Abstract
Mounting evidence indicates that marginal biotin deficiency is not rare, contrary to previous assumptions. Accordingly, robust indicators of biotin status would be useful. In a study of 10 healthy adults, we recently provided evidence that abnormally increased plasma concentration of 3-hydroxyisovaleryl carnitine (3HIA-carnitine) is a sensitive indicator of marginal biotin deficiency. We sought to determine whether urinary excretion of 3HIA-carnitine (expressed as the ratio to urinary creatinine) significantly increases in marginal biotin deficiency. Marginal, asymptomatic biotin deficiency was induced experimentally in the same 10 healthy adults (8 women) by feeding undenatured egg white with meals for 28 d. Biotin status was repleted by a mixed general diet plus biotin supplementation. Urinary excretion of 3HIA-carnitine was determined by liquid chromatography-tandem MS on d 0, 14, and 28 (depletion) and on d 35 and 50 (repletion). Mean urinary 3HIA-carnitine concentration increased with depletion (P < 0.0001; d 0 vs. 28) and decreased with repletion (P = 0.0002; d 28 vs. 50). Urinary 3HIA-carnitine excretion was greater than the upper limit of normal in 9 of 10 participants by d 14 and decreased to within normal limits by d 50 in all participants. This study provides evidence that urinary excretion of 3HIA-carnitine is an early and sensitive indicator of marginal biotin deficiency. The ease of collection of untimed urine samples and application of a new analytical method with simplified sample preparation suggest that urinary 3HIA-carnitine is likely to be a useful indicator for large population studies.
Introduction
Biotin is a water-soluble vitamin classified in the B complex group. Marginal biotin deficiency is not rare and occurs in clinical circumstances such as pregnancy (1, 2), protein energy malnutrition (3), and long-term therapy with certain anticonvulsants (4–8). Accordingly, early and sensitive indicators of biotin status are likely to be clinically useful (9).
Biotin is a covalently bound prosthetic group for 5 mammalian carboxylases. The 5 carboxylases are propionyl-CoA carboxylase (PCC; EC 6.4.1.3),7 β-methylcrotonyl-CoA carboxylase (MCC; EC 6.4.1.4), pyruvate carboxylase (EC 6.4.1.1), and 2 isoforms of acetyl-CoA carboxylase (EC 6.4.1.2). Each carboxylase catalyzes an essential step in intermediary amino acid metabolism.
In certain genetic inborn errors of metabolism (10–12), reduced activity of MCC impairs catalysis of an essential step in the mitochondrial catabolism of the branched chain amino acid leucine. Metabolic impairment shunts MCC through enoyl-CoA hydratase to produce 3-hydroxyisovaleryl-CoA (13), which can inhibit cellular respiration if further metabolism and detoxification does not occur. It is generally thought that 3-hydroxyisovaleryl-CoA is detoxified by carnitine acetyltransferase, as 3-hydroxyisovaleryl carnitine (3HIA-carnitine) is produced and transported across mitochondrial membranes via carnitine-acylcarnitine translocase (14). As cellular excretion occurs, acyl carnitines are moved across the mitochondrial membrane by carnitine-acylcarnitine translocase and leave the cell, appearing in plasma and urine (15) in increased amounts.
Because nutritional biotin deficiency also causes impairment of MCC activity and increased urinary excretion of 3-hydroxyisovaleric acid (3-HIA) (16, 17) and plasma concentration of 3HIA-carnitine (18), we hypothesize that urinary excretion of 3HIA-carnitine also increases in response to biotin deficiency. Here, we report an assessment of the validity of using urinary excretion of 3HIA-carnitine as an indicator of marginal biotin deficiency.
This paper adds to the data from the published methods paper (19) by more than doubling the number of participants, by quantitating the increase weekly over 4 wk rather than just the beginning and end of depletion, and by adding observations concerning the response of urinary 3HIA-carnitine to repletion.
Participants and Methods
This research, conducted October 1999 through July 2000, was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences. Written informed consent was obtained from each participant as part of the ongoing consent process. Specific details of the design of this study have previously been published (18, 20). Ten participants (8 women) completed the study. Demographic information about the participants has also previously been published (18, 20).
Biotin depletion phase.
To ensure biotin sufficiency on d 0 of the study, participants consumed a 300-μg (1.2 μmol) biotin supplement (10 times the recommended daily allowance) (21) daily for 7 d (loading phase) starting on approximately d −14. This biotin supplement was discontinued at d −7. Participants consumed a multivitamin supplement without biotin from d −7 through d 27.
Participants were admitted on d −1 to the UAMS Clinical Research Center (CRC). The biotin depletion regimen consisted of a dietitian-planned, low-biotin diet plus consumption of an egg white beverage with meals starting on d 0 as previously described (18). Participants spent evenings and nights in the CRC but were free to leave the CRC on weekdays. Lunches with egg white drinks were provided for these meals. Participants continued the depletion regimen through d 27.
Biotin repletion phase.
To investigate biotin repletion through biotin supplementation compared with initial self-selected diet alone, participants were randomized to an “immediate” supplement group (n = 5) and a “late” supplement group (n = 5) after the d-28 blood sample was obtained and the 24-h urine collection was completed. The immediate supplement group resumed a self-selected, mixed general diet on d 28 and consumed a 30-μg biotin supplement daily from d 28 through d 50. The late supplement group resumed a self-selected mixed general diet at d 28 but did not start the biotin supplement until d 35. Participants in this group consumed the biotin supplement from d 35 through d 50.
Blood sample collection.
Peripheral venous blood was collected on d 0, 14, 28, 35, and 50 as previously described (22). Peripheral blood lymphocytes were obtained as previously described (22).
Markers of successful induction of biotin deficiency.
Lymphocyte PCC activities were measured as previously described (22). MCC would be the most metabolically proximate enzyme to our new putative marker. However, activity of the biotin-dependent carboxylases per milligram of tissue protein is decreased in peripheral blood lymphocytes compared with liver (23,24) and measurement of MCC activity using the number of white cells that can be reasonably obtained from adult volunteers is problematic, because absolute MCC activity is ∼5-fold less than absolute PCC activity. These considerations dictated our choice of PCC, which we previously validated as a marker of marginal biotin deficiency (22, 25).
Urinary 3-HIA was quantitated by GC-MS as previously described (16). Concentration of plasma 3HIA-carnitine was measured as previously described (26).
Determination of urinary excretion of 3HIA-carnitine.
Urinary 3HIA-carnitine was diluted and quantitated by liquid chromatography-tandem MS (LC-MS/MS) as previously described (19) using a urine sample preparation method that omits the solid phase extraction procedure that is required in plasma sample preparation (26). Briefly, urine was diluted 30-fold with deionized water. Separation and quantitation was performed as previously described (19, 26). Appropriate analytical calibration and quality control standards were prepared and run with study urine samples as previously reported (19). Urinary excretion of 3HIA-carnitine is reported in mmol/mol creatinine rather than as excretion rates per time, because determination of urinary indicators in untimed urine samples is more practical in population studies. We have previously observed that excretion normalized by creatinine is approximately as sensitive as excretion rate (16). Urinary creatinine was determined by the picric acid method as previously described (27).
Statistical analyses.
Differences in mean lymphocyte PCC activity, urinary 3-HIA, plasma 3HIA-carnitine, and urinary excretion of 3HIA-carnitine during both the depletion and repletion phases of the study were tested for significance by 1-way ANOVA with repeated measures and Dunnett’s post hoc testing, as previously described (18). The variances at the progressive time points tended to decrease with time for biotin indices that decrease and tended to increase with time for biotin indices that increase. To determine whether this could have altered our conclusions concerning the significance of the differences between the means over time, we also performed a log-transformation of the original data sets followed by 1-way ANOVA with repeated measures and Dunnett’s post hoc testing. The heteroskedasticity of the data were removed by the log-transformation; significances of the differences between the means over time did not meaningfully change. For simplicity of presentation, we have presented only the analyses of the original data sets. All tests were performed using either KaleidaGraph (version 4.1.1; Synergy Software) or SAS PROC MIXED (version 9.2; SAS Institute) software.
Relationships between urinary excretion of 3HIA-carnitine and other indicators of biotin status were examined by regression analyses. Specifically, mixed-effect regression models were used to look at the pairwise associations between urinary excretion of 3HIA-carnitine and plasma concentrations of 3HIA-carnitine, urinary excretion of 3HIA-carnitine and urinary excretion of 3-HIA, and urinary excretion of 3HIA-carnitine and lymphocyte PCC activity.
Normal ranges.
The term normal range is used to denote values from biotin-sufficient normal participants in whom biotin sufficiency was established by loading and washout as previously described (18). The normal ranges for lymphocyte PCC activities, urinary excretion of 3-HIA, and plasma concentrations of 3HIA-carnitine were previously described (18).
No prior data were available for the urinary excretion of 3HIA-carnitine from a group of participants subjected to a biotin-loading and washout protocol. Consequently, the range for urinary excretion of 3HIA-carnitine was chosen as the full range of values at d 0 (biotin sufficiency) of the participants in this study.
Results and Discussion
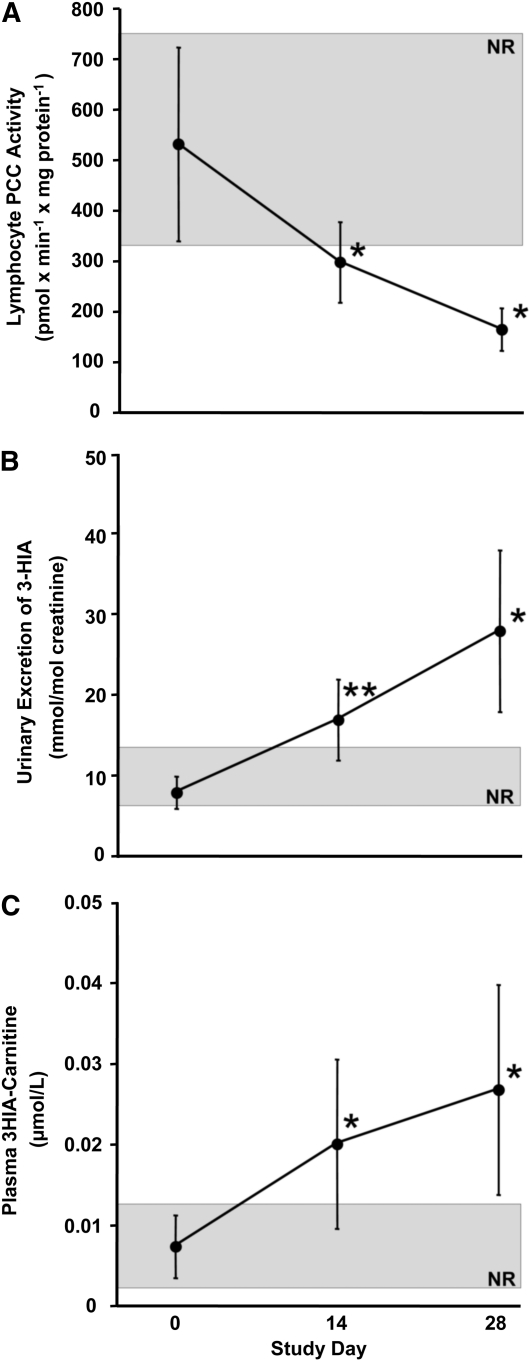
At d −1 of the study, participants were biotin sufficient as judged by 3 established indicators of biotin status (Table 1). Marginal biotin deficiency was successfully induced in the depletion phase of the study as judged by decreased PCC activities (Fig. 1A), increased urinary excretion of 3-HIA (Fig. 1B), and increased plasma concentration of 3HIA-carnitine (Fig. 1C). Diagnostic sensitivity improved over the course of the depletion phase of the study (Table 2).
TABLE 1.
Biotin sufficiency of study participants prior to starting the biotin depletion phase of the study as judged by validated indicators of biotin status1
| Indicator of biotin status | Study participants, n = 10 | Normal, free-living Individuals, n ≥ 20 |
| Lymphocyte PCC, −1 x mg protein−1) | 534 ± 190 | 510 ± 156 |
| Urinary excretion, mmol/mol creatinine | 8.5 ± 2.3 | 8.1 ± 2.7 |
| Plasma 3HIA-carnitine, nmol/L | 7 ± 4 | 10 ± 4 |
Participants tested at d −1 after undergoing biotin loading of 300 g (1.2 μmol)/d for 7 d and washout for 7 d.
FIGURE 1.
Lymphocyte PCC activity (A), urinary excretion of 3-HIA (B), and plasma concentration of 3HIA-carnitine (C) in participants who consumed raw egg whites for 28 d. Values are means ± SD, n = 10. *Different from d 0, P < 0.003; **different from d 0, P < 0.05. The shaded rectangle denotes the normal range (NR).
TABLE 2.
Comparison of diagnostic sensitivity of various indicators of biotin deficiency during the depletion phase of the study1
| Indicator of biotin status | d 14 | d 28 |
| % | ||
| Lymphocyte PCC activity | 70 | 100 |
| Urinary excretion of 3-HIA | 80 | 100 |
| Plasma concentration of 3HIA-carnitine | 70 | 90 |
| Urinary excretion of 3HIA-carnitine | 90 | 90 |
| Urinary excretion of 3HIA-carnitine | 30 | 60 |
Diagnostic sensitivity is defined as the percentage of participants above the normal range at the time of the measurement.
No signs or symptoms of frank biotin deficiency (e.g. hair loss, skin rash, mental changes) were reported by any participant at any time in the study. No signs of frank biotin deficiency were observed by the study physician (D.M.M.) or CRC nurses.
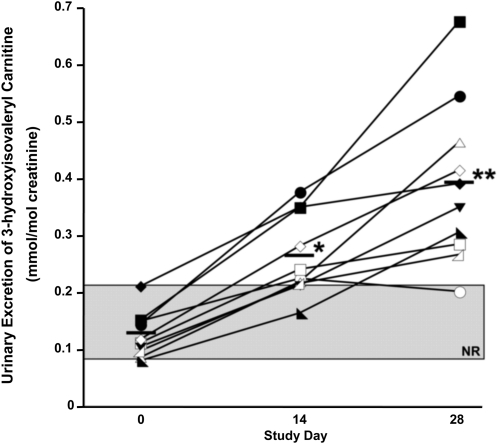
During biotin depletion, mean urinary excretion of 3HIA-carnitine (expressed per mole of urinary creatinine) increased ∼3-fold from d 0 (Fig. 2). Diagnostic sensitivity at d 14 and 28 was similar to other indicators of biotin deficiency (Table 1). When expressed per 24 h, the mean urinary excretion of 3HIA-carnitine also increased ∼3-fold (data not shown), but the diagnostic sensitivity was substantially less (Table 1).
FIGURE 2.
Effects of biotin depletion on urinary excretion of 3HIA-carnitine. Values shown are individual participant data denoted by individual symbols, n = 10. Means shown as solid bars. *Different from d 0, P < 0.001; **different from d 0, P < 0.0001. The shaded rectangle denotes the normal range (NR).
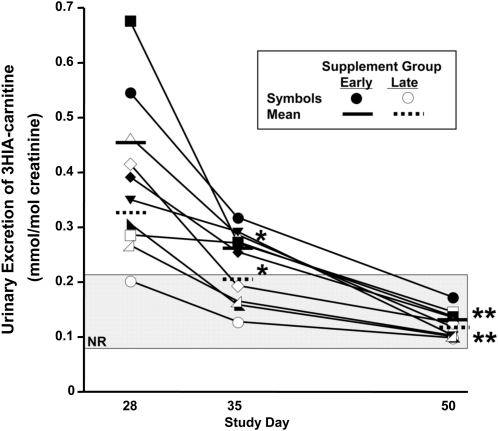
In the immediate supplement group (Fig. 3), urinary excretion of 3HIA-carnitine (mmol/mol creatinine) strikingly decreased by d 35 (P = 0.008). By d 50, urinary excretion of 3HIA-carnitine had returned to within the normal range for all 5 participants (P = 0.0004 by d 50).
FIGURE 3.
Effects of biotin repletion on urinary excretion of 3HIA-carnitine. Values shown are individual participant data denoted by individual symbols, n = 10. *Different from d 28, P < 0.02; **different from d 28, P < 0.001. The shaded rectangle denotes the normal range (NR).
In the late supplement group (Fig. 3), urinary excretion of 3HIA-carnitine decreased (P = 0.02) after 1 wk of consuming a mixed general diet without biotin supplementation. By d 50 (after 15 d of biotin), urinary excretion of 3HIA-carnitine returned to normal in all 5 participants (P = 0.0009).
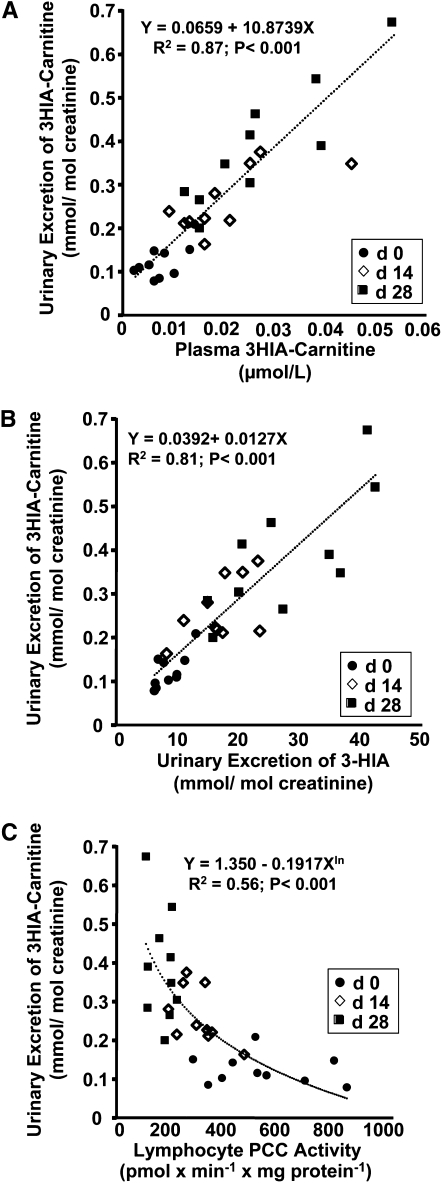
We examined the dependence of urinary excretion of 3HIA-carnitine on the plasma concentration of 3HIA-carnitine (Fig. 4A). By mixed effects regression modeling, we observed a significant linear relationship in which the plasma 3HIA carnitine concentration predicts 87% of the variability of urinary excretion of 3HIA-carnitine. We also examined the relationship of urinary 3HIA-carnitine to urinary 3-HIA (Fig. 4B). We observed a significant linear relationship in which urinary 3HIA-carnitine predicts 81% of the variability in urinary 3-HIA excretion. To investigate the relationship of urinary 3HIA-carnitine to a more direct indicator of tissue biotin status, we plotted urine 3HIA-carnitine excretion against lymphocyte PCC activity for all participants at all time points (Fig. 4C). The observed curvilinear relationship between urinary excretion of 3HIA-carnitine and lymphocyte PCC activity is reasonably well fit by log transformation of urinary 3HIA-carnitine and PCC activity. The regression model indicates that ~56% of the variation in urinary excretion of 3HIA-carnitine can be explained by the equation given in the figure inset.
FIGURE 4.
Correlations between urinary excretion of 3HIA-carnitine (A–C) and plasma concentration of 3HIA-carnitine (A), urinary excretion of 3-HIA (B), and lymphocyte PCC activity (C) during biotin depletion. Values shown are individual participant data from time intervals, n = 10. The formulas for the regressions are provided in each panel, and the goodness of fit is indicated by R2. ur, urine; pl- plasma.
These relationships are all consistent with a model for metabolic perturbation that begins with biotin depletion and the consequent reduction of tissue activities of the biotin-dependent carboxylases followed by accumulation of 3-methylcrotonyl-CoA in the mitochondria and the formation of increased amounts of 3HIA-carnitine and 3-HIA. Likely, 3HIA-carnitine is transported from the mitochondria by carnitine-acylcarnitine translocase.
Properly diagnosing marginal biotin deficiency is potentially important, because recent clinical studies have provided evidence that marginal biotin deficiency is more common than previously thought. For example, our studies provide evidence that marginal, asymptomatic biotin deficiency is a common occurrence in normal human pregnancy (1, 2, 28, 29) and is teratogenic in some mammals (28, 30). Moreover, in both children and adults, marginal biotin deficiency appears to be a frequent consequence of long-term therapy with certain anticonvulsants (4–8). Smoking and malnutrition have also been reported to cause impaired biotin status (3, 31, 32).
This study provides initial evidence that the urinary excretion of 3HIA-carnitine is an early and sensitive indicator of marginal biotin deficiency. Diagnostic sensitivity was similar to that of lymphocyte PCC activity (currently the best validated indicator of marginal biotin status in humans), urinary 3-HIA, and plasma concentration of 3HIA-carnitine. The utility of urinary excretion of and plasma concentration of 3HIA-carnitine in application to population studies is likely to be superior to that of lymphocyte PCC activity, because considerations related to lymphocyte isolation, sample storage, and assay characteristics, as previously reviewed (18), make assaying lymphocyte PCC time-consuming, problematic, and costly for populations studies. In contrast, collection of untimed urine samples and storage at −70°C is technically straightforward. 3HIA-carnitine stored frozen in urine, plasma, or blood on filter paper stored at room temperature is likely stable for months or years (33, 34). Quantitation by LC-MS/MS is both precise and accurate in plasma (26) and urine (19). Quantitation requires small volumes (10 μL) of urine.
The relationships reported here between urinary 3HIA-carnitine and plasma 3HIA-carnitine, urinary 3-HIA, and lymphocyte PCC activity are consistent with current understanding of the metabolic pathogenesis of biotin deficiency. Theoretically, direct measurement of lymphocyte and hepatic MCC activity would provide a measurement of biotin status that is more proximate to the metabolic block that leads to increased synthesis and excretion of 3HIA-carnitine. However, in freshly isolated lymphocytes, MCC activity is substantially lower compared with PCC activity and quantitation with our current assay is problematic.
Acyl CoA compounds are compartmentalized in the mitochondria. Increased concentration of 3-hydroxyisovaleryl CoA leads to a disruption of the esterified-CoA:free-CoA ratio and ultimately to mitochondrial toxicity (10, 35). Detoxification of this metabolic end product is performed by 2 mechanisms (13). The first is the transfer of the 3-hydroxyisovaleryl moiety to carnitine, most likely by the enzyme carnitine acetyltransferase forming 3HIA-carnitine (14). The other is deacylation of the 3-hydroxyisovaleryl-CoA, 3HIA-carnitine, or both to form 3-HIA. Which is the predominant pathway (CoA or carnitine) leading to the formation of 3-HIA is not clear. Both 3-HIA and 3HIA-carnitine are able to cross the outer mitochondrial membrane and leave the cell to be transported in plasma and finally excreted in urine (15, 36).
Direct relationships between urinary 3HIA-carnitine, plasma 3HIA-carnitine, and urinary 3-HIA are not surprising, even at marginal degrees of biotin deficiency. Rapidly rising urinary 3HIA-carnitine in response to decreasing activity of lymphocyte PCC as an index of hepatic MCC activity is reasonable as well and is consistent with the very high urinary excretion of 3HIA-carnitine and 3-HIA observed when MCC activity is reduced to the profound degree seen in inborn errors (e.g. 5% of normal activity).
This study has several important limitations. Confidence in any generalization of conclusions concerning diagnostic sensitivity to larger populations is limited by the small total number of participants studied. Likewise, the number of participants for each of the “normal ranges” is small from a statistical sampling standpoint. The normal range reported in this study was assessed in healthy adults after biotin loading and washout; on d 0, urinary excretion of 3HIA-carnitine ranged from 0.08 to 0.2 mmol/mol creatinine. Even by d 28 when the participants were marginally biotin deficient, urinary excretion of 3HIA-carnitine ranged from only 0.2 to 0.68 mmol/mol creatinine. Whether these ranges are representative of healthy adults who have not undergone biotin loading and washout remains to be established.
We cannot completely exclude the possibility that a decrease in leucine intake contributed to the decrease in urinary 3HIA-carnitine during the repletion period. We controlled the total protein intake from other sources during the study to produce a diet that was approximately normal in protein intake [∼2.0 g/(kg ⋅ d)] with a normal energy from protein (∼14% of energy from protein). However, in the uncontrolled environment of a self-selected diet that was used for the repletion period, a decrease in protein intake and consequently a decrease in 3HIA carnitine excretion are theoretically possible.
Moreover, 3HIA-carnitine data from the study reported here provide no evidence concerning diagnostic specificity in other clinical situations. Whether abnormally increased urinary excretion of 3HIA-carnitine will be observed in other clinical situations in which biotin status is not impaired remains unknown. For example, both heterozygous MCC deficiency and genetic deficiencies of enzymes catalyzing steps in the catabolism of leucine distal to the MCC step might well cause increased plasma concentrations and urinary excretion of 3HIA-carnitine. Moreover, all participants reported here were well nourished, had normal protein intake during the study, and therefore, likely were carnitine sufficient. The effect of mild to moderate carnitine deficiency on plasma concentrations and urinary excretion of 3HIA-carnitine is currently not known.
Contrary to initial expectation, the diagnostic sensitivity of urinary 3HIA-carnitine expressed per 24-h urine is substantially inferior to the diagnostic sensitivity of urinary 3HIA-carnitine expressed per mass of urinary creatinine. We speculate that this loss of diagnostic sensitivity reflects that the 24-h urine collections were not always complete, despite instructing the participants on proper collection of timed urine samples.
In summary, the initial study provides evidence that urinary excretion of 3HIA-carnitine expressed per mole of urinary creatinine appears to be a sensitive, practical indicator of marginal biotin deficiency that has advantages relative to currently validated indices of biotin status, especially PCC activity. The application of the LC-MS/MS method for urine 3HIA-carnitine used here potentially allows for the assessment of marginal biotin deficiency in humans subjected to a variety of phenotypical factors that can cause marginal biotin deficiency. Determination of specificity and robustness awaits application in future larger population studies.
Acknowledgments
We thank Kim Stuckey, Jeanne Poppelreiter, Melain Raguseo, Mike Ruckle, and Teresa Evans for study coordination and laboratory technical work. We also thank Teresa Evans for measurements of urinary excretion of 3-HIA. We thank Suzanne Owen for measurements of urinary excretion of 3HIA-carnitine and Joel Bradley, Ron Trolard, and Rosemarie Bachand of Cambridge Isotope Laboratories for the generous gift of authentic 3HIA-carnitine and deuterated-3HIA-carnitine standards used in this work. D.M.M., J.M., and T.D.H. designed research; S.L.S., T.D.H., A.B., N.I.M., C.L.H., and D.M.M. conducted research; J.M. provided essential equipment for research; S.L.S. and H.J.S. analyzed data; S.L.S. and D.M.M. wrote the paper; and D.M.M. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH R37DDK36823, R37DDK36823-26S1 (D.M.M.); NIH ARRA Supplement RO1DK079892-01A2S1 (D.M.M.); Arkansas Biosciences Institute, Arkansas Tobacco Settlement Proceeds Act of 2000 (D.M.M.); CDC Cooperative Agreement contract 200-2007-21729 and Bioterrorism Cooperative agreement U90/CCU616974-07 (J.H.M.). The project described was supported by award no. UL1RR029884 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the NIH.
Abbreviations used: CRC, Clinical Research Center; 3-HIA, 3-hydroxyisovaleric acid; 3HIA-carnitine, 3-hydroxyisovaleryl carnitine; LC-MS/MS, liquid chromatography-tandem MS; MCC, β-methylcrotonyl-CoA carboxylase; PCC, propionyl-CoA carboxylase.
Literature Cited
- 1.Mock DM, Stadler DD. Conflicting indicators of biotin status from a cross-sectional study of normal pregnancy. J Am Coll Nutr. 1997;16:252–7 [DOI] [PubMed] [Google Scholar]
- 2.Mock DM, Stadler DD, Stratton SL, Mock NI. Biotin status assessed longitudinally in pregnant women. J Nutr. 1997;127:710–6 [DOI] [PubMed] [Google Scholar]
- 3.Velazquez A, Martin-del-Campo C, Baez A, Zamudio S, Quiterio M, Aguilar JL, Perez-Ortiz B, Sanchez-Ardines M, Guzman-Hernandez J, et al. Biotin deficiency in protein-energy malnutrition. Eur J Clin Nutr. 1998;43:169–73 [PubMed] [Google Scholar]
- 4.Krause KH, Berlit P, Bonjour JP. Impaired biotin status in anticonvulsant therapy. Ann Neurol. 1982;12:485–6 [DOI] [PubMed] [Google Scholar]
- 5.Krause KH, Berlit P, Bonjour JP, Schmidt-Gayk H, Schellenberg B, Gillen J. Vitamin status in patients on chronic anticonvulsant therapy. Int J Vitam Nutr Res. 1982;52:375–85 [PubMed] [Google Scholar]
- 6.Krause KH, Kochen W, Berlit P, Bonjour JP. Excretion of organic acids associated with biotin deficiency in chronic anticonvulsant therapy. Int J Vitam Nutr Res. 1984;54:217–22 [PubMed] [Google Scholar]
- 7.Mock DM, Dyken ME. Biotin catabolism is accelerated in adults receiving long-term therapy with anticonvulsants. Neurology. 1997;49:1444–7 [DOI] [PubMed] [Google Scholar]
- 8.Mock DM, Mock NI, Lombard KA, Nelson RP. Disturbances in biotin metabolism in children undergoing long-term anticonvulsant therapy. J Pediatr Gastroenterol Nutr. 1998;26:245–50 [DOI] [PubMed] [Google Scholar]
- 9.Said HM. Biotin bioavailability and estimated average requirement: why bother? Am J Clin Nutr. 1999;69:352–3 [DOI] [PubMed] [Google Scholar]
- 10.Roschinger W, Millington DS, Gage DA, Huang ZH, Iwamoto T, Yano S, Packman S, Johnston K, Berry SA, et al. 3-Hydroxyisovalerylcarnitine in patients with deficiency of 3-methylcrotonyl CoA carboxylase. Clin Chim Acta. 1995;240:35–51 [DOI] [PubMed] [Google Scholar]
- 11.Van Hove JL, Josefsberg S, Freehauf C, Thomas JA, Thuy LP, Barshop BA, Woontner M, Mock DM, Chiang PW, et al. Management of a patient with holocarboxylase synthetase deficiency. Mol Genet Metab. 2008;95:201–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf B. Disorders of biotin metabolism. : Scriver CR, Beaudet AL, Sly WS, Valle D, The metabolic and molecular basis of inherited disease. 8th ed New York: McGraw-Hill, Inc; 2001. p. 3151–77 [Google Scholar]
- 13.Sweetman L, Williams JC. Branched chain organic acidurias. : Scriver CR, Beaudet AL, Sly WS, Valle D, The metabolic and molecular basis of inherited disease. 7th ed New York: McGraw-Hill, Inc; 1995 [Google Scholar]
- 14.Zammit VA, Ramsay RR, Bonomini M, Arduini A. Carnitine, mitochondrial function and therapy. Adv Drug Deliv Rev. 2009;61:1353–62 [DOI] [PubMed] [Google Scholar]
- 15.Pande SV. A mitochondrial carnitine acylcarnitine translocase system. Proc Natl Acad Sci USA. 1975;72:883–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mock NI, Malik MI, Stumbo PJ, Bishop WP, Mock DM. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased status in experimental biotin deficiency. Am J Clin Nutr. 1997;65:951–8 [DOI] [PubMed] [Google Scholar]
- 17.Mock DM, Jackson H, Lankford GL, Mock NI, Weintraub ST. Quantitation of urinary 3-hydroxyisovaleric acid using deuterated 3-hydroxyisovaleric acid as internal standard. Biomed Environ Mass Spectrom. 1989;18:652–6 [DOI] [PubMed] [Google Scholar]
- 18.Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Spencer HJ, Moran JH, Mock DM. Plasma concentration of 3-hydroxyisovaleryl carnitine is an early and sensitive indicator of marginal biotin deficiency in humans. Am J Clin Nutr. 2010;92:1399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horvath TD, Stratton SL, Bogusiewicz A, Owen SO, Mock DM, Moran JH. Quantitative measurement of urinary excretion of 3-hydroxyisovaleryl carnitine by LC-MS/MS as an indicator of biotin status in humans. Anal Chem. 2010;82:9543–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mock DM, Henrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am J Clin Nutr. 2002;76:1061–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NRC Dietary reference intakes tables: the complete set (PDF). In: Food and Nutrition Board Institute of Medicine, editor Dietary reference intakes. Washington, DC: The National Academies; 2005 [Google Scholar]
- 22.Mock D, Henrich C, Carnell N, Mock N, Swift L. Lymphocyte propionyl-CoA carboxylase and accumulation of odd-chain fatty acid in plasma and erythrocytes are useful indicators of marginal biotin deficiency. J Nutr Biochem. 2002;13:462. [DOI] [PubMed] [Google Scholar]
- 23.Mock DM, Mock NI. Lymphocyte propionyl-CoA carboxylase activity is an early and sensitive indicator of biotin deficiency in rats, but urinary excretion of 3-hydroxyisopropionic acid is not. J Nutr. 2002;132:21945–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sealey WM, Stratton SL, Hansen DK, Mock DM. Marginal maternal biotin deficiency in CD-1 mice reduces fetal mass of biotin-dependent carboxylases. J Nutr. 2005;135:973–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratton SL, Bogusiewicz A, Mock MM, Mock NI, Wells AM, Mock DM. Lymphocyte propionyl-CoA carboxylase and its activation by biotin are sensitive indicators of marginal biotin deficiency in humans. Am J Clin Nutr. 2006;84:384–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath TD, Stratton SL, Bogusiewicz A, Pack L, Moran JM, Mock DM. Quantitative measurement of plasma 3-hydroxyisovaleryl carnitine by LC-MS/MS as a novel biomarker of biotin status in humans. Anal Chem. 2010;82:4140–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mock DM. Rayon balls and disposable-diaper material selectively adsorb creatinine. Am J Clin Nutr. 1992;55:326–30 [DOI] [PubMed] [Google Scholar]
- 28.Mock DM. Marginal biotin deficiency is common in normal human pregnancy and is highly teratogenic in the mouse. J Nutr. 2009;139:154–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stratton SL, Matthews NI, Bogusiewicz A, Zhu S, Mock DM. Evidence that biotin status is impaired in human pregnancy. FASEB J. 2009;23:103.6 [Google Scholar]
- 30.Zempleni J, Mock DM. Marginal biotin deficiency is teratogenic. Proc Soc Exp Biol Med. 2000;223:14–21 [DOI] [PubMed] [Google Scholar]
- 31.Velazquez A, Teran M, Baez A, Gutierrez J, Rodriguez R. Biotin supplementation affects lymphocyte carboxylases and plasma biotin in severe protein-energy malnutrition. Am J Clin Nutr. 1995;61:385–91 [DOI] [PubMed] [Google Scholar]
- 32.Sealey WM, Teague AM, Stratton SL, Mock DM. Smoking accelerates biotin catabolism in women. Am J Clin Nutr. 2004;80:932–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strnadova KA, Holub M, Muhl A, Heinze G, Ratschmann R, Mascher H, Stockler-Ipsiroglu S, Waldhauser F, Votava F, et al. Long-term stability of amino acids and acylcarnitines in dried blood spots. Clin Chem. 2007;53:717–22 [DOI] [PubMed] [Google Scholar]
- 34.Shigematsu Y, Bykov IL, Liu YY, Nakai A, Kikawa Y, Sudo M, Fujioka M. Acylcarnitine profile in tissues and body fluids of biotin-deficient rats with and without L-carnitine supplementation. J Inherit Metab Dis. 1994;17:678–90 [DOI] [PubMed] [Google Scholar]
- 35.Pasquali M, Monsen G, Richardson L, Alston M, Longo N. Biochemical findings in common inborn errors of metabolism. Am J Med Genet C Semin Med Genet. 2006;142C:64–76 [DOI] [PubMed] [Google Scholar]
- 36.Mock DM, Quirk JG, Mock NI. Marginal biotin deficiency during normal pregnancy. Am J Clin Nutr. 2002;75:295–9 [DOI] [PMC free article] [PubMed] [Google Scholar]