Abstract
As the prevalence of osteoporosis is expected to increase over the next few decades, the development of novel therapeutic strategies to combat this disorder becomes clinically imperative. These efforts draw extensively from an expanding body of knowledge pertaining to the physiologic mechanisms of skeletal homeostasis. To this body of knowledge, we contribute that cells of hematopoietic lineage may play a crucial role in balancing osteoblastic bone formation against osteoclastic resorption. Specifically, our laboratory has previously demonstrated that megakaryocytes (MK) can induce osteoblast (OB) proliferation in vitro, but do so only when direct cell-to-cell contact is permitted. To further investigate the nature of this interaction, we have effectively neutralized several adhesion molecules known to function in the analogous interaction of MKs with another cell-type of mesenchymal origin - the fibroblast (FB). Our findings implicate the involvement of fibronectin/RGD-binding integrins including α3β1 (VLA-3) and α5β1 (VLA-5) as well as glycoprotein IIb (CD41), all of which are known to be expressed on MK membranes. Furthermore, we demonstrate that interleukin (IL)-3 can enhance MK-induced OB activation in vitro, as demonstrated in the MK-FB model system. Taken together, these results suggest that although their physiologic and clinical implications are very different, these two models of hematopoietic-mesenchymal cell activation are mechanistically analogous in several ways.
Keywords: Megakaryocytes, Osteoblasts, Integrins, CD41, IL-3
Skeletal fragility has emerged as a major limitation to quality of life as we age. Osteoporosis and the ensuing hip, wrist, and vertebral fractures are significant sources of morbidity and pain among the elderly: such a fracture can be the sentinel event that transforms a relatively healthy, independent senior citizen into a person requiring significant assistance for daily living. This downward spiral is evidenced by a one-year post-hip fracture mortality of 24 percent (National Osteoporosis Foundation). As the prevalence of osteoporosis is expected to increase over the next few decades, the development of novel therapeutic strategies to combat this disorder becomes clinically imperative. These efforts draw extensively from an expanding body of knowledge pertaining to the physiologic mechanisms of skeletal homeostasis. To this body of knowledge, we contribute that cells of hematopoietic lineage may play a crucial role in balancing osteoblastic bone formation against osteoclastic resorption.
Over the past decade, a new paradigm has emerged wherein MKs have been found to play an important role in skeletal homeostasis. In brief, data demonstrate that MKs may act to stimulate bone formation by expressing/secreting bone-related proteins, and by directly enhancing OB proliferation and differentiation (Thiede et al., 1994; Kelm et al., 1992; Breton-Gorius et al., 1992; Chenu and Delmas, 1992; Frank et al., 1993; Sipe et al., 2004; Kacena et al., 2004; Ciovacco et al., 2009; Miao et al., 2004; Bord et al., 2005; Ciovacco et al., in press). Simultaneously, MKs may regulate bone resorption by expressing/secreting several factors known to be involved in osteoclastogenesis, and recent studies demonstrate that MKs can inhibit osteoclast (OC) formation in vitro (Ciovacco et al., in press; Bord et al., 2003; Bord et al., 2004; Beeton et al., 2006; Pearse et al., 2001; Chagraoui et al., 2003; Kartsogiannis et al., 1999; Jiang et al., 1994; Soslau et al., 1997; Wickenhauser et al., 1995a; Wickenhauser et al., 1995b; Kacena et al., 2006). Specifically, our laboratory has demonstrated that MKs induce OB activation in vitro via a mechanism(s) requiring direct physical contact between the two cell types (Kacena et al., 2004), whereas MKs inhibit OC development in vitro via the elaboration of an as-yet unidentified soluble factor(s) (Kacena et al., 2006). The net result, as demonstrated in vivo, is that increases in MK number can lead to concomitant increases in bone mass (Kacena et al., 2004; Kacena et al., 2005; Suva et al., 2008; Frey et al., 1998a; Frey et al., 1998b; Yan et al., 1996; Yan et al., 1995; Villeval et al., 1997).
In the present study, we have focused our efforts on characterization of the contact-dependent mechanism(s) by which MKs induce OB proliferation/differentiation. To this end, we have effectively neutralized several adhesion molecules known to function in the analogous interaction of MKs with another cell-type of mesenchymal origin - the FB (Wickenhauser et al., 2000; Schmitz et al., 1998). Furthermore, we have explored the effect of IL-3 on our MK-OB model system, as this cytokine has been shown to enhance MK-induced FB activation in vitro (Schmitz et al., 1999; Schmitz et al., 1995). Here we examine these new data which may offer insight as to the mechanism(s) of this interaction.
Materials and Methods
Preparation of neonatal calvarial cells (OB) and Fetal Liver Derived MKs and Experimental Conditions
C57BL/6 murine calvarial cells of the OB lineage were prepared by sequential collagenase digestion as previously described (Horowitz et al., 1994; Wong and Cohn, 1975). Cells collected from fractions 3–5 were used as the starting population for OB/osteoprogenitor culture. To isolate MKs, livers from 13- to 15-day-old embryos (C57BL/6 mice) were collected, and single cell suspensions were prepared and cultured in DMEM with 10% FCS and 1% conditioned medium (CM) from a murine TPO-secreting fibroblast cell line. MKs were then separated from other cell-types (90–95% pure MK population) using a one-step albumin gradient (Drachman et al., 1997). 2500 OB/well were co-cultured with 5000 MK/well in 96-well tissue culture plates (optimal, pre-tested).
Ethylenediaminetetraacetic acid (EDTA, Sigma, St. Louis, 0.0125mM and 0.125mM) and soluble tetrapeptide Arg-Gly-Asp-Ser (RGDS; Sigma, 0.0125mM and 0.625mM) were titrated into MK-OB co-cultures and OB control cultures. The following neutralizing antibodies were purchased: integrin α3 chain/CD49c (10μg/mL; polyclonal; R&D Systems, Minneapolis, MN), integrin α5 chain/CD49e (20μg/mL; clone: 5H10-27 MFR5; BD Biosciences Pharmingen, San Diego, CA), and glycoprotein (gp) IIb/integrin αIIb chain/CD41 (10ng/mL; clone: MWReg30; BD Biosciences Pharmingen). Goat IgG fraction was added to separate controls at equivalent concentrations to assure that blocking was antigen-specific. Finally, recombinant murine IL-3 (R&D Systems, 10 ng/ml and 30 ng/ml) was also titrated into co-cultures and controls.
Proliferation Analysis
Proliferation was measured by β-scintillation count following tritiated (3H)-thymidine incorporation (1 μCi/well; 5–8 Ci/mmol; added 16 hours prior to freezing of culture at day 4). Because MKs are non-adherent, they were removed by washing (4×) prior to freezing and subsequent harvesting to ensure that OB proliferation alone was measured (Kacena et al., 2004).
Statistics
Unless otherwise stated, all data are presented as the Mean ± SD. For all studies one-way ANOVA was used to determine significant differences (p<0.05). All analyses were performed with the Statistical Package for Social Sciences (SPSS 6.1.1; Norusis/SPSS Inc., Chicago, IL) software and were two-tailed with a level of significance set at 0.05. Experiments are always repeated, in some cases multiple times. Within individual experiments, data points are based on a minimum of triplicate samples.
Results
Effect of EDTA on MK-OB Cultures
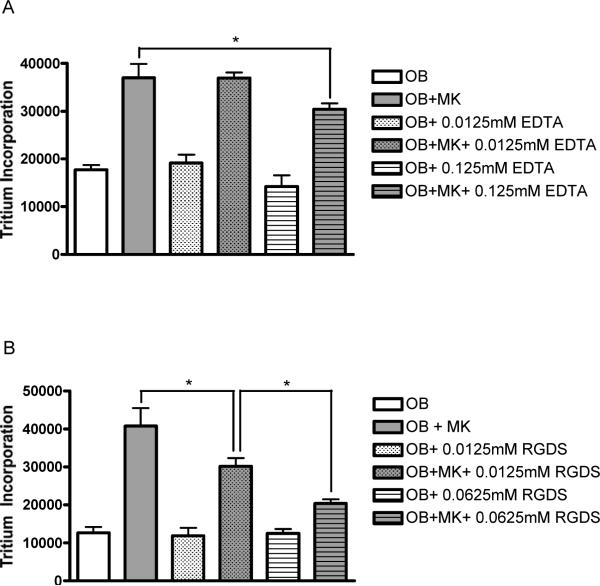
The bivalent cation chelator EDTA was titrated into MK-OB co-cultures and OB controls to examine the effect of non-selective integrin inhibition on MK-induced OB activation. While the addition of EDTA at 12.5μM failed to inhibit MK-induced activation, 125μM EDTA significantly reduced tritium incorporation in co-cultures by 18% without affecting OB controls (Fig. 1A). At higher concentrations tested, EDTA began to inhibit OB mono-culture proliferation (data not shown).
Figure 1.
Integrin involvement in MK-induced OB activation. (A) 12.5μM EDTA was not sufficient to disrupt MK-induced OB proliferation, however 125μM EDTA diminished MK-induced proliferation by 18%. Tritium incorporation by OB monocultures was not affected at either concentration reported. (B) Soluble tetrapeptide RGDS caused a dose-dependant inhibition of MK-induced proliferation with 12.5μM and 62.5μM decreasing tritium incorporation in co-cultures by 26% and 50%, respectively. Again, OB monoculture proliferation remained unaffected at both concentrations reported. * Denotes a significant difference between groups examined (p<0.05).
Effect of RGDS on MK-OB Cultures
To elucidate the involvement of Arg-Gly-Asp (RGD)-binding receptors in our model system, the soluble tetrapeptide Arg-Gly-Asp-Ser (RGDS; Sigma, St. Louis, MO) was titrated into co-cultures and OB controls. RGDS caused dramatic, dose-dependent inhibition of proliferation in co-cultures, with 12.5μM and 62.5μM, respectively, diminishing MK-induced activation by 26% and 50%, respectively, without affecting OB controls (Fig. 1B). Further increases in RGDS concentration did not result in greater inhibition of proliferation in co-cultures.
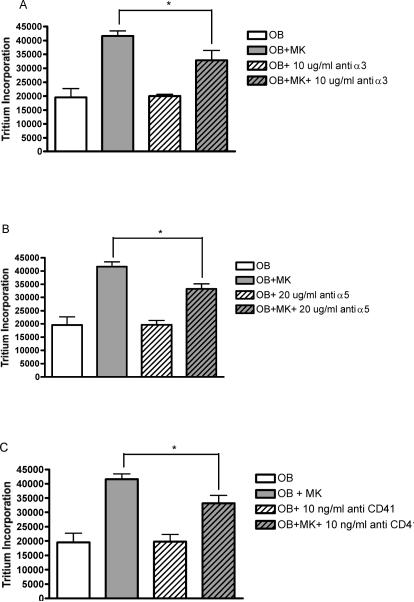
Effect of anti-α3, anti-α5, and anti-CD41 on MK-OB Cultures
Neutralizing antibodies directed against the following specific adhesion molecules were applied to co-cultures and OB controls: integrin α3 chain/CD49c, integrin α5 chain/CD49e, and glycoprotein (gp) IIb/integrin αIIb chain/CD41. Each of the neutralizing antibodies tested yielded virtually identical results, causing moderate, yet significant, reductions in co-culture proliferation without affecting OB monocultures. Specifically, maximal suppression of 21% by anti-α3 was seen at 10μg/mL, 20% by anti-α5 at 20μg/mL, and 20% by anti-CD41 at 10ng/mL (Fig. 2). Non-specific IgG did not affect tritium incorporation in co-cultures or OB monocultures when added at respective control concentrations (data not shown).
Figure 2.
Involvement of specific adhesion molecules in MK-induced OB activation. Application of neutralizing antibodies against integrin α3 chain (A), integrin α5 chain (B), and CD41 (C) each diminished MK-induced OB proliferation by approximately 20%, without affecting tritium incorporation in OB monocultures. * Denotes a significant difference between groups examined (p<0.05).
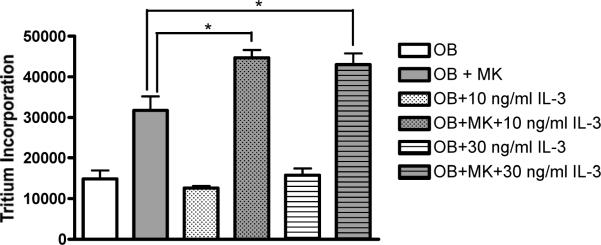
Effect of IL-3 on MK-OB Cultures
Lastly, recombinant murine IL-3 was titrated into co-cultures and controls to explore the effect of this cytokine on MK-induced OB proliferation. Ten ng/mL and 30 ng/mL IL-3 enhanced MK-induced OB proliferation by 41% and 37%, respectively, while OB monocultures remained unaffected (Fig. 3). Further increasing IL-3 concentration did not yield additional enhancement of MK-induced activation.
Figure 3.
IL-3 enhances MK-induced OB activation. At 10ng/mL and 30ng/mL, IL-3 enhanced MK-induced OB proliferation by 41% and 37%, respectively. Tritium incorporation by OB monocultures remained unaffected at both concentrations reported. * Denotes a significant difference between groups examined (p<0.05).
Discussion
Our laboratory has previously demonstrated that MKs can induce OB proliferation, but do so only when direct cell-to-cell contact is permitted (Kacena et al., 2004). To further investigate the nature of this interaction, we have systematically disrupted known mechanisms of MK-FB adhesion/signaling in our own MK-OB co-culture model system. We began our investigation with the addition of EDTA to co-cultures and controls. This chelating agent reduces the availability of bivalent cations necessary for proper dimerization and ligand-binding of integrin heterodimers (Ruoslahti, 1991; Hynes, 1992) and has been shown to inhibit MK-FB adherence and signaling in vitro (Schmitz et al., 1998). The ability of EDTA to diminish MK-induced OB proliferation without affecting OB monocultures thus implicates integrin involvement in MK-OB adherence/signaling as well. Refining the scope of our investigation, we next examined the role of RGD receptors in our model system. The conformation of the RGD sequence of fibronectin is approximated in soluble form by the tetrapeptide RGDS (Pierschbacher and Ruoslahti, 1987). Therefore, our finding that the addition of this tetrapeptide inhibits MK-induced proliferation without affecting OB monocultures implicates specifically, although not exclusively, RGD-binding integrins. These data are again consistent with those pertaining to MK-FB adherence/signaling (Schmitz et al., 1998). The respective roles of MK-expressed, fibronectin-binding integrins α3β1 (VLA-3) and α5β1 (VLA-5; an RGD receptor integrin) were then examined by application of neutralizing antibodies to co-cultures and controls. This resulted in significant reductions in MK-induced proliferation. As OB monocultures were not affected by these antibodies, and non-specific IgG affected neither co-cultures nor OB controls, we conclude that these specific integrins directly contribute to MK-OB adhesion/activation, as demonstrated in the MK-FB model system (Schmitz et al., 1998). Employing the same technique and reasoning, we next elucidated the involvement of the MK-expressed glycoprotein CD41. This molecule, also known as gpIIb, is retained on the surfaces of mature platelets where it complexes with CD61 (gpIIIa) forming a heterodimeric receptor capable of recognizing a host of extracellular proteins (fibrinogen, fibronectin, von Willebrand factor, vitronectin, etc.) with affinities modulated by the state of platelet activation. In agreement with MK-FB interaction findings (Wickenhauser et al., 2000), we conclude that this glycoprotein plays an important role in MK-induced adherence/activation.
Lastly, our data demonstrate that IL-3 significantly enhances MK-induced OB proliferation without affecting OB monocultures. Although we have not demonstrated that this enhancement is contingent upon direct cell-cell contact, Schmitz et al. (Schmitz et al., 1995) showed that IL-3 could not enhance FB proliferation when MK-FB co-cultures were divided by cell-impermeable membranes. Furthermore, the prerequisite of direct cell-cell contact in no way excludes signaling via soluble factors as a mechanism for MK-induced mesenchymal cell activation. To the contrary, Schmitz et al. (Schmitz et al., 1995) speculate that adhesion may serve principally to expose FBs to supra-threshold levels of MK-derived growth factors such as PDGF and TGFβ. While many such details of our own model - and skeletal homeostasis in general - remain obscure, we remain optimistic that our efforts will contribute to identification of pathways which when engaged will function as an anabolic stimulator of bone formation for the treatment of osteoporosis and other bone loss diseases.
Acknowledgements
We wish to thank Dr. Yougen Xi, Mrs. Tracy Nelson, and Mr. Thomas Gibson for their assistance with these studies. This work was supported by the Richard K. Gershon Fellowship (JML), by NIH/NIAMS grants AR47342 (MCH) and AR055269 (MAK), by a Pilot and Feasibility Award from the Yale Core Center for Musculoskeletal Disorders AR46032 (MAK), and by the Department of Orthopaedics and Rehabilitation at Yale University School of Medicine and the Department of Orthopaedic Surgery at Indiana University School of Medicine.
Grants: NIH/NAIMS:AR47342, NIH/NIAMS:AR055269, NIH/NIAMS:AR46032
References
- Beeton CA, Bord S, Ireland D, Compston JE. Osteoclast formation and bone resorption are inhibited by megakaryocytes. Bone. 2006;39:985–990. doi: 10.1016/j.bone.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Bord S, Frith E, Ireland DC, Scott MA, Craig JI, Compston JE. Megakaryocytes modulate osteoblast synthesis of type-l collagen, osteoprotegerin, and RANKL. Bone. 2005;36:812–819. doi: 10.1016/j.bone.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Bord S, Frith E, Ireland DC, Scott MA, Craig JI, Compston JE. Synthesis of osteoprotegerin and RANKL by megakaryocytes is modulated by oestrogen. Br J Haematol. 2004;126:244–251. doi: 10.1111/j.1365-2141.2004.05024.x. [DOI] [PubMed] [Google Scholar]
- Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32:136–141. doi: 10.1016/s8756-3282(02)00953-5. [DOI] [PubMed] [Google Scholar]
- Breton-Gorius J, Clezardin P, Guichard J, Debili N, Malaval L, Vainchenker W, Cramer EM, Delmas PD. Localization of platelet osteonectin at the internal face of the alpha-granule membranes in platelets and megakaryocytes. Blood. 1992;79:936–941. [PubMed] [Google Scholar]
- Chagraoui H, Sabri S, Capron C, Villeval JL, Vainchenker W, Wendling F. Expression of osteoprotegerin mRNA and protein in murine megakaryocytes. Exp Hematol. 2003;31:1081–1088. doi: 10.1016/s0301-472x(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Chenu C, Delmas PD. Platelets contribute to circulating levels of bone sialoprotein in human. J Bone Miner Res. 1992;7:47–54. doi: 10.1002/jbmr.5650070108. [DOI] [PubMed] [Google Scholar]
- Ciovacco WA, Cheng Y-H, Horowitz MC, Kacena MA. Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J Cell Biochem. doi: 10.1002/jcb.22456. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciovacco WA, Goldberg CG, Taylor AF, Lemieux JM, Horowitz MC, Donahue HJ, Kacena MA. The role of gap junctions in megakaryocyte-mediated osteoblast proliferation and differentiation. Bone. 2009;44:80–86. doi: 10.1016/j.bone.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman JG, Sabath DF, Fox NE, Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89:483–492. [PubMed] [Google Scholar]
- Frank JD, Balena R, Masarachia P, Seedor JG, Cartwright ME. The effects of three different demineralization agents on osteopontin localization in adult rat bone using immunohistochemistry. Histochemistry. 1993;99:295–301. doi: 10.1007/BF00269102. [DOI] [PubMed] [Google Scholar]
- Frey BM, Rafii S, Crystal RG, Moore MA. Adenovirus long-term expression of thrombopoietin in vivo: a new model for myeloproliferative syndrome and osteomyelofibrosis. Schweiz Med Wochenschr. 1998a;128:1587–1592. [PubMed] [Google Scholar]
- Frey BM, Rafii S, Teterson M, Eaton D, Crystal RG, Moore MA. Adenovector-mediated expression of human thrombopoietin cDNA in immune-compromised mice: insights into the pathophysiology of osteomyelofibrosis. J Immunol. 1998b;160:691–699. [PubMed] [Google Scholar]
- Horowitz MC, Fields A, DeMeo D, Qian HY, Bothwell AL, Trepman E. Expression and regulation of Ly-6 differentiation antigens by murine osteoblasts. Endocrinology. 1994;135:1032–1043. doi: 10.1210/endo.135.3.7520861. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jiang S, Levine JD, Fu Y, Deng B, London R, Groopman JE, Avraham H. Cytokine production by primary bone marrow megakaryocytes. Blood. 1994;84:4151–4156. [PubMed] [Google Scholar]
- Kacena MA, Gundberg CM, Nelson T, Horowitz MC. Loss of the transcription factor p45 NF-E2 results in a developmental arrest of megakaryocyte differentiation and the onset of a high bone mass phenotype. Bone. 2005;36:215–223. doi: 10.1016/j.bone.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Nelson T, Clough ME, Lee SK, Lorenzo JA, Gundberg CM, Horowitz MC. Megakaryocyte-mediated inhibition of osteoclast development. Bone. 2006;39:991–999. doi: 10.1016/j.bone.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res. 2004;19:652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, Niforas P, Ng KW, Martin TJ, Gillespie MT. Localization of RANKL (receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25:525–534. doi: 10.1016/s8756-3282(99)00214-8. [DOI] [PubMed] [Google Scholar]
- Kelm RJ, Jr, Hair GA, Mann KG, Grant BW. Characterization of human osteoblast and megakaryocyte-derived osteonectin (SPARC) Blood. 1992;80:3112–3119. [PubMed] [Google Scholar]
- Miao D, Murant S, Scutt N, Genever P, Scutt A. Megakaryocyte-bone marrow stromal cell aggregates demonstrate increased colony formation and alkaline phosphatase expression in vitro. Tissue Eng. 2004;10:807–817. doi: 10.1089/1076327041348473. [DOI] [PubMed] [Google Scholar]
- Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci U S A. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J Biol Chem. 1987;262:17294–17298. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz B, Thiele J, Otto F, Farahmand P, Henze F, Frimpong S, Wickenhauser C, Fischer R. Evidence for integrin receptor involvement in megakaryocyte-fibroblast interaction: a possible pathomechanism for the evolution of myelofibrosis. J Cell Physiol. 1998;176:445–455. doi: 10.1002/(SICI)1097-4652(199809)176:3<445::AID-JCP1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Schmitz B, Thiele J, Witte O, Kaufmann R, Wickenhauser C, Fischer R. Influence of cytokines (IL-1 alpha, IL-3, IL-11, GM-CSF) on megakaryocyte-fibroblast interactions in normal human bone marrow. Eur J Haematol. 1995;55:24–32. doi: 10.1111/j.1600-0609.1995.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Schmitz B, Wickenhauser C, Thiele J, Frimpong S, Brockbals C, Selbach B, Mueller C, Fischer R. Megakaryocyte induced fibroblast proliferation is enhanced by costimulation with IL-6/IL-3 and dependent on secretory and adhesion events. Leuk Res. 1999;23:723–729. doi: 10.1016/s0145-2126(99)00091-0. [DOI] [PubMed] [Google Scholar]
- Sipe JB, Zhang J, Waits C, Skikne B, Garimella R, Anderson HC. Localization of bone morphogenetic proteins (BMPs)-2, -4, and -6 within megakaryocytes and platelets. Bone. 2004;35:1316–1322. doi: 10.1016/j.bone.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Soslau G, Morgan DA, Jaffe JS, Brodsky I, Wang Y. Cytokine mRNA expression in human platelets and a megakaryocytic cell line and cytokine modulation of platelet function. Cytokine. 1997;9:405–411. doi: 10.1006/cyto.1996.0182. [DOI] [PubMed] [Google Scholar]
- Suva LJ, Hartman E, Dilley JD, Russell S, Akel NS, Skinner RA, Hogue WR, Budde U, Varughese KI, Kanaji T, Ware J. Platelet dysfunction and a high bone mass phenotype in a murine model of platelet-type von Willebrand disease. Am J Pathol. 2008;172:430–439. doi: 10.2353/ajpath.2008.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiede MA, Smock SL, Petersen DN, Grasser WA, Thompson DD, Nishimoto SK. Presence of messenger ribonucleic acid encoding osteocalcin, a marker of bone turnover, in bone marrow megakaryocytes and peripheral blood platelets. Endocrinology. 1994;135:929–937. doi: 10.1210/endo.135.3.8070388. [DOI] [PubMed] [Google Scholar]
- Villeval JL, Cohen-Solal K, Tulliez M, Giraudier S, Guichard J, Burstein SA, Cramer EM, Vainchenker W, Wendling F. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90:4369–4383. [PubMed] [Google Scholar]
- Wickenhauser C, Hillienhof A, Jungheim K, Lorenzen J, Ruskowski H, Hansmann ML, Thiele J, Fischer R. Detection and quantification of transforming growth factor beta (TGF-beta) and platelet-derived growth factor (PDGF) release by normal human megakaryocytes. Leukemia. 1995a;9:310–315. [PubMed] [Google Scholar]
- Wickenhauser C, Lorenzen J, Thiele J, Hillienhof A, Jungheim K, Schmitz B, Hansmann ML, Fischer R. Secretion of cytokines (interleukins-1 alpha, -3, and -6 and granulocyte-macrophage colony-stimulating factor) by normal human bone marrow megakaryocytes. Blood. 1995b;85:685–691. [PubMed] [Google Scholar]
- Wickenhauser C, Schmitz B, Baldus SE, Henze F, Farahmand P, Frimpong S, Thiele J, Fischer R. Selectins (CD62L, CD62P) and megakaryocytic glycoproteins (CD41a, CD42b) mediate megakaryocyte-fibroblast interactions in human bone marrow. Leuk Res. 2000;24:1013–1021. doi: 10.1016/s0145-2126(00)00063-1. [DOI] [PubMed] [Google Scholar]
- Wong GL, Cohn DV. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975;72:3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Fletcher F, Hartley C, McElroy P, Sun Y, Xia M, Mu S, Saris C, Hill D, Hawley RG, McNiece IK. Chronic exposure to retroviral vector encoded MGDF (mpl-igand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood. 1995;86:4025–4033. [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Hill D, Chen Y, Fletcher F, Hawley RG, McNiece IK. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88:402–409. [PubMed] [Google Scholar]