph node mets | 75 | 0.041 | |||
| neg | 25% (9) | 56% (10) | 52% (11) | ||
| pos | 75% (27) | 44% (8) | 48% (10) |
Associations between stromal Cav-1 expression and categorical variables is tested using Fisher's exact test. Associations with continuous variables are tested using the Kruskal-Wallis test, a nonparametric test analogous to the ANOVA F-test for a 1-way design. For tumor size (cm), the three numbers reported are 1st quartile, median and 3rd quartile.
Table 3.
Association of the presence of LN metastasis with negative stromal Cav-1
| N | Stromal Cav-1 | p value | ||
| neg (0) | pos (1 + 2) | |||
| (N = 37) | (N = 46) | |||
| Lymph node mets | 75 | 0.018 | ||
| neg | 25% (9) | 54% (21) | ||
| pos | 75% (27) | 46% (18) | ||
Association is tested using Fisher's exact test.
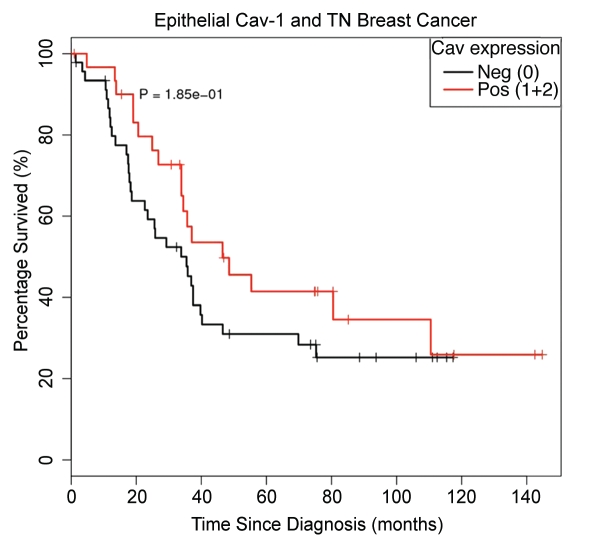
Interestingly, in a parallel analysis, the levels of tumor epithelial Cav-1 had no prognostic significance (p = 1.9 × 10−1). In this cohort, 85 patients were scored, with 50 patients showing an absence of epithelial Cav-1 staining, and 33 patients showing moderate-to-strong epithelial Cav-1 staining (Fig. 3). Median survival rates were 35.4 months [epithelial Cav-1 (−)] versus 46.5 months [epithelial Cav-1 (+)]; 5-year survival rates were 30.95% [epithelial Cav-1 (−)] versus 41.45% [epithelial Cav-1 (+)] (Table 4).
Figure 3.
Kaplan-Meier analysis of epithelial Cav-1 levels does not predict overall survival in triple-negative breast cancer patients. Interestingly, the levels of tumor epithelial Cav-1 had no prognostic significance (p = 1.9 × 10−1). In this cohort, 85 patients were scored, with 50 patients showing an absence of epithelial Cav-1 staining, and 33 patients showing moderate-to-strong epithelial Cav-1 staining. Median survival rates were 35.4 months [epithelial Cav-1 (−)] versus 46.5 months [epithelial Cav-1 (+)]; 5-year survival rates were 30.95% [epithelial Cav-1 (−)] versus 41.45% [epithelial Cav-1 (+)] (see Table 4).
Table 4.
Overall survival (OS) summaries based on epithelial Cav-1 status
| Survival | Epi Cav −ve | Epi Cav +ve |
| Median survival | 35.4 mo | 46.5 mo |
| 5-year survival | 30.95% | 41.45% |
The p value for testing differences in the two survival curves is 0.185 using the log-rank test.
Thus, the prognostic value of Cav-1 immunostaining in TN breast cancer patients is compartment-specific, and selective for an absence of Cav-1 staining in the stromal fibroblast compartment.
Associations between epithelial Cav-1 expression and lack of steroid hormone receptor positivity and the expression of basal markers were previously reported by others.6 However, in this previous study, epithelial Cav-1 positivity was not associated with survival in a multivariate analysis,6 which is consistent with our new findings.
Comparison of stromal Cav-1 staining with other risk factors in TN breast cancers.
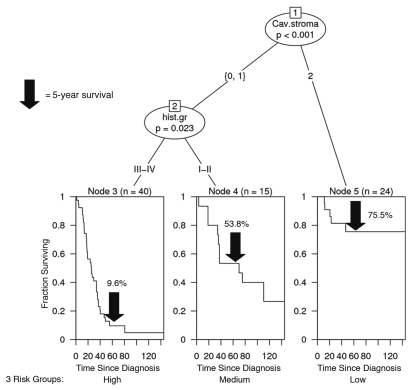
Next, we used a recursive-partitioning algorithm to assess which factors are most predictive of overall survival in TN breast cancer patients. In this analysis, we included tumor size, histologic grade, whether the patient received surgery, radiotherapy or chemotherapy, CK5/6, EGFR, p53 and Ki67 status, as well as the stromal Cav-1 score in this predictive algorithm. The results are summarized in Figure 4.
Figure 4.
A comparison of stromal Cav-1 staining with other risk factors in triple negative breast cancers. A recursive-partitioning algorithm was used to assess which factors are most predictive of overall survival in TN breast cancer patients. In this analysis, we included tumor size, histologic grade, whether the patient received surgery, radiotherapy or chemotherapy, CK5/6, EGFR, P53 and Ki67 status, as well as the stromal Cav-1 score in this predictive algorithm. There are essentially three risk groups identified in this model: (1) Low-risk, high stromal Cav-1 staining (score = 2); (2) Medium-risk, low stromal Cav-1 staining (score = 0 or 1), with low-histologic grade (I or II); and (3) High-risk, low stromal Cav-1 staining (score = 0 or 1), with high-histologic grade (III or IV). For the low-risk group, >50% of the patients remained alive during the follow-up period (nearly 12 years). For the medium-risk, and low-risk groups, the median survival was 69.8 months, and 25.6 months, respectively (see Table 5). This analysis indicates that stromal Cav-1 expression is the most important prognostic factor for overall survival in TN breast cancer (p < 0.001).
We see that there are essentially three risk groups in this model:(1) Low-risk, high stromal Cav-1 staining (score = 2); (2) Medium-risk, low stromal Cav-1 staining (score = 0 or 1), with low-histologic grade (I or II); and (3) High-risk, low stromal Cav-1 staining (score = 0 or 1), with high-histologic grade (III or IV).
For the low-risk group, if we look at median survival, >50% of the patients remained alive during the follow-up period (nearly 12 years). For the medium-risk, and highrisk groups, the median survival was 69.8 months, and 25.6 months, respectively (Table 5). Similarly, the 5-year survival rates for the low-, medium- and high-risk groups were 75.5, 53.8 and 9.6%, respectively.
Table 5.
Overall survival (OS) summaries based on risk groups defined by recursive partitioning
| Survival | Risk group | ||
| Low | Medium | High | |
| Median survival | >50% alive | 69.8 mo | 25.6 mo |
| 5 year survival | 75.5% | 53.8% | 9.6% |
The p value for testing differences in the three survival curves defined by the risk groups is 1.34 × 10−6 using the log-rank test.
As such, this analysis indicates that stromal Cav-1 expression is the most important prognostic factor for overall survival in TN breast cancer (p < 0.001).
Prognostic value of stromal caveolin-1 immunostaining for predicting clinical outcome in basal-like breast cancer patients.
Next, we determined the prognostic value of stromal Cav-1 in basal breast cancer patients, which represent a sub-set of TN breast cancer cases. Basal-like breast cancers are typically ER (−), PR (−) and HER2 (−), but are found to express other specific markers, such as either CK5/6 or EGFR. As such, we selected the TN patients who stained positively for either CK5/6 or EGFR, for inclusion as basal-like breast cancer patients in this analysis.
Using this approach, 57 of the TN cases were re-classified as basal-like breast cancers and the remaining 25 TN cases were excluded from this analysis (Table 6). It is important to note that classification of these tumors as basal-like breast cancers was not significantly associated with either epithelial or stromal Cav-1 status (Table 6). Interestingly, the basal-like breast cancer patients in our study were significantly associated with p53-positive expression status (Table 6); this is consistent with previous studies showing an association between p53-positivity and basal-like breast cancer.7
Table 6.
Association of molecular and pathological markers by basal-like breast cancer (BLBC) status
| N | BLBC (N = 57) | TNBC (N = 25) | p value | |
| Tumor size | 66 | 1.500/2.000/4.000 | 1.400/2.100/3.125 | 0.642 |
| Stromal Cav-1 | 83 | 0.649 | ||
| 0 | 47% (27) | 40% (10) | ||
| 1 | 23% (13) | 32% (8) | ||
| 2 | 30% (17) | 28% (7) | ||
| Epithelial Cav-1 | 83 | 0.464 | ||
| neg (0) | 63% (36) | 52% (13) | ||
| pos (1 + 2) | 37% (21) | 48% (12) | ||
| Lymph node mets | 74 | 0.799 | ||
| neg | 40% (21) | 43% (9) | ||
| pos | 60% (32) | 57% (12) | ||
| Histologic grade | 85 | 0.768 | ||
| I–II | 19% (11) | 24% (6) | ||
| III–IV | 81% (46) | 76% (19) | ||
| p53 status | 69 | 0.016 | ||
| neg | 36% (17) | 70% (14) | ||
| pos (>0%) | 64% (30) | 30% (6) | ||
| p53 status | 69 | 0.255 | ||
| neg | 64% (30) | 80% (16) | ||
| pos (>70%) | 36% (17) | 20% (4) |
Associations with categorical variables are tested using the Fisher exact test, and associations with continuous variables are tested using the Wilcoxon rank-sum test.
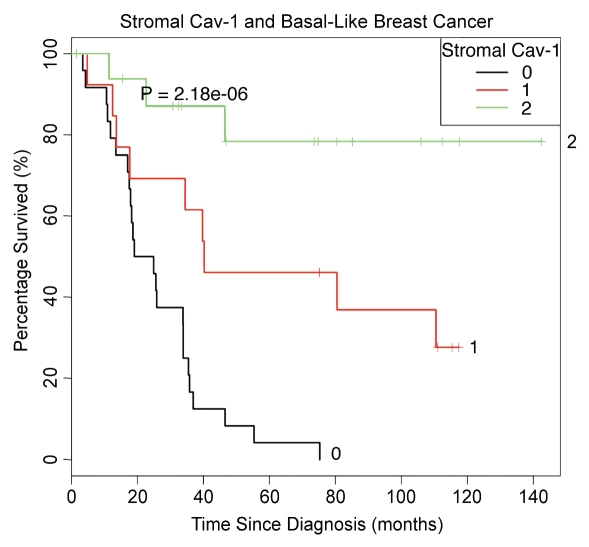
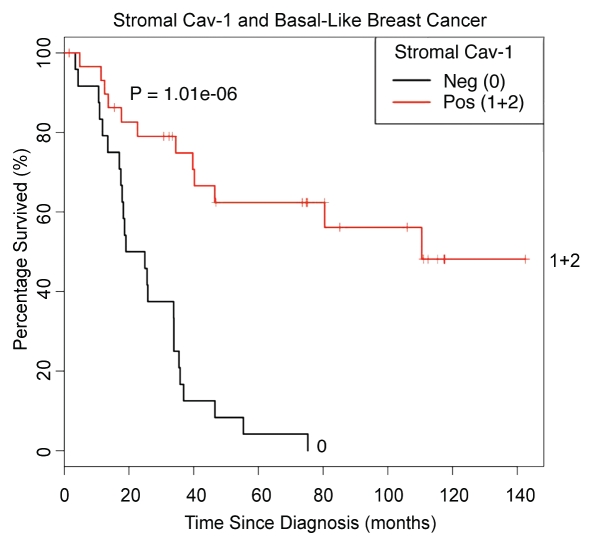
Figures 5 and 6 show the Kaplan-Meier analysis of stromal Cav-1 in basal-like breast cancer patients, which is highly statistically significant (p = 2.2 × 10−6). Thus, stromal Cav-1 status also has strong prognostic significance in TN patients with the basal-like breast cancer phenotype.
Figure 5.
Kaplan-Meier analysis of stromal Cav-1 levels predicts overall survival in basal-like triple-negative breast cancer patients. We also determined the prognostic value of stromal Cav-1 in basal breast cancer patients. For this purpose, we selected the TN patients who stained positively for either CK5/6 or EGFR, for inclusion as basal-like breast cancer patients in this analysis. Using this approach, 57 of the TN breast cancer cases were re-classified as basal-like breast cancers. Note that Kaplan-Meier analysis of stromal Cav-1 status in basal-like breast cancer patients was highly statistically significant (p = 2.2 × 10−6). As such, stromal Cav-1 status also has strong prognostic significance in TN patients with the basal-like breast cancer phenotype.
Figure 6.
Kaplan-Meier analysis of stromal Cav-1 levels predicts overall survival in basal-like triple negative breast cancer patients. As in Figure 5, except that patients that recieved a stromal Cav-1 score of 1 or 2 were considered as a single group (See 1 + 2), and then compared with patients that received a stromal Cav-1 score of 0. Note that Kaplan-Meier analysis of stromal Cav-1 status in basal-like breast cancer patients was highly statistically significant (p = 1 × 10−6).
Discussion
The definition of triple negative (TN) breast cancer is a diagnosis of exclusion and is based on the simultaneous absence of three commonly used breast cancer diagnostic biomarkers, namely estrogen receptor-alpha (ER-alpha), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2/Neu).8 In total, TN breast cancer patients account for ∼10–20% of all the cases of invasive ductal carcinoma (IDC). TN breast cancer is a particularly lethal sub-type of breast cancer as most patients have been shown to have a relatively poor prognosis in the first 5 years post-diagnosis. However, a significant portion of TN breast cancer patients do not relapse, even without adjuvant systemic therapy.9,10 Thus, new prognostic and/or predictive factors need to be identified to guide treatment decisions in this potentially heterogeneous group of breast cancer patients.
A subset of TN breast cancers has a basal-like phenotype, and are associated with positivity for high-molecular-weight (basal) cytokeratins 5/6 (CK 5/6) and/or epidermal growth factor receptor expression (EGFR).9 Basal-like breast cancers were originally described in the 1970's as tumors originating from myo-epithelial cells; however, they gained widespread recognition after being characterized as a distinct group by gene expression profiling studies.11,12 Basal-like breast cancer is also thought to be a particularly lethal sub-type of breast cancer.8,13 The presence of a fibrotic focus, a scar-like area found in the center of an invasive breast tumor, was associated with poor clinical outcome in basal-like breast cancers.14 In contrast, a dense lymphocytic infiltrate was associated with better survival in several studies.15,16
Here, we identified stromal Cav-1 as a new predictive biomarker for both the TN and basal-like breast cancer sub-types. TN patients, with high-levels of stromal Cav-1, had a good clinical outcome, with >50% of the patients remaining alive during the follow-up period. In striking contrast, the median survival for TN patients with moderate stromal Cav-1 staining was 33.5 months. Similarly, the median survival for TN patients with absent stromal Cav-1 staining was 25.7 months. A comparison of 5-year survival rates yielded a similar pattern. TN patients with high stromal Cav-1 had a good 5-year survival rate, with 75.5% of the patients remaining alive. In contrast, TN patients with moderate or absent stromal Cav-1 levels had progressively worse 5-year survival rates, with 40 and 9.4% of the patients remaining alive. Virtually identical results were obtained with CK5/6 (+) and/or EGFR (+) TN breast cancer cases, indicating that a loss of stromal Cav-1 is also a strong prognostic factor for basal-like breast cancers. However, in a parallel analysis, the levels of tumor epithelial Cav-1 had no prognostic significance.
Our current biomarker studies with stromal Cav-1 in triple-negative and basal-like breast cancers could also have possible implications for the treatment stratification of this patient population. Recently, using a basal-like “triple-negative” human breast cancer cell line (MDA-MB-231 cells), we showed that Cav-1-deficient cancer-associated fibroblasts can significantly promote tumor growth and angiogenesis, in part, by recruiting Cav-1 (+) endothelial precursor cells.17 More specifically, in this new xenograft model, Cav-1-deficient cancer-associated fibroblasts resulted in a 2.5-fold increase in tumor mass, and a 3.1-fold increase in micro-vessel density.17 A more detailed molecular analysis revealed that Cav-1-deficient cancer-associated fibroblasts show a proteomic shift towards aerobic glycolysis, with the stromal upregulation of multiple glycolytic enzymes, such as LDH and the M2-isoform of pyruvate kinase.17 We have termed this new phenomenon the “Reverse Warburg Effect.”18 Thus, we validated the expression of glycolytic enzymes in the stromal fibroblasts of human breast cancer patients that lack stromal Cav-1 immunostaining.17 In accordance with these findings, we next used well-established glycolysis inhibitors to target Cav-1-deficient cancer-associated fibroblasts, which resulted in a dramatic 4.5-fold reduction in “triple-negative” breast cancer tumor growth.17 Thus, chemically-induced metabolic restriction with glycolysis inhibitors may be a new therapeutic strategy for targeting triple-negative and basal-like human breast cancers, in patients with a loss of stromal Cav-1.17 As such, stromal Cav-1 levels could be used both for (i) prognostic purposes, as well as (ii) treatment stratification, allowing a more personalized approach to breast cancer diagnosis and therapy.
Materials and Methods
Archived clinical materials.
Cases for the study where obtained from the Surgical Pathology files at the Thomas Jefferson University with Institutional Review Board approval. The study population consisted of 85 triple negative breast carcinomas with follow up ranging from 12 up to 144 months (median follow-up time 33.8 months). All 85 cases were invasive ductal carcinomas of no special type. Out of 85 triple negative breast cancers, 57 were basal-like. The median patient age was 57 (age range = 33.92). Clinical and pathological variables were determined following well-established criteria. All invasive carcinomas were graded according to the method, described by Elston and Ellis.19 For inclusion in this study as TN breast cancer, expression of estrogen, progesterone receptors was not detected or present in <1% of tumor cells, with satisfactory positive control. HER2 was scored 0–1+ or 2+, and an absence of HER2 amplification by fluorescent in situ hybridization was required for negativity. Expression of CK5/6 and/or EGFR, in addition to absence of hormone receptors and HER2 expression, was required to classify cases as basal-like breast cancer. This panel of four antibodies (CK5/6, ER, EGFR and HER2) was previously shown to have 100% specificity and 76% sensitivity for the basal-like phenotype.20
Antigen retrieval and caveolin-1 (Cav-1) immunohistochemistry.
Cav-1 expression in the tumor stroma was assessed using a standard immunoperoxidase method (Biocare Medical Mach 3 Rabbit HRP-polymer, Concord, CA), using a rabbit polyclonal anti-Cav-1 antibody (BD Biosciences, diluted 1:4,000). The staining was scored semiquantitatively as negative (0; no staining), weak (1; either diffuse weak staining or strong staining in less than 30% of stromal cells per core), or strong (2; defined as strong staining of 30% or more of the stromal cells).5,21 Any expression in epithelial tumor cells was considered a positive Cav-1 staining. Briefly, paraffin-embedded blocks containing TN breast cancer specimens were fixed in 10% neutral-buffered formalin and processed for immunohistochemical analysis. Tissue sections were deparaffinized in xylene, re-hydrated in ethanol, re-hydrated with water, and washed in 1% phosphate-buffered saline. Prior to primary antibody application, slides underwent antigen retrieval. Slides were steamed for 30 min in DAKO Target Retrieval Solution pH 6.0 (cat#S1699). Cooled at room temperature for 20 min and washed with TBS buffer 5 times. Sections were then blocked with 3% (v/v) H2O2 for 10 min. Primary antibody anti-Cav-1 (BD Biosciences) was applied to slides and incubated for 60 min using a 1/4,000 dilution. The IHC process was finalized with application of suitable immune complexes such as Mach3 Rabbit HRP-Polymer (Biocare Medical cat# M3R531L) and visualized with Dako Liquid DAB+ Substrate-Chromogen Solution (DAKO cat# K3468) (diaminobenzidine tetrahydrochloride) for 5 min.
EGFR and CK5/6 immuno-staining.
Tissue sections for expression of CK5/6 and EGF-R antibodies were prepared using a standard histology de-paraffinization protocol. Prior to primary antibody application, slides went under antigen retrieval. Slides were treated with proteolytic enzyme for 5 min (DAKO cat# S3007) for EGFR samples and for CK5/6 were steamed for 30 min in DAKO Target Retrieval Solution pH 6.0 (cat#S1699). Sections for both, EGFR as well as CK5/6 were then blocked with 3% (v/v) H2O2 for 10 min following with avidin-biotin block 20 min each. Primary antibodies for EGFR (DAKO cat#M3563, diluted 1:100) and CK5/6 (DAKO cat#M7237, diluted 1:50) were applied to the slides and incubated for 60 min. The IHC process was finalized with application of suitable immune complexes, such as LSAB + HRP (DAKO cat# S0690) and visualized with Dako Liquid DAB+ Substrate-Chromogen Solution (DAKO cat# K3468) (diaminobenzidine tetrahydrochloride) for 5 min. For CK5/6, positivity was defined as the positive detection of any invasive malignant cells.22 Similarly, any staining for EGFR present in tumor cells was considered positive.9
Statistical analysis.
We evaluated the association of stromal and epithelial Cav-1 expression with various pathological measures (T-stage, N-stage, histological grade, primary tumor size) and molecular markers (p53, Ki67, EGFR, CK5/6), using the Fisher exact test (for binary and categorical variables) and either Kruskal-Wallis test (for the Cav-1 expression score) or Wilcoxon rank-sum test (for Cav-1 expression status). The association of Cav-1 expression with overall survival was evaluated using Kaplan-Meier curves and log-rank tests, univariately. Multivariate analysis of the association of various molecular and pathological markers with overall survival was done using a recursive binary partitioning algorithm based on conditional inference, where each binary split is determined by a log-rank test.23 We considered a p value of less than 5% to be statistically significant. A secondary aim of this study was to evaluate differences between TN and basal-like breast cancers. Associations of various pathological and molecular measures with basal-like breast cancer status were evaluated with Wilcoxon rank-sum tests or Fisher exact tests, as appropriate.
Figure 2.
Kaplan-Meier analysis of stromal Cav-1 levels predicts overall survival in triple negative breast cancer Patients. Of the 85 TN breast cancers examined, 83 could be semi-quantitatively scored for stromal Cav-1 levels. Interestingly, 24 patients showed high levels of Cav-1 stromal staining, while 22 showed a lower, intermediate level of staining, and 37 showed an absence of Cav-1 stromal staining. The results of this analysis were highly statistically significant (p = 2.8 × 10−6). Patients with high-levels of stromal Cav-1 (score = 2), had a good clinical outcome, with >50% of the patients remaining alive during the follow-up period (nearly 12 years). In contrast, the median survival for patients with moderate stromal Cav-1 staining (score = 1) was 33.5 months. Similarly, the median survival for patients with absent stromal Cav-1 staining (score = 0) was 25.7 months (see Table 1).
Acknowledgements
M.P.L. and his laboratory were supported by grants from the NIH/NCI (R01-CA-080250; R01-CA-098779; R01-CA-120876; R01-AR-055660), and the Susan G. Komen Breast Cancer Foundation. A.K.W. was supported by a Young Investigator Award from Breast Cancer Alliance, Inc., and a Susan G. Komen Career Catalyst Grant. F.S. was supported by grants from the W.W. Smith Charitable Trust, the Breast Cancer Alliance (BCA) and a Research Scholar Grant from the American Cancer Society (ACS). Funds were also contributed by the Margaret Q. Landenberger Research Foundation (to M.P.L.).
This project is funded, in part, under a grant with the Pennsylvania Department of Health (to M.P.L.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/11983
References
- 1.Witkiewicz AK, Casimiro MC, Dasgupta A, Mercier I, Wang C, Bonuccelli G, et al. Towards a new “stromalbased” classification system for human breast cancer prognosis and therapy. Cell Cycle. 2009;8:1654–1658. doi: 10.4161/cc.8.11.8544. [DOI] [PubMed] [Google Scholar]
- 2.Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams TM, Lee H, Cheung MW, Cohen AW, Razani B, Iyengar P, et al. Combined loss of INK4a and caveolin-1 synergistically enhances cell proliferation and oncogene-induced tumorigenesis: Role of INK4a/CAV-1 in mammary epithelial cell hyperplasia. J Biol Chem. 2004;279:24745–24756. doi: 10.1074/jbc.M402064200. [DOI] [PubMed] [Google Scholar]
- 4.Mercier I, Casimiro MC, Wang C, Rosenberg AL, Quong J, Allen KG, et al. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: Implications for the response to hormonal therapy. Cancer Biol Ther. 2008;7:1212–1225. doi: 10.4161/cbt.7.8.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174:2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsheikh SE, Green AR, Rakha EA, Samaka RM, Ammar AA, Powe D, et al. Caveolin 1 and Caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br J Cancer. 2008;99:327–334. doi: 10.1038/sj.bjc.6604463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakha EA, Elsheikh SE, Aleskandarany MA, Habashi HO, Green AR, Powe DG, et al. Triple-negative breast cancer: Distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15:2302–2310. doi: 10.1158/1078-0432.CCR-08-2132. [DOI] [PubMed] [Google Scholar]
- 8.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 9.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 10.Jumppanen M, Gruvberger-Saal S, Kauraniemi P, Tanner M, Bendahl PO, Lundin M, et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. Breast Cancer Res. 2007;9:16. doi: 10.1186/bcr1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161–220. [PubMed] [Google Scholar]
- 12.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 13.Rakha EA, El-Rehim DA, Paish C, Green AR, Lee AH, Robertson JF, et al. Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. Eur J Cancer. 2006;42:3149–3156. doi: 10.1016/j.ejca.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Van den Eynden GG, Smid M, Van Laere SJ, Colpaert CG, Van der Auwera I, Bich TX, et al. Gene expression profiles associated with the presence of a fibrotic focus and the growth pattern in lymph node-negative breast cancer. Clin Cancer Res. 2008;14:2944–2952. doi: 10.1158/1078-0432.CCR-07-4397. [DOI] [PubMed] [Google Scholar]
- 15.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9:65. doi: 10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 17.Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, et al. The Reverse Warburg Effect: Glycolysis Inhibitors Prevent the Tumor Promoting Effects of Caveolin-1 Deficient Cancer Associated Fibroblasts. Cell Cycle. 2010;9:1960–1971. doi: 10.4161/cc.9.10.11601. [DOI] [PubMed] [Google Scholar]
- 18.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 19.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41:154–161. [PubMed] [Google Scholar]
- 20.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 21.Witkiewicz AK, Dasgupta A, Nguyen KH, Liu C, Kovatich AJ, Schwartz GF, et al. Stromal caveolin-1 levels predict early DCIS progression to invasive breast cancer. Cancer Biol Ther. 2009;8:1167–1175. doi: 10.4161/cbt.8.11.8874. [DOI] [PubMed] [Google Scholar]
- 22.Abd El-Rehim DM, Pinder SE, Paish CE, Bell J, Blamey RW, Robertson JF, et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004;203:661–671. doi: 10.1002/path.1559. [DOI] [PubMed] [Google Scholar]
- 23.Hothorn T, Hornik K, Zeileis A. Unbiased recursive partitioning: A conditional inference framework. J Comput Graph Stat. 2006;15:651–674. [Google Scholar]