Abstract
Matrix metalloproteinases (MMPs) play a well-defined role in later stages of tumor progression. However, there has been evidence that they also contribute to earlier stages of malignant transformation. The Wnt signaling transduction pathway plays a critical role in development and in the pathogenesis of many epithelial cancers. Here we have used Wnt1-induced epithelial-mesenchymal transition (EMT) in C57MG murine mammary epithelial cells to study the role of MMPs in this early step of malignant progression. Overexpression of Wnt1 in C57MG cells promoted EMT, the translocation of β-catenin from the cell membrane to the nucleus and its transcriptional activity, cell proliferation and cell motility. Simultaneously, we observed an increased expression of stromelysin-1 (MMP-3) and a 5.5-fold increase in MMP-3 promoter activity in C57MG cells expressing Wnt1 compared with C57MG cells. Treatment of Wnt-overexpressing cells with MMP inhibitor AG3340 decreased MMP-3 expression. We also found evidence that MMP-3 and Wnt3a cooperate in enhancing the transcriptional activity of β-catenin in C57MG cells. Consistently, the effects of Wnt1 on EMT, proliferation and migration were inhibited by MMP inhibitors, or upon downregulation of MMP-3 by siRNA. These results suggest that MMP-3 is both a direct transcriptional target and a necessary contributor of the Wnt/β-catenin signaling pathway.
Key words: Wnt1, epithelial mesenchymal transition, MMPs
Introduction
Members of the matrix metalloproteinase (MMP) family of proteases have been implicated in a large variety of physiological and pathological conditions associated with intense tissue remodeling.1 In cancer these enzymes play a complex role in multiple steps of tumor progression including transformation, local invasion, intravasation and extravasation, angiogenesis and distant metastasis.2,3 Although it was initially thought that the primary function of these enzymes is to degrade proteins of the extracellular matrix (ECM), it is now well recognized that MMPs have a much broader spectrum of activity that includes the proteolytic processing of cytokines, growth factors, growth factor receptors, and cell adhesion molecules that can have both positive and negative effects on tumor progression.4,5 In the mammary gland, MMPs play an important role in normal development, controlling branching morphogenesis that occurs during puberty, pregnancy and post lactational involution.6–8 MMPs have also been shown to be involved in malignant transformation of the mammary gland. Overexpression of MMPs like MMP-3 (stromelysin-1) and MMP-7 (matrilysin-1) in the mammary gland of transgenic mice, results in premature differentiation and increased incidence of mammary tumor formation.9–11 A potential molecular basis for such an effect has been elucidated by the demonstration that some MMPs like MMP-3 and MMP-7 promote epithelial to mesenchymal transition (EMT), an early step in malignant transformation of epithelial cancers.9,12 Furthermore, it has been demonstrated that some MMPs including MMP-7 and MMP-14 (MT1-MMP) are transcriptionally upregulated by β-catenin, a protein that typically translocates to the nucleus during EMT and is also the target of Wnt-mediated signaling, suggesting thus a connection between MMPs and this oncogenic pathway.13,14
Wnts are a large family of secreted glycoproteins involved in cell-cell signaling of a variety of developmental processes such as differentiation, cell polarity and cell adhesion.15,16 Members of the Wnt family of proteins have also been implicated in cancer. For example, inappropriate activation of Wnt has been linked to the initiation of specific human cancers17–19 and Wnt1, Wnt2, Wnt3, Wnt3a and Wnt 9b induce the morphological transformation of mammary epithelial cells in culture.20,21 Overexpression of Wnt1 in murine mammary epithelial cells in vitro promotes EMT and cell proliferation.22 Mice in which Wnt1 is overexpressed under the control of the murine mammary tumor virus (MMTV) promoter show marked alveolar and ductal hyperplasia in the mammary gland and develop mammary adenocarcinoma.23,24 Wnt proteins signal through several transduction pathways. The canonical pathway inhibits the phosphorylation of β-catenin by glycogen synthase kinase-3β (GSK-3β) and its subsequent proteasomal degradation, thus redirecting β-catenin toward the nucleus25 where it associates with the Lymphoid Enhancer Factor-T-Cell Factor (LEF/TCF) family of DNA-binding proteins,26 and activates the transcription of a large variety of genes such as c-myc, cyclin D1, mmp-7 and mt1-mmp.13,14,27,28 Considering that several MMPs are transcriptionally upregulated by β-catenin and that a feature of Wnt-mediated signaling is the translocation of β-catenin to the nucleus, the overexpression of MMPs in Wnt-transformed cells can be anticipated. Accordingly, we previously reported an upregulation of the expression of several MMPs in the mammary tumors of MMTV/Wnt1 transgenic mice. We also demonstrated that when crossed with mice overexpressing Tissue Inhibitor of Metalloproteinases (TIMP)-2 under the same MMTV promoter, double transgenic mice develop fewer tumors with an increased latency,29 suggesting therefore that MMPs may also play a contributory role in Wnt1-mediated malignant transformation. Here we have used Wnt1 overexpressing C57MG mouse mammary epithelial cells to demonstrate that MMPs are both targets and contributors to Wnt-induced EMT.
Results
Wnt1 transformation upregulates MMP-3 expression in C57MG cells.
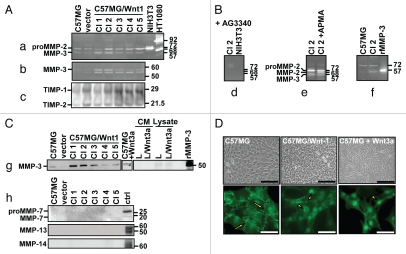
We began our investigation by examining the effect of Wnt1 transformation on the expression of MMPs and TIMPs in C57MG cells. We transfected C57MG cells with the plasmid pMIRB-Wnt1-HA and established five stable (C57MG/Wnt1) clones which were characterized for the expression of MMPs and TIMPs using several approaches including gelatin, casein and reverse gelatin zymographies, as well as western and northern blotting (Fig. 1). By zymography we demonstrated the presence of a 72 kDa gelatinolytic band in the supernatant of both C57MG and C57MG/Wnt1 cells and a 57 kDa band more abundantly present in the supernatant of C57MG/Wnt1 clones (Fig. 1A). A casein gel analysis revealed two caseinolytic bands of 54 kDa and 44 kDa in the supernatant of C57MG/Wnt1 clones, suggestive of representing the pro form and activated form of stromelysin-1 (MMP-3), an MMP with known caseinolytic activity (Fig. 1B). By reverse gelatin zymography we detected the presence of TIMP-1 and TIMP-2, but their expression was not consistently influenced by Wnt1 transformation (Fig. 1C). Confirmation that the gelatinolytic bands represented MMP activity was obtained by incubating parallel gels in the presence of 20 µg/ml of AG3340 (Fig. 1D). We then documented that the 72 kDa band represents proMMP-2 by showing that incubation with APMA induced a partial shift to a 68 kDa form (Fig. 1E). Further evidence indicating that the 57 kDa band overexpressed in Wnt1-transfected clones represents MMP-3 was obtained by demonstrating that in gelatin zymographies, it co-migrated with active recombinant MMP-3 (Fig. 1F), and by showing an increase in MMP-3 expression in clones overexpressing Wnt1, in particular clones 1, 2 and 3, by western blot (Fig. 1G). To demonstrate that MMP-3 overexpression in C57MG/Wnt1 cells was the specific result of an increase in Wnt activity, we treated C57MG cells with the supernatant of mouse L fibroblasts producing Wnt3a, and showed an overexpression of MMP-3 upon treatment (Fig. 1G, middle) whereas MMP-3 was not present in the supernatant of L or L/Wnt3a cells (Fig. 1G, right). Using western blot analysis, we found no evidence for the production of other MMPs including MMP-7, MMP-13 and MMP-14 in either parent cells or in Wnt1-transformed cells (Fig. 1H). We then documented that, consistent with the role of Wnt1 in promoting EMT, the increase in MMP-3 expression in C57MG/Wnt1 clones and C57MG cells treated with Wnt3a was associated with morphological changes characterized by the presence of elongated cells that piled up and acquired a mesenchymal-like phenotype. Furthermore, Wnt transformation or treatment of C57MG cells with Wnt3a was associated with the translocation of β-catenin from the cell membrane to the nucleus (Fig. 1I). Thus altogether these data demonstrated that induction of EMT in mammary epithelial cells by Wnt1 transfection or treatment with Wnt3a is associated with a specific increase in MMP-3 expression.
Figure 1.
Wnt1 transformation upregulates MMP-3 expression in C57MG cells and induces β-catenin translocation. (A) Conditioned media from C57MG and C57MG/Wnt1 clones (25 µg/lane) was loaded on gelatin (a), casein (b) and reverse gelatin zymographies (c). Conditioned media from NIH3T3 and HT1080 cells were used as positive controls for mouse MMP-2 and human MMP-2 and MMP-9 expression, respectively. (B) Gel was incubated overnight in the presence of AG3340 (20 µg/ml) (d). Samples were incubated with AP MA(10 µM) prior to electrophoresis (e). Recombinant mouse MMP-3 was loaded in one lane as positive control (f). (C) Western blot analysis of conditioned media (20 µg/lane) as in (A), of conditioned media from C57MG cells treated with Wnt3a, and of conditioned media and cell lysates of L and L/Wnt3a cells (g). Recombinant human MMP-7 and limb extracts from 15-day-old mouse embryos were used as positive controls for MMP-7, MMP-13 and MMP-14 respectively (h). (D) Photomicrographs of C57MG, C57MG/Wnt1 and C57MG cells treated with Wnt3a (top). Immunolocalization of β-catenin detected in the cell membrane (arrows) and in the nuclei (arrowheads) (bottom). Bar = 100 µm in phase contrast and 20 µm in fluorescence.
MMP-3 is a transcriptional target of Wnt signaling.
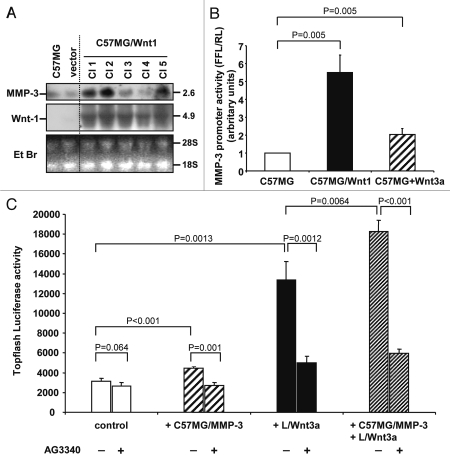
We next asked the question whether the increased MMP-3 expression associated with Wnt1 transfection was the result of transcriptional upregulation. By northern blot analysis we first demonstrated an increase in MMP-3 mRNA expression in C57MG/Wnt1 clones that correlated with Wnt1 mRNA expression (Fig. 2A). A similar analysis for MMP-9 and MMP-7 expression did not reveal any presence of their mRNA (data not shown). We then tested the effect of Wnt1 transfection and treatment with Wnt3a on the activity of a 1.3 kb murine MMP-3 promoter driving the expression of a firefly luciferase reporter gene in C57MG. The data indicated a 5.5-fold increase in MMP-3 promoter activity in C57MG/Wnt1 cells, and a 2-fold increase in Wnt3a-treated C57MG cells when compared to untreated cells (Fig. 2B), and are consistent with MMP-3 being a transcriptional target of Wnt.
Figure 2.
MMP-3 is a transcriptional target of Wnt signaling. (A) Northern blot analysis of total RNA extracted from C57MG and C57MG/Wnt1 clones (20 µg/lane) (top and middle). Staining of the gel with ethidium bromide (bottom). (B) Dual luciferase reporter assay for C57MG, C57MG/Wnt1 and C57MG cells treated with Wnt3a, 48 h after transfection with pGL2-SL1-luc plasmid. The data are representative of five experiments performed in triplicate. (C) Luciferase assay for the induction of Topflash reporter by MMP-3 and Wnt3a in the presence or absence of AG3340 (42 µg/ml). CT7 cells were co-cultured for 18 h with C57MG control cells, or C57MG/MMP-3 cells, or L/Wnt3a cells, or a combination of those three cell types. The data represent the mean (±SD) of four determinations from a representative experiment.
MMP-3 cooperates with Wnt to increase the transcriptional activity of β-catenin.
The demonstration that MMP-3 is a transcriptional target of Wnt signaling raises the question whether MMP-3 is just a consequence of Wnt1 signaling or whether it could play a contributory role in Wnt-induced EMT. To address this question, we first co-cultured MMP-3 producing C57MG cells with CT7 cells containing the Topflash reporter plasmid reflecting the transcriptional activity of β-catenin and demonstrated a 1.5-fold increase in firefly luciferase activity that was not observed in the presence of AG3340 (Fig. 2C). Consistent with our previous data, co-culture of L/Wnt3a cells and CT7 cells also increased the Topflash activity in CT7 cells and this effect was suppressed in the presence of AG3340. Furthermore, co-culturing both L/Wnt3a cells and MMP-3 overexpressing C57MG cells with CT7 cells increases the Topflash luciferase activity in CT7 cells beyond the level observed with L/Wnt3a cells, and this effect was also suppressed in the presence of AG3340. These data thus suggest that MMP-3 cooperates with Wnt, enhancing Wnt-mediated signaling and β-catenin transcriptional activity. The observation that this effect is suppressed in the presence of an MMP inhibitor suggests that inhibition of MMPs may reverse Wnt-induced EMT.
Reversal of Wnt1-induced EMT and β-catenin transcriptional activity by MMP inhibition.
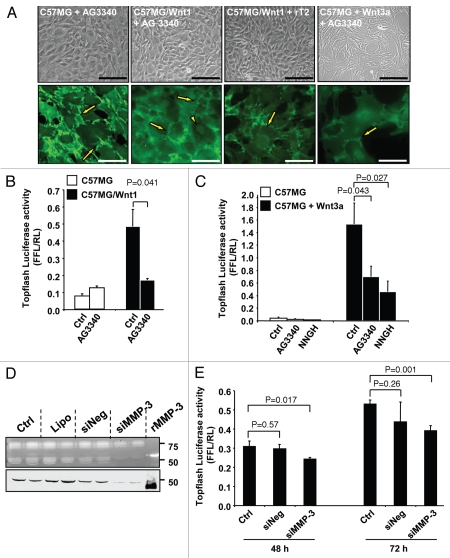
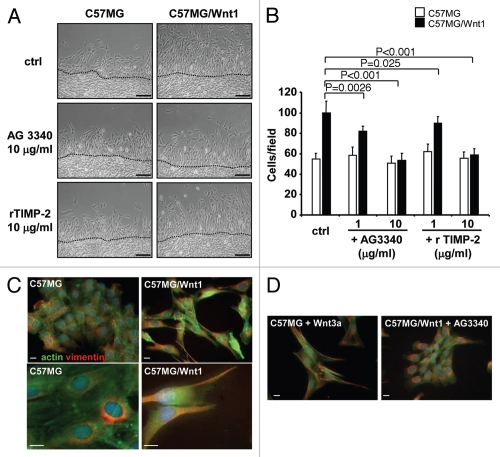
To test this hypothesis we examined the effect of two broad-spectrum MMP inhibitors, TIMP-2 and AG3340, on Wnt-induced EMT and translocation of β-catenin (Fig. 3A). These experiments revealed that in the presence of AG3340 (10 µg/ml) or rTIMP-2 (10 µg/ml), C57MG/Wnt1 cells and Wnt3a-treated C57MG cells reversed to an epithelial phenotype typical of the untransfected cells. These changes were associated with a corresponding shift in localization of β-catenin from the nucleus to the cell surface suggesting an inhibition of β-catenin transcriptional activity. This was confirmed by demonstrating a significant inhibition of the Topflash reporter activity in C57MG/Wnt1 or Wnt3a-treated C57MG cells in the presence of AG3340 or NNGH, an MMP-3 inhibitor (Fig. 3B and C). Further evidence supporting the demonstration that inhibition of MMP-3 downregulates the transcriptional activity of β-catenin was obtained by downregulating MMP-3 in C57MG/Wnt1 cells by siRNA and testing these cells for activity of the Topflash reporter construct. The data (Fig. 3D and E) show a 22 and 27% inhibition of the Topflash reporter activity at 48 and 72 h respectively in cells in which MMP-3 expression was downregulated by 90% by siRNA transfection that was not observed in cells transfected with the control siRNA. This inhibition is lower than the one observed in the presence of NNGH, but significant (p < 0.001). The data thus indicate a reversal of EMT and an inhibition of β-catenin transcriptional activity induced by Wnt when MMP-3 is inhibited or downregulated, suggesting that MMP-3 actively contributes to Wnt-mediated cell transformation.
Figure 3.
MMP inhibition reverses β-catenin nuclear translocation and transcriptional activity. (A) Photomicrographs of C57MG, C57MG/Wnt1 and C57MG cells treated with Wnt3a and cultured in the presence of AG3340 or rTIMP-2. Immunolocalization of β-catenin detected in the cell membrane (arrows) and in the nuclei (arrowheads) (bottom). Bar = 100 µm in phase contrast and 20 µm in fluorescence. The localization of β-catenin in these cells in the absence of AG3340 is shown in Figure 1D. (B and C) Topflash promoter activity in C57MG, C57MG/Wnt1 and C57MG cells treated with Wnt3a in the absence or presence of MMP inhibitors AG3340 and NNGH. The data represent the mean of FFL/RL ratio of three samples and are representative of three independent experiments. (D) Casein zymography (top) and western blot (bottom) on conditioned media of C57MG/Wnt1 cells transfected with MMP-3 siRNA. (Ctrl) untransfected controls; (Lipo) cells treated with lipofectamine alone; (siNeg) cells transfected with non-silencing siRNA; (siMMP-3) cells transfected with MMP-3 siRNA; (rMMP-3) recombinant MMP-3 used as positive control. (E) Topflash promoter activity of C57MG/Wnt1 cells transfected with indicated siRNA at 48 and 72 h time points. The data represent the mean (±SD) of three samples.
Reversal of Wnt1-induced proliferation and migration by MMP inhibition.
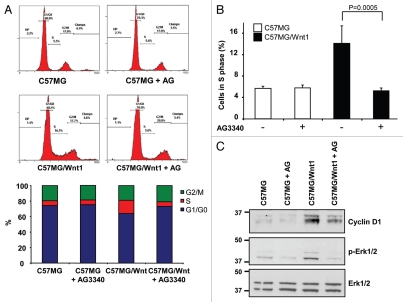
To further confirm the contributory role of MMP-3 in Wnt-mediated signaling, we tested the effect of AG3340 and rTIMP-2 on two other functions typically associated with Wnt-mediated EMT, cell proliferation and cell migration. Using FACS analysis on PI-stained cells, we documented a 2.9-fold increase in the percentage of C57MG/Wnt1 cells in S phase when compared with untreated cells (Fig. 4A and B) and an absence of increase when cells were treated with AG3340. Inhibition of entry of C57MG/Wnt1 cells into S phase by AG3340 corresponded to a decrease in expression of cyclin D1 and Erk ½ phosphorylation (Fig. 4C). The effect of AG3340 on Wnt1-induced migration was then examined using an in vitro wound assay. As anticipated, the migration of C57MG/Wnt1 cells was increased by 1.8-fold when compared with C57MG cells. However, when treated with AG3340 or rTIMP-2, the number of C57MG/Wnt1 cells that migrated was similar to the number of migratory cells from untreated or treated C57MG cells (Fig. 5A and B). Since the acquisition of a mesenchymal-like phenotype is typically associated with cytoskeleton reorganization and a change in vimentin expression,30 we examined the effect of Wnt1 in C57MG/Wnt1 and C57MG treated with Wnt3a on the expression and cellular distribution of actin and vimentin and whether it would be influenced by MMP inhibition. Although vimentin was present in both C57MG and C57MG/Wnt1 cells, we observed a significant difference in its subcellular localization, from being predominantly present in the perinuclear area in C57MG cells to being primarily located in cellular processes in association with actin filaments in C57MG/Wnt1 cells that underwent EMT (Fig. 5C). This was confirmed by demonstrating a similar change in vimentin distribution in C57MG cells upon treatment with Wnt3a (Fig. 5C). It therefore seems that Wnt1-induced EMT in C57MG cells, does not affect vimentin expression which is present in untransfected cells, but rather has a significant effect on its organization by promoting its association with actin. Consistent with our other observations, we demonstrated that the effect of Wnt1 on the cellular distribution of vimentin was reversed in the presence of AG3340 in C57MG/Wnt1 cells (Fig. 5D). Thus, altogether the data point to an active contributory role for MMP-3 in Wnt1-induced EMT. This role includes the promotion of cell-cell dissociation, cell proliferation and migration all associated with β-catenin translocation to the nucleus and increased β-catenin transcriptional activity.
Figure 4.
MMP inhibition reduces cell proliferation in Wnt1-transformed cells. (A) Analysis of cell cycle distribution by FACS on nuclei stained with propidium iodide. Representative distribution of C57MG and C57MG/Wnt1 cells in the absence or presence of AG3340 (10 µg/ml) added 24 h before cell harvesting (top). Representation of cell cycle distribution from above samples (bottom). (B) The data show the average percentage (±SD) of cells in S phase in triplicate cultures, and is representative of five independent experiments. (C) Western blot for cyclin D1, p-Erk1/2 and Erk1/2 expression in total cell lysates of C57MG and C57MG/Wnt1 cells cultured in the absence or presence of AG3340 (10 µg/ml) for 48 h.
Figure 5.
MMP inhibition reduces cell migration and is associated with vimentin/actin organization in Wnt1-transformed cells. (A) Photomicrographs of C57MG and C57MG/Wnt1 cells obtained 24 h after a scratch wound in the absence or presence of MMP inhibitors as indicated. Bar = 100 µm (top). (B) The data represent the average number (±SD) of cells migrating into the wound from ten microscopic fields examined in three separate dishes, and are representative of two independent experiments (bottom). (C) Immunolocalization of actin (green) and vimentin (red) in C57MG, C57MG/Wnt 1 analyzed by epifluorescence (top) and confocal (bottom). Bar = 10 µm. (D) Immunolocalization of actin (green) and vimentin (red) in C57MG cells treated with Wnt3a and in C57MG/Wnt1 cells treated with AG3340 (10 µg/ml). Bar = 10 µm.
Inhibition of MMP s inhibits transcriptional upregulation of MMP-3.
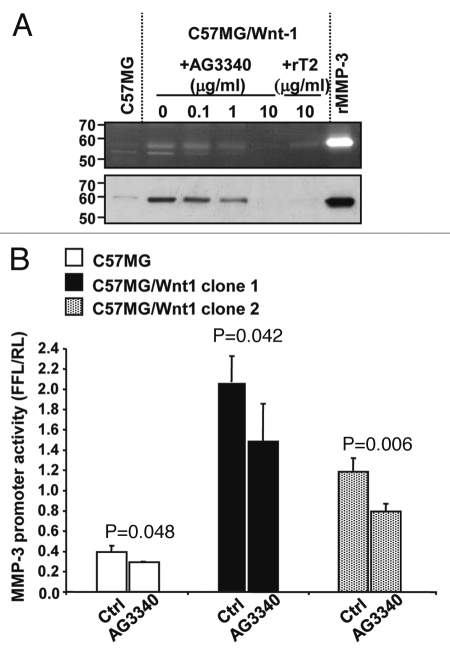
Our data thus demonstrate that MMP-3 is both a target and a regulator of Wnt-mediated activity. We therefore tested whether inhibition of MMP-3 activity in C57MG/Wnt1 cells would also inhibit MMP-3 transcription and expression. We first determined whether the production of MMP-3 by C57MG/Wnt1 cells would be decreased upon treatment with AG3340 or rTIMP-2. Casein gel zymography and western blot analysis of the supernatant of C57MG/Wnt1 cells demonstrated a significant decrease in the presence of MMP-3 in the supernatant of cells treated with AG3340 or rTIMP-2 that is consistent with these inhibitors inhibiting Wnt1-induced MMP-3 production (Fig. 6A). Second, we examined whether there would be a decrease in MMP-3 promoter activity in C57MG/Wnt1 cells in the presence of MMP inhibition. Consistent with our hypothesis, the data revealed a significant decrease in MMP-3 promoter activity in C57MG/Wnt1 cells upon treatment with AG3340 (Fig. 6B). These data thus demonstrate that by downregulating Wnt activity, MMP inhibition also decreases the production of MMP-3 in Wnt1-transformed cells.
Figure 6.
MMP inhibition downregulates Wnt-induced MMP-3 expression. (A) Casein zymography (top) and western blot (bottom) of conditioned media obtained from C57MG and C57MG/Wnt1 cells cultured for four days in the absence or presence of AG3340 or rTIMP-2 at indicated concentrations. Recombinant MMP-3 was loaded as positive control. (B) MMP-3 promoter activity in C57MG and C57MG/Wnt1 cells from clone 1 and clone 2 treated with AG3340 (50 µg/ml). The data represent the mean FFL/RL ratio (±SD) from triplicate samples and are representative of four experiments showing similar results.
Preventive treatment with MMP inhibitor prevents and delays the onset of Wnt1-induced mammary tumors in mice.
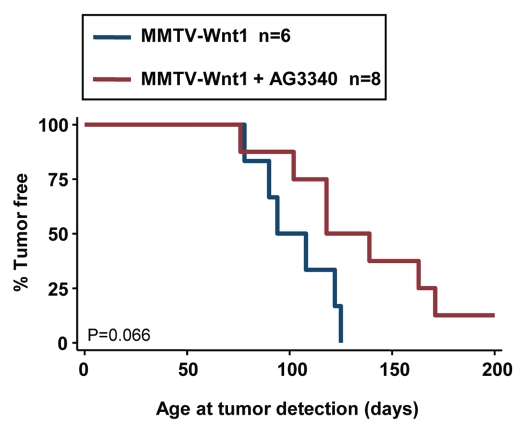
In support of the concept that MMPs are active contributor of Wnt-mediated transformation in vivo, we previously demonstrated that MMTV-Wnt1 mice overexpressing TIMP-2 develop fewer mammary tumors with an increased latency. Here we tested whether the preventive administration of AG3340 to young MMTV-Wnt1 females would have a similar effect. In this experiment, we treated mice with AG3340 at a dose of 50 mg/kg, twice a week, from six weeks of age until tumor development. All mice were paired for breeding and females bore one or more litters. The data indicated a shift in the median time to tumor development from 90 days in control mice to 120 days in AG3340-treated mice with one treated mouse remaining tumor-free (Fig. 7). Although having a p value higher than 0.05 (p = 0.066), the trend suggests that inhibition of MMP with a synthetic inhibitor delays Wnt-mediated tumor formation and is consistent with our in vitro data demonstrating that MMP inhibition downregulates Wnt1-mediated EMT.
Figure 7.
Preventive treatment with MMP inhibitor delays tumor development. Multiparous MMTV-Wnt1 female mice received i.p. injections of AG3340 (50 mg/kg) twice a week from week 6 to tumor development, and were monitored for mammary tumor formation in comparison to untreated littermates. The data are a Kaplan-Meier analysis of the percentage of tumor-free mice over time.
Discussion
Our data provide evidence that MMP-3 is both a transcriptional target and a regulator of Wnt signaling. EMT is an early event of malignant transformation of epithelial cells during which cells lose their polarity and contact with neighboring cells, and acquire a mesenchymal-like and motile phenotype, critical for local invasion.31,32 EMT can be induced by extra cellular factors commonly associated with carcinoma progression, including Wnt.33 Wnt-induced EMT is typically associated with a translocation of β-catenin from the plasma membrane to the nucleus where it forms transcription regulatory complexes with LEF/TCF proteins. MMPs like MMP-7,13 MT1-MMP,14 and MMP-26,34 have been shown to be among the target genes upregulated by β-catenin/LEF/TCF. We report here that MMP-3 is transcriptionally upregulated by Wnt signaling adding therefore this MMP to the list of β-catenin regulated MMPs. Prieve and Moon, similarly reported a modulation in MMP-3 expression upon Wnt transformation of C57MG cells, but attributed the effect to the non-canonical Wnt/Ca2+ pathway, because Wnt5a but not Wnt1 increased MMP-3 expression in C57MG cells.35 However, their observations were based on DNA microarray and RT-PCR analysis and were not confirmed by protein expression or promoter regulation analysis. Here we demonstrate an upregulation of MMP-3 upon activation of Wnt signaling in C57MG cells and provide evidence for the involvement of the canonical Wnt pathway, as both Wnt1 transfection and Wnt3a treatment lead to MMP-3 upregulation, β-catenin nuclear translocation, and upregulation of the mouse MMP-3 promoter.
We then provide evidence in several experiments that MMP-3 is not only a target of Wnt signaling but also a regulator of and contributor to Wnt signaling by demonstrating that the effects of Wnt on C57MG cells could be reversed in the presence of MMP inhibitors or upon MMP-3 downregulation by siRNA. Although experiments using broad spectrum inhibitors of MMPs like AG3340, NNGH or TIMP-2 could not rule out a contributory role for other MMPs also secreted by C57MG cells, in particular MMP-2, experiments using MMP-3 siRNA are consistent with a regulatory role for MMP-3. Our data however do not rule out the possibility that in other systems, MMPs other than MMP-3 might play a similar role. Several investigators have previously suggested that MMPs including MMP-3 can be causal in EMT in a Wnt-independent manner. Sternlicht et al. have shown that overexpression of MMP-3 in the mammary gland of transgenic mice induced formation of mammary tumors.11 Radisky et al. showed that overexpression of MMP-3 in murine mammary epithelial SCp2 cells induces EMT through increased expression of Rac1b and induction of the transcription factor Snail by reactive oxygen species. More recently, Orlichenko and Radisky, have shown a similar effect for MMP-9 but not MMP-2.36 We did not observe changes in Snail expression in C57MG and C57MG/Wnt1 cells in the absence or presence of AG3340 (data not shown) suggesting therefore a different mechanism.37,38 The mechanism by which MMP-3 regulates Wnt signaling in C57MG cells is presently unknown but currently investigated in our laboratory. Our coculture data and our data with MMP inhibitors indicate that it involves an extracellular and a proteolytic function of MMP-3. Several possibilities should be considered. Because some MMPs have been shown to cleave the ectodomain of E-cadherin, it is conceivable that by cleaving E-cadherin, MMP-3 might promote the release of β-catenin, which in conjunction with Wnt would increase its transcriptional activity. C57MG cells however do not express E-cadherin. β-catenin also associates with other transmembrane proteins, such as glycoprotein MUC1,39 which could be cleaved by MMP-3, but we could not find evidence for the expression of this glycoprotein in C57MG cells. Another possibility is that MMP-3 may have a regulatory function on the interaction between Wnt and its receptors Frizzled and lipoprotein receptor-related proteins LRP5/6. The binding of Wnt to LRP and Frizzled is controlled by several inhibitory proteins including Dickkopf-1 (Dkk-1), soluble Frizzled related proteins and Kremen.40,41 Cleavage of these proteins by MMP-3 would result in increased Wnt signaling. For example, Dkk-1 has been recently identified among potential targets of MT1-MMP in a degradome study of MDA-MB breast cancer cells.42
We previously reported that overexpression of TIMP-2 in the mammary glands of MMTV-Wnt1 mice inhibited tumor formation suggesting that MMP inhibition could prevent or delay malignant transformation of epithelial cells.29 Here, using the same MMTV-Wnt1 transgenic tumor model, we examined the effect of a preventive treatment using low dose (50 mg/kg) of a small MMP inhibitor, AG3340, that was administered twice a week. In previous experiments we had used higher doses (100 mg/kg) of the inhibitor administered twice daily by gastric gavage.43 Although the difference between the treated group and the untreated group was not statistically significant, this low dose preventing treatment seemed to delay the onset of mammary tumor by 30 days, and one multiparous MMTV-Wnt1 treated mouse remained tumor-free past 8 months of age. These observations indicating that a low dose MMP inhibitor administered in a preventative manner impairs Wnt1-induced tumors formation and progression are consistent with our previous report and with a contributory role of MMPs in Wnt1-induced tumorigenesis.
Finally, the observation that in the presence of MMP inhibitors, C57MG/Wnt1 cells reverse to an epithelial phenotype and thus undergo mesenchymal to epithelial transition (MET), suggests that MMP may control this process in vivo. As EMT promotes local invasion and intravasation of metastatic epithelial cancer cells, MET may need to occur in order for these cells to grow at the metastatic site.44,45 Downregulation of MMPs like MMP-3 or inhibition of MMPs by natural inhibitors in the metastatic niche, may thus contribute to the progression of established metastatic tumor cells. This may in part explain the lack of therapeutic effect of MMP inhibitors in cancer patients.5
In summary, by demonstrating that MMP-3 is both a target and a regulator of Wnt signaling and that MMP inhibition can promote MET in Wnt-transformed cells, our data provide additional insight into the complex role that MMPs play in cancer progression.
Materials and Methods
Cell culture.
Murine mammary epithelial cells C57MG were cultured in DMEM (Cellgro, Mediatech Inc., Herndon, VA) supplemented with 10% fetal calf serum (FCS) (Omega Scientific Inc., Tarzana, CA), 2 mM L-glutamine, and 1% penicillin/streptomycin (Irvine Scientific, Santa Ana, CA). Wnt1-HA was received from J. Kitajewski (Columbia University, New York, NY)20 and cloned into pMIRB (received from Ornitz D, Washington University, St. Louis, MO).46 C57MG cells were transfected with the pMIRB-Wnt1 HA plasmid using Lipofectamine (Life Technologies, Inc., Gaithersburg, MD). Stable clones were selected in the presence of 500 µg/ml G418 (Bio Whitaker, Walkersville, MD) and screened for Wnt1 expression by northern blots. To evaluate the sustained expression of Wnt1 in our transfected clones, RT-PCR were performed using the following primers: 5′-CAT CGA GTC CTG CAC CTG-3′; 5′-TGG GCG ATT TCT CGA AGT AG-3′.47 In some conditions, cells were cultured in the presence of the natural MMP inhibitor rTIMP-2 (10 µg/ml),48 the synthetic broad spectrum MMP inhibitors AG3340 (10 µg/ml) (Prinomastat, Agouron/Pfizer, Inc., San Diego, CA), or N-isobutyl-N-(4-methoxyphenylsulfonyl)glycyl hydroxamic acid (NNGH, 50 µM)49,50 (Enzo Life Sciences, Inc., Plymouth Meeting, PA). Immortalized mouse fibroblast L cells and Wnt3a-expressing L cells (ATCC, Manassas, VA) were maintained in DMEM containing 10% FCS. Conditioned media were prepared according to the protocol recommended by ATCC.
Cell transfections and reporter assays.
C57MG and C57MG/Wnt1 cells were co-transfected with the plasmid pGL2-SL1-luc (2 µg/ml) containing a full length 1.3 kb murine MMP-3 promoter inserted upstream of a firefly luciferase reporter gene (gift of Dr. Bissell M, Lawrence Berkeley National Laboratory, Berkeley, CA) and the Renilla luciferase reporter plasmid pRL-SV40 (25 ng/ml) used as internal control. Cells were also co-transfected with the TCF-luciferase reporter pTopflash (250 ng/ml) (Upstate, Lake Placid, NY) and pRL-SV40. The luciferase activity was measured in 20 µl of cell lysates using the Dual-Luciferase™ reporter assay (Promega, Madison, MI) and the results were expressed as the ratio of firefly over Renilla luciferase activity (FFL/RL). In some experiments, C57MG cells were stably transfected with pTopflash (renamed CT7 cells) or a MMP-3 cDNA containing plasmid LPCX (C57MG/MMP3).
SiRNA transfection.
Silencing of mouse MMP-3 (Gene accession number NM-010809) was performed using siRNA transfection. The MMP-3 target sequences were 5′-AAC ATG GAG ACT TTG TCC CTT-3′, 5′-AAG ATT GTG TGT CGT TTA TTA-3′, and 5′-AAG ATG TGA AGC AAT TTA TTA-3′. The siRNA duplexes were obtained from Qiagen Inc. (Valencia, CA) and were transfected together into C57MG/Wnt1 cells at a concentration of 50 nM each using Lipofectamine 2000 (Invitrogen).
Zymographies and western blots.
Analyses were performed on conditioned media or whole cell lysates obtained in RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100 and 0.1% SDS). Typically, aliquots containing 25–30 µg of protein each were resolved by SDS-PAGE. SDS-PAGE zymographies were performed in gels containing either 1% gelatin or casein as substrate as previously described.29 For western blots, gels were transferred to a nitrocellulose membrane, blocked overnight at 4°C in TBS/0.05% Tween (TBST) containing 5% dry milk, incubated with the primary antibodies for 2 h at room temperature, washed in TBST and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Proteins were detected using the ECL reagent (Amersham Life Science, Buckinghamshire, UK). The following primary antibodies were used: an anti-mouse MMP-3 polyclonal antibody and an anti-mouse MMP-13 polyclonal antibody (gifts of Dr. Ch. Peeters-Joris, Catholic University of Louvain, Louvain, Belgium); an anti-human MMP-7 monoclonal antibody crossreacting with murine MMP-7 (gift of Dr. L.M. Matrisian); an anti-human MT1-MMP polyclonal antibody (Chemicon International, Temecula, CA); an anti-human β-catenin polyclonal antibody and an anti-human cyclin D1 polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); anti-Wnt3a polyclonal antibody, anti-phospho-p44/42 and anti-p44/42 MAP kinases (Erk1/2) polyclonal antibodies (Cell Signaling Technology, Inc., Beverly, MA).
Northern blot.
Total RNA was isolated from C57MG and C57MG/Wnt1 cells using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Aliquots of 20 µg were separated by electrophoresis in 1% agarose gels containing 18% formaldehyde, and blotted onto Hybond-N filters (Amersham Pharmacia Biotech, Buckinghamshire, England). Northern hybridizations were carried out according to standard procedures using [α-32P] dCTP-labeled cDNA fragments for mouse Wnt1 and MMP-3 as probes.
Cell proliferation and cell motility assays.
For FACS® analysis, cells were seeded into 100-mm plates and cultured to confluence in the presence or absence of 10 µg/ml AG3340. Cells were washed in PBS, resuspended in 70% ethanol and stored overnight at 4°C. Cells were incubated with 20 µg/ml RNase A for 30 min at 37°C, centrifuged, and resuspended in PBS containing 40 µg/ml propidium iodide. The analysis was performed on an EPICS Elite ESP cell sorter (Beckman Coulter, Inc., Brea, CA) using the Expo 32 (version 1.2) software. To assess cell migration, confluent monolayers of C57MG or C57MG/Wnt1 cells were scratch-wounded with a pipette tip to create a cell-free area. Cells were incubated for 24 h in the presence or absence of MMP inhibitors, and wound closure reflecting cell migration was documented by photography using an Olympus IMT-2 microscope equipped with an Olympus DPII digital camera. For each condition, 10 microscopic fields were selected along the wound and cells that had migrated from the wound edge to the cell-free space were counted.
Immunofluorescence.
C57MG cells, C57MG cells treated with Wnt3a, and C57MG/Wnt1 stable clones were cultured in Lab-Tek II chamber slides (Nalgen Nunc International, Naperville, IL), fixed 10 min with 4% paraformaldehyde in PBS, permeabilized 5 min with 0.1% Triton X-100 in PBS, and blocked 10 min with 15% FCS in PBS. Cells were incubated with the primary antibody for 1 h at 37°C, washed in 0.1% Triton X-100/PBS then incubated with the secondary antibody for 45 min at room temperature. The primary antibodies used were an anti-human β-catenin polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), an anti-mouse vimentin monoclonal antibody (clone LN-6, Sigma), and an anti-actin (Sigma) antibody. Slides were mounted in Vectashield with DAPI (Vector Laboratories, Inc., Burlingame, CA) and examined under epifluorescence. Images were acquired with a Zeiss Axiovert 200 M microscope equipped with a 40x Plan Neofluar objective and Semrock Brightline filters for DAPI, GFP, Texas Red, Cy5, and a Hamamatsu ORCA ER digital camera. Confocal images were obtained with a CARV II Spinning disc confocal (BD Biosciences, San Jose, CA) and IP Lab 4.0 software (Scanalytics, Rockville, MD). Digital images were processed in Adobe Photoshop for adjustments of brightness and contrast.
Effect of MMP inhibition on tumor formation.
MMTV-Wnt1 mice were bred and observed for mammary tumor formation in comparison to littermates receiving intra peritoneal injections at a dose of 50 mg/kg of AG3340 twice a week. The mice were monitored for the development of a small tumor nodule by weekly palpation and the age at tumor onset was recorded. The protocol was approved by the CHLA Institutional Animal Care and Use Committee (protocol number 53-05).
Statistical analysis.
The two-sample t-test was used to compare the percentage of cells in S phase in C57MG and in C57MG/Wnt1 cells, to assess the effect of MMP inhibitors on C57MG/Wnt1 cell migration, and to compare transcriptional activity under different conditions. Kaplan-Meier plots and the log-rank test were used to compare the age of the MMTV-Wnt1 mice treated or not treated with AG3340 at the time of tumor detection and the probability of being tumor free.
Acknowledgements
We thank Dr. JunQing Qian for the preparation of the pMIRB-Wnt1 HA plasmid, Drs. Lynn M. Matrisian, Mina Bissell and Chantal Peeters-Joris for providing the antibody against MMP-7 and MMP-7 recombinant protein, the pGL2-SL1-Luc plasmid and the antibodies against MMP-3 and MMP-13 respectively. We thank Dr. David R. Shalinsky for providing the AG3340. We also thank Mrs. J. Rosenberg for her help formatting the manuscript. This work was supported by RO1 CA 42919 grant from the National Institutes of Health to Y.A. DeClerck.
Abbreviations
- EMT
epithelial mesenchymal transition
- MET
mesenchymal to epithelial *transition
- MMP
matrix metalloproteinase
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/12193
References
- 1.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 2.Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25:45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 3.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 4.McCawley LJ, Matrisian LM. Matrix Metalloproteinases: they're not just for matrix anymore. Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 6.Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112:439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, et al. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell. Biol1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph-Owen LA, Chan R, Muller WJ, Matrisian LM. The matrix metalloproteinase matrilysin influences early-stage mammary tumorigenesis. Cancer Res. 1998;58:5500–5506. [PubMed] [Google Scholar]
- 11.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 13.Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJH, Rubinfeld B, Polakis P, et al. The metalloproteinase matrilysin is a target of β-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002;21:5861–5867. doi: 10.1038/sj.onc.1205755. [DOI] [PubMed] [Google Scholar]
- 15.Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 16.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 17.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 18.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 19.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- 21.Qian J, Jiang Z, Li M, Heaphy P, Liu YH, Shackleford GM. Mouse Wnt9b transforming activity, tissue-specific expression, and evolution. Genomics. 2003;81:34–46. doi: 10.1016/s0888-7543(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 22.Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 25.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 26.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 27.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 28.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 29.Blavier L, Lazaryev A, Dorey F, Shackleford GM, DeClerck YA. Matrix metalloproteinases play an active role in Wnt1-induced mammary tumorigenesis. Cancer Res. 2006;66:2691–2699. doi: 10.1158/0008-5472.CAN-05-2919. [DOI] [PubMed] [Google Scholar]
- 30.Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelialmesenchymal transition in human breast cancer—observations in vitro and in vivo. Cells Tissues Organs. 2007;185:191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 31.Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105:425–431. doi: 10.1016/s0092-8674(01)00365-8. [DOI] [PubMed] [Google Scholar]
- 32.Vincent-Salomon A, Thiery JP. Host microenvironment in breast cancer development: epithelial-mesenchymal transition in breast cancer development. Breast Cancer Res. 2003;5:101–106. doi: 10.1186/bcr578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 34.Marchenko GN, Ratnikov BI, Rozanov DV, Godzik A, Deryugina EI, Strongin AY. Characterization of matrix metalloproteinase-26, a novel metalloproteinase widely expressed in cancer cells of epithelial origin. Biochem J. 2001;356:705–718. doi: 10.1042/0264-6021:3560705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prieve MG, Moon RT. Stromelysin-1 and mesothelin are differentially regulated by Wnt-5a and Wnt-1 in C57 mg mouse mammary epithelial cells. BMC Dev Biol. 2003;3:2. doi: 10.1186/1471-213X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25:593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 37.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson CM, Khauv D, Bissell MJ, Radisky DC. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem. 2008;105:25–33. doi: 10.1002/jcb.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 40.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 41.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 42.Butler GS, Dean RA, Tam EM, Overall CM. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol. 2008;28:4896–4914. doi: 10.1128/MCB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, et al. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 45.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 46.Chellaiah AT, McEwen DG, Werner S, Xu J, Ornitz DM. Fibroblast growth factor receptor (FGFR) 3. Alternative splicing in immunoglobulin-like domain III creates a receptor highly specific for acidic FGF/FGF-1. J Biol Chem. 1994;269:11620–11627. [PubMed] [Google Scholar]
- 47.Ziegler S, Rohrs S, Tickenbrock L, Moroy T, Klein-Hitpass L, Vetter IR, et al. Novel target genes of the Wnt pathway and statistical insights into Wnt target promoter regulation. FEBS J. 2005;272:1600–1615. doi: 10.1111/j.1742-4658.2005.04581.x. [DOI] [PubMed] [Google Scholar]
- 48.Boone TC, Johnson MJ, DeClerck YA, Langley KE. cDNA cloning and expression of a metalloproteinase inhibitor related to tissue inhibitor of metalloproteinases. Proc Natl Acad Sci USA. 1990;87:2800–2804. doi: 10.1073/pnas.87.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacPherson LJ, Bayburt EK, Capparelli MP, Carroll BJ, Goldstein R, Justice MR, et al. Discovery of CGS 27023A, a non-peptidic, potent and orally active stromelysin inhibitor that blocks cartilage degradation in rabbits. J Med Chem. 1997;40:2525–2532. doi: 10.1021/jm960871c. [DOI] [PubMed] [Google Scholar]
- 50.Calderone V, Fragai M, Luchinat C, Nativi C, Richichi B, Roelens S. A high-affinity carbohydrate-containing inhibitor of matrix metalloproteinases. Chem Med Chem. 2006;1:598–601. doi: 10.1002/cmdc.200600020. [DOI] [PubMed] [Google Scholar]