Abstract
Male infertility accounts for ~40% of cases of failure to conceive. Testes have a strict zinc (Zn) requirement and severe Zn deficiency compromises spermatogenesis, sperm viability, and motility, compromising fertility in men. Despite the high prevalence of marginal Zn deficiency in humans, less emphasis has been placed on understanding the consequences on male reproduction. Swiss Webster mice were used to visualize Zip protein expression during spermatogenesis using immunohistochemistry. Data suggest Zip5 imports Zn into Sertoli cells and spermatocytes, augmented by Zip10 (primary spermatocytes) and Zip8 (secondary spermatocytes). Zip6, 8, and 10 expression was retained in round spermatids, although Zip8 and Zip10 expression disappears during spermatid maturation. Zip1 and Zip6 expression was detected in mature, elongated spermatids. Zip14 was detected in undifferentiated spermatogonia and Leydig cells. Mice fed diets (n = 10/group) reduced in Zn concentration [marginal-Zn diet (MZD), 10 mg Zn/kg; low-Zn diet (ZD), 7 mg Zn/kg] for 30 d had >35% lower liver Zn concentrations than mice fed the control diet (C; 30 mg Zn/kg) (P < 0.05). Plasma Zn and testosterone concentrations and the testes Zn concentration and weight were not significantly lower than in controls. Plasma Zn was greater in the ZD group than in the C and MZD groups. Mice fed ZD had a reduced number of terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells (~50%; P < 0.05), compromised seminiferous tubule structure, and reduced Zip10 and Zip6 abundance (>50%; P < 0.5) compared with mice fed C. Our data provide compelling evidence that reduced Zn intake may be associated with infertility in men, perhaps independent of decreased levels of circulating Zn or testosterone, which warrants further investigation in human populations.
Introduction
Zinc (Zn) is required for well over 300 different biological processes, including DNA transcription, protein translation, cell proliferation and differentiation, and apoptosis. Prasad et al. (1) first reported severe Zn deficiency in humans from the Middle East in the early 1960s that resulted from low intake of bioavailable Zn (2). Primary clinical consequences noted in severely Zn-deficient men included delayed sexual maturation and infertility. These observations seem reasonable given the high Zn uptake required by the testes, prostate, and epididymis (3) to maintain optimal function. Zn depletion studies in men (2.7–5 mg Zn/d) result in oligospermia, which coincides with poor Leydig cell function and lower testosterone concentration and can be reversed by Zn supplementation (4). Numerous studies have explored effects of severe Zn deficiency on male reproductive function in animal models, including rodents (5–9), rabbits (10, 11), and bulls (12). It is important to note that most of these studies utilized a diet severely deficient in Zn (<0.5 μg Zn/g diet), which results in food cycling and requires a pair-fed control group for statistical comparison. In fact, Mason et al. (13) noted that the Zn-deficient treatment used in their study was so severe that 20% of the experimental rats died. Although effects of severe Zn deficiency on male fertility may be profound, the relevance of these observations to male reproduction in most human populations may not be of clinical importance to the general population. In contrast, recent data from Song et al. (9) suggested that reproductive function in male rats may be sensitive to a more marginal Zn deficiency (~6 μg Zn/g diet), which reduces prostate Zn concentration and increases oxidative damage (8). These observations may have important implications in human fertility as national food balance data currently estimates that the percentage of individuals at risk of inadequate Zn intake in North Africa, the Eastern Mediterranean, United States, and Canada at ~10% and in Southeast Asia, the prevalence of Zn deficiency is ~33% (14). Thus, although severe Zn deficiency does manifest in a small percentage of individuals, ∼20% of the world’s population is estimated to be at risk for marginal Zn intake.
Zn plays a key role in spermatogenesis from several perspectives. Zn is located primarily in the Leydig cells, the late type B spermatogonia, and the spermatids. Zn is essential for the production and secretion of testosterone from the Leydig cells (15, 16), which, in conjunction with follicle stimulating hormone, is a key regulator of spermatogenesis (17). As a result, Zn deficiency has been associated with reduced function of the luteinizing hormone receptor (18), reduced steroid synthesis (16), and Leydig cell damage (19) emanating from oxidative stress (6, 7). Zn is quite high in the developing spermatocytes due to the need for Zn during DNA condensation and meiosis (20). Zn also facilitates the packaging of DNA in spermatids (21) and is purported to prolong spermatozoa life span once released postejaculation (22–25). Despite the clear reliance on Zn for optimal spermatogenesis, testicular function, and fertility, there is little understanding of how the testes acquire Zn from circulation, how Zn is transported through the testes into the maturing gametes during spermatogenesis, or how marginal Zn deficiency affects this process. Zn import is regulated through the Zip family of mammalian Zn transporters (Zip1-Zip14), which were identified as a result of gene sequence homology with known Zn transporters (ZRT1, IRTl-like p rotein) found in plants and yeast. Zip proteins facilitate Zn uptake into the cytoplasm, either into the cell from the extracellular milieu or into the cytoplasm across an intracellular membrane. To our knowledge, expression of only 2 Zn importers, Zip8 and Zip14, has been documented in testes (26). However, cell-specific localization and contribution of Zip8 and Zip14 to overall testicular Zn metabolism and spermatogenesis have not been elucidated.
Our understanding of how testes regulate Zn transfer to optimize male fertility is clearly limited. The first objective of this study was to characterize mechanisms through which specific cell types in the testes acquire Zn. Secondly, given the fact that marginal-to-low Zn intake is prevalent in many human populations, we explored effects of marginal-to-low Zn intake on the management of testicular Zn metabolism and function using Swiss Webster mice.
Materials and Methods
Mice.
This study was approved by the IACUC Committee at The Pennsylvania State University, which is accredited by the American Association for the Accreditation of Laboratory Animal Care. Outbred, male, Swiss Webster mice were obtained commercially (Charles River) and housed individually on wire-bottom racks in polycarbonate cages. Mice (n = 10 mice/diet) were fed commercially available purified diets (MP Biomedicals) for 30 d based on AIN93, which were identical except for the Zn concentration: control diet (C4; 30 mg Zn/kg diet), marginal-Zn diet (MZD; 10 mg Zn/kg diet), or a low-Zn diet (ZD; 7 mg Zn/kg diet). Food intake and body weight were measured 3 times/wk. After 30 d, mice were feed deprived for 3 h and killed by CO2 asphyxiation between 0800 and 1200 h. Blood was collected by cardiac puncture into heparinized tubes and plasma was separated by centrifugation at 1000 × g for 10 min at 4°C. Liver was removed and snap-frozen in liquid nitrogen. Testes were dissected, weighed, and snap-frozen in liquid nitrogen, stored in RNA later, or fixed in Bouin’s fixative.
Tissue Zn analysis.
Plasma was diluted in 0.1 mol/L nitric acid and tissues were wet-ashed in concentrated nitric acid. The Zn concentration was determined by atomic absorption spectrophotometry as previously described (27).
Real time relative RT-PCR.
Total RNA was isolated from homogenized testes following the manufacturer’s instructions (Life Technologies). RNA integrity was assessed following electrophoresis through agarose (2%) followed by ethidium bromide staining. cDNA synthesis, real-time relative PCR, and data analysis were preformed as previously described (28). We focused on Zip1, Zip5, Zip6, Zip8, Zip10, and Zip14, because these Zip proteins have been localized to the cell membrane of other cell types (29, 30). Gene-specific primers were designed using Primer Express software (Perkin Elmer Applied Biosystems) to span introns to avoid coamplification of genomic DNA (Supplemental Table 1). Relative mRNA expression was determined by real-time relative RT-PCR by using the SYBR Green detection system (Perkin Elmer Applied Biosystems). Data are expressed as the fold relative to value in C mice.
Immunoblot analysis.
One whole testis was homogenized in HEPES-EDTA buffer containing protease inhibitors and total membrane fractions were isolated and proteins were immunoblotted as previously described (28). Primary antibodies included: anti-rabbit Zip5 (1:1000 Aviva Systems Biology); anti-rabbit Zip6 (1:1000; generous gift from Dr. Liping Huang, University of California Davis); anti-rabbit Zip8 (1:1000; generous gift from Dr. Mitchell Knutson, University of Florida Gainesville); anti-rabbit Zip10 (1:1000 ProSci); anti-rabbit Zip14 (1:1000 Sigma-Aldrich); and anti-chicken Zip1 (1:4000). Proteins were detected with donkey, anti-rabbit IgG or anti-chicken IgY (1:30,000) conjugated to HRP (Amersham Pharmacia Biotech), visualized with Super Signal Femto Chemiluminescent Detection System (Pierce) and exposed to autoradiography film. Relative band density was quantified using the Carestream Gel Logic 212 Pro.
Histology.
Whole, fixed testes were serially dehydrated in ethanol, embedded in paraffin, and sectioned (5 μm) onto positively charged glass slides. Sections were stained with hematoxylin and eosin to evaluate testes morphology. Zn transporter localization was examined by immunohistochemistry using the ABC Vectastain kit (Vector). Primary antibody concentrations were as follows: Zip1 (1:750), Zip5 (1:300), Zip6 (1:750), Zip8 (1:500), Zip10 (1:1500), and Zip14 (1:1500). Following immunostaining, tissue sections were counterstained with toluene blue.
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay.
Apoptosis was identified using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stain (Trevigen). Following immunostaining, sections were counterstained with toluene blue. The number of TUNEL-positive apoptotic cells was visually quantified under 10× magnification. Data are the mean number of apoptotic cells/section from 3 mice/group.
Testosterone assay.
The plasma testosterone concentration was measured by using ELISA (R&D Systems Testosterone Parameter Assay kit).
Statistical analysis.
Data are expressed as mean ± SD. Data were analyzed by 1-way ANOVA and post-tested using Tukey’s test or t test as noted. Statistical analysis was conducted using GraphPad Prism 3.02 and significance was accepted at P < 0.05.
Results
Plasma and liver Zn concentrations.
Reduced Zn intake did not affect total food intake or body weight gain (data not shown). In addition, testes weight and testes and plasma Zn concentrations of mice fed MZD and ZD were not significantly lower than in mice fed C (Table 1). Plasma testosterone concentrations did not differ among the groups, but there was a large degree of variability in all groups. It was interesting to note that the plasma Zn concentration in the mice fed ZD was significantly higher compared with mice fed C and MZD. Zn deficiency was documented by the significantly lower liver Zn concentration in mice fed both MZD and ZD compared with mice fed C (Table 1).
TABLE 1.
Plasma, liver, and testes Zn concentrations, testes weight, and plasma testosterone in mice fed a C, MZD, or ZD diet for 30 d1
| Control | MZD | ZD | |
| Plasma Zn, μmol/L | 3.9 ± 0.4b | 3.4 ± 0.3b | 5.1 ± 0.5a |
| Liver Zn, μmol/g | 0.44 ± 0.05a | 0.29 ± 0.03b | 0.24 ± 0.02b |
| Testes Zn, nmol/g | 0.24 ± 0.02 | 0.27 ± 0.03 | 0.26 ± 0.02 |
| Testes weight, g | 0.54 ± 0.08 | 0.56 ± 0.03 | 0.55 ± 0.07 |
| Plasma testosterone, nmol/L | 51.0 ± 62.0 | 33.6 ± 42.0 | 80.1 ± 64.8 |
Values are means ± SD, = 7–10. Means in a row with superscripts without a common letter differ, P < 0.05.
Testis morphology.
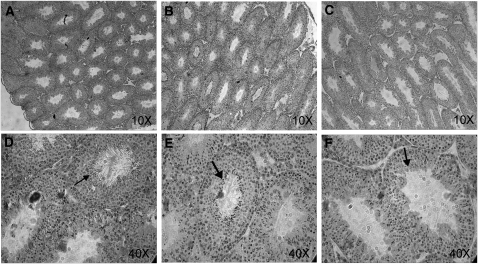
Despite the lack of significant differences in testes weight or total Zn concentration, testes morphology was compromised in mice fed ZD. We first noted that the overall density of seminiferous tubules appeared to be less in mice fed ZD compared with mice fed C (Fig. 1) in tissue sections stained with hematoxylin and eosin. Although it was not possible to quantitatively determine the number of seminiferous tubules, the seminiferous tubules appeared less histologically dense in the mice fed ZD compared with mice fed MZD or C. The seminiferous tubules from ZD mice also featured an irregular germinal epithelium. No noticeable effect was observed in mice fed MZD. We hypothesized that a reduced number of seminiferous tubules and irregular germinal epithelium in mice fed ZD would result from enhanced apoptosis. However, TUNEL staining was primarily detected in the Leydig cells and not the cells within the seminiferous tubules (Supplemental Fig. 1). There were fewer TUNEL-positive apoptotic cells in mice fed ZD (13 ± 5 apoptotic cells/field) compared with mice fed C (30 ± 4 apoptotic cells/field) (P < 0.05), but the difference was not significant in those fed MZD (16 ± 9 apoptotic cells/field). Mice fed C also appeared to have more seminiferous tubules in which the entire population of spermatids stained positive for TUNEL compared with mice fed MZD or ZD. We presume this is not an artifact as TUNEL staining was conducted on all tissues at the same time under identical conditions. Two further observations were that mice fed ZD appeared to have fewer sperm compared with mice fed C and contained entire seminiferous tubules in which all sperm appeared to be tailless (Fig. 1). Reduced TUNEL staining of spermatids within an entire seminiferous tubule may reflect the fewer number of spermatids in mice fed ZD.
FIGURE 1.
Testicular morphology in mice fed C (A,D), MZD (B,E), or ZD (C,F) diet for 30 d. Representative sections of testes (5 μm) were stained with hematoxylin and eosin; 10× or 40× magnification is shown. Arrows illustrate mature spermatids, which are less evident with decreased dietary Zn. Note the disorganization of the seminiferous tubule structure, particularly in the mice fed the ZD diet (F).
Development of a model of testicular Zn import.
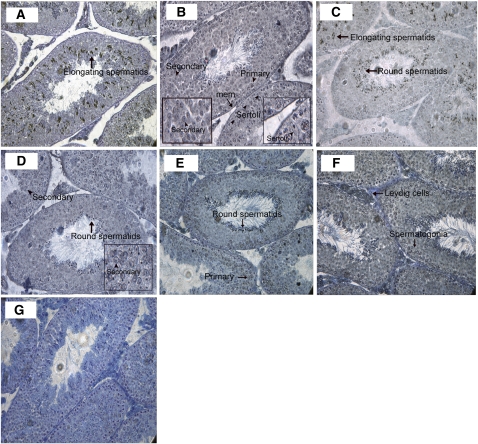
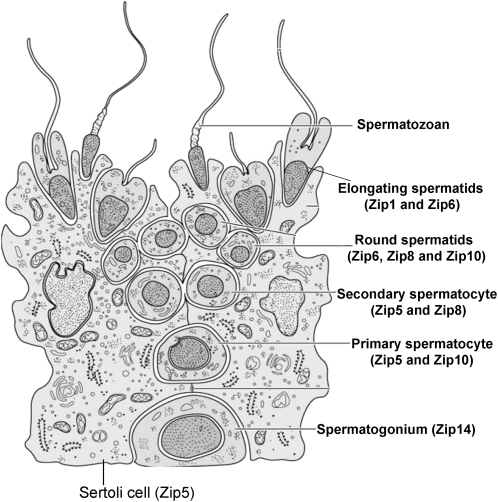
To our knowledge, expression of Zip proteins in testes has not been documented. We first aimed to localize expression of Zn importers to specific cell types within the seminiferous tubule using immunohistochemistry in control mice (Fig. 2). Our data clearly indicated that expression of specific Zn importers is restricted to specific cell-types within the seminiferous tubule. Zip1 expression was restricted to elongating spermatids. We detected Zip5 on cell membrane of Sertoli cells and both primary and secondary spermatocytes. Zip6 was localized to the round and elongating spermatids. Zip8 staining was particularly dominant on the cell membrane of secondary spermatocytes as well as in the round spermatids. Zip10 was detected on the cell membrane of primary spermatocytes and round spermatids. Within the seminiferous tubule, Zip14 expression appeared restricted to the spermatogonia but was also clearly detected on the cell membrane of the Leydig cells. Identification of the localization of Zn importers in specific cell-types permitted the development of a working model to better understand the transfer of Zn through the seminiferous tubule during spermatogenesis (Fig. 3).
FIGURE 2.
Localization of Zn importers in distinct cell types within the testes of mice fed the C diet for 30 d. Representative sections of testes (5 μm) are shown. Zn importers (Zip1, Zip5, Zip6, Zip8, Zip10, and Zip4) were detected by immunohistochemistry and visualized with DAB staining (black). Sections were counter-stained with toluene blue, magnification 40×. Images illustrated that Zip1 expression (A) was restricted to elongating (mature) spermatids (arrow). Zip5 expression (B) was detected on the membrane (mem) encapsulating the seminiferous tubule (arrow), Sertoli cells (arrowhead and inset), and both primary (arrowhead) and secondary (arrowhead and inset) spermatocytes. Zip6 expression (C) was detected in immature, round (arrow), and elongating (arrow) spermatids; Zip8 (D) was detected in secondary spermatocytes (arrowhead and inset) and in round spermatids (arrow). Zip10 (E) was detected in primary spermatocytes (arrow) and in round spermatids (arrow). Zip14 (F) was detected in spermatogonia (arrow) and on the cell membrane of Leydig cells (arrow). (G) Negative control.
FIGURE 3.
Model of Zn import into developing germ cells during spermatogenesis.
Zn importer expression.
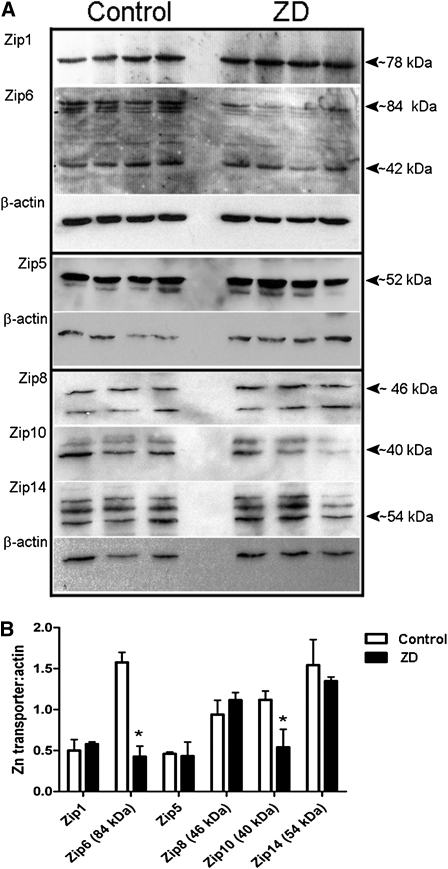
As noted above, although the total Zn concentration of the testes was not significantly affected, seminiferous tubule morphology was disrupted in the mice fed ZD. We determined whether Zip mRNA expression was altered, but it did not differ from controls in mice fed ZD (data not shown). However, protein abundance of Zip6 and Zip10 was reduced by >50% in mice fed ZD compared with those fed C (P < 0.05) (Fig. 4A,B).
FIGURE 4.
Protein abundance of Zn transporters in testes of mice fed the ZN or C diet for 30 d. (A) Representative immunoblots of Zip1, Zip5, Zip6, Zip8, Zip10, and Zip14 in total membrane fractions isolated from testes (50 μg protein/lane, n = 3–4 individual testes) from mice fed a C or ZD diet. Two proteins immunoreacted with Zip6 antibody (Zip6a, ~84 kDa and Zip6b, ~42 kDa); 2 proteins immunoreacted with Zip10 antibody (~40 and 44 kDa); 3 proteins immunoreacted with Zip14 antibody (~54, 56, and 58 kDa). Immunoblots were stripped and reprobed for β-actin as a loading control. (B) Quantitative analysis of band density. Data are means, n = 3–4. *Different from C, P < 0.05.
Discussion
Infertility affects ~9% of couples and paternal infertility is estimated to account for 30–40% of cases of failure to conceive (31). Assisted reproductive technologies, while increasingly common, are incredibly expensive and in the United States are estimated to require ~50% of a couple’s disposable income (31). Therefore, understanding the biological explanations for male sub- or infertility and developing potential interventions has important health and economic implications. Spermatogenesis is under exquisite control to synchronize germ cell maturation. It has been understood for some time that severe Zn deficiency compromises spermatogenesis, sperm viability, and motility, resulting in reduced fertility in men, which is associated with reduced angiotensin converting enzyme activity (32) and oxidative damage (33). We first employed immunohistochemistry to generate a model to visualize how the testes transfer Zn to different cell types during spermatogenesis. The primary Zn importer into testes appears to be Zip5, which has previously been detected in small intestine and villous yolk sac (34). To our knowledge, this is the first documentation of testicular expression of Zip5 and definitive localization to the plasma membrane of Sertoli cells suggests it plays a role in Zn acquisition from circulation. Expression of the Zn exporter ZnT1 has also been detected specifically in Sertoli cells (35,36) where it is predicted to play a key role in Zn export from Sertoli cells to the developing germ cells.
Our data suggest that Zn is imported into spermatogonium (a primitive undifferentiated germ cell) by Zip14. Three Zip14 isoforms appear to be expressed in testes, which may reflect differences in alternatively spliced variants and/or glycosylation patterns (37). The localization of Zip14 to the Leydig cell membrane suggests it plays a role in providing Zn for testosterone production in this cell type, which is critical for hormonal regulation of spermatogenesis. It is currently unknown if each isoform is expressed in a specific cell type. Recent studies indicate that Zip14 transports iron, manganese, and cadmium as well as Zn (26). Cadmium exposure has been associated with infertility (38) and reduced testosterone (39). We speculate that the ability to reverse these effects with Zn supplementation may partially reflect competition between Zn and cadmium for import into Leydig cells through Zip14.
Zip5 localization suggests that Zip5 also plays a role in Zn import into primary and secondary spermatocytes. Our data suggest that Zn accumulation in spermatocytes (40) may be assisted through the developmental regulation of Zip8 and Zip10; however, it is currently not understood why the need for stage-specific regulation of Zip8 and Zip10 during spermatocyte maturation may be required. Spermatid maturation reflects nuclear condensation, which changes the shape of the nuclei (spermatids) followed by the loss of cytoplasm (spermatozoa). An elegant study by Kehr et al. (41) using X-ray fluorescent microscopy illustrated that Zn concentration remains high in these late germ cells and is particularly enriched in the nucleus of spermatids and both the nucleus and tail of early sperm (41), which is required for chromatin condensation and motility (42). Our data indicated that Zip6, Zip8, and Zip10 may play key roles in this Zn accumulation, because they are all specifically expressed in round spermatids. During maturation, Zip6 expression was maintained, expression of Zip8 and Zip10 was lost, and Zip1, which appears exclusively restricted to elongated spermatids, was noted. This may reflect the development/loss of specific Zn-requiring processes and/or the elimination of specific cell membrane domains. For example, Zip8 and Zip10 may be required by spermatocytes and immature spermatids for mechanisms that utilize Zn for nuclear condensation and thus are no longer necessary once that process is complete. Moreover, one could envision the loss of cell membrane during the final stage of spermatozoan maturation might eliminate expression of Zip8 and Zip10 on that membrane domain. In contrast, Zip1, which appears to be exclusively expressed in elongating spermatids, and/or Zip6 may be required for different stage-specific processes such as facilitating Zn import from prostatic fluid for viability and/or motility once spermatozoa are ejaculated. Clearly, more studies are needed to understand the stage-specific regulation of Zn importers during spermatogenesis.
Studies in humans and various models have determined that severe Zn deficiency negatively affects male reproduction by inducing testicular atrophy and spermatocyte loss, increasing spermatogenic cell apoptosis, and decreasing seminiferous tubule diameter. A recent report indicates that severe Zn deficiency in rats also alters the unique bilipid composition of the testes (5), which may compromise sperm viability. In fact, men who were placed on a Zn-restricted diet for 24–40 wk experienced oligospermia (<20 million sperm in 1 mL ejaculate) (6). However, food intake data suggest that marginal Zn deficiency is much more prevalent than severe Zn deficiency and may contribute to sub- and infertility (22). A recent report indicated that poor Zn nutrition and smoking are important risk factors for low sperm quality and idiopathic male infertility (24), perhaps from impaired antioxidant defense and compromised DNA repair mechanisms (6). Although fertility per se was not assessed in our current study, we observed subtle effects of reduced Zn intake on testicular Zn metabolism. Reduced Zn intake did not affect testes weight or total Zn concentration, consistent with a previous report of rats with marginal Zn intake (9 mg Zn/kg diet for 3 wk) (5). However, unlike this previous report (5), our data suggest that seminal tubule structure and spermatogenesis were impaired in mice fed our ZD, as has been observed in studies of more severe Zn deficiency. These differences may reflect species variation or our dietary treatment (7 mg Zn/kg for 30 d). It is important to note that direct indicators of sperm viability or motility were not assessed in our study and require further investigation. Importantly, these impairments in spermatogenesis do not appear to be associated with reduced circulating levels of testosterone or apparent differences in the number of Leydig cells as has been documented in severe Zn deficiency (43). These data must be interpreted with caution, because precise markers of Leydig cell number and function were not assessed. Testicular impairments may be further exacerbated by prostate damage and reduced prostate Zn concentration (8) during marginal Zn deficiency. Together, these studies suggest that marginal Zn intake in men may compromise fertility long before effects of Zn deficiency such as reduced plasma Zn or testosterone concentrations are evident.
Our data suggest that Zn redistribution within the testes may have compromised spermatogenesis. Maintenance of Zip5 expression during moderate Zn deficiency may have maintained Zn import into testes from circulation; however, changes in Zip5 localization or differential loss or gain in specific cell types in response to Zn intake cannot be disregarded. In addition, Zn metabolism in specific cell types within the testes may have subtly altered oxidative stress within specific germ cells within the testes (6). For example, Zip6 and Zip10 abundance were significantly reduced by >50%, suggesting that Zn import into primary spermatocytes (through Zip10) and spermatids (through both Zip6 and Zip10) may have been compromised. We speculate that changes in hormones (44) and cytokines (45) may have contributed to these observations.
In summary, elucidation of the localization of Zn import proteins in specific cell types within the testes has allowed us to develop a model and explore how this complex reproductive tissue transfers Zn during spermatogenesis across the tightest barrier (blood-testes) in biology. Although further studies are needed to develop a complete understanding of Zn transfer from circulation to provide for spermatozoa maturation, our observational study provides a platform to understand how Zn is transferred from Sertoli cells into the developing gametes during spermatogenesis. Moreover, our data, combined with data from Song et al. (8, 9), provide compelling evidence that marginal Zn deficiency may be associated with compromised testes function and perhaps infertility in men.
Supplementary Material
Acknowledgments
We thank Farnaz Foolad, Amy Kaucher, Suzie Reding, and Becky Crouse for technical expertise. T.C., N.H.M., and S.L.K. designed the research; T.C. and N.H.M. conducted the research; T.C. and S.L.K. analyzed the data; T.C., N.H.M., and S.L.K. wrote the paper; and S.L.K. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by interdepartmental funds and the NIH (HD058614 to S.L.K.).
Supplemental Figure 1 and Supplemental Table 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: C, control; MZD, marginal-zinc diet; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; ZD, low-zinc diet.
Literature Cited
- 1.Prasad AS, Miale A, Jr, Farid Z, Sandstead HH, Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia, hepatosplenomegaly, dwarfism, and hypognadism. J Lab Clin Med. 1963;61:537–49 [PubMed] [Google Scholar]
- 2.Prasad AS, Schulert AR, Miale A, Jr, Farid Z, Sandstead HH. Zinc and iron deficiencies in male subjects with dwarfism and hypogonadism but without ancylostomiasis, schistosomiasis or severe anemia. Am J Clin Nutr. 1963;12:437–44 [DOI] [PubMed] [Google Scholar]
- 3.Vera-Gil A, Castejón MP, Barral M, Recreo P, Castejón MP. Location of Zn in the testicle of the rat. Acta Anat (Basel). 1991;141:70–3 [DOI] [PubMed] [Google Scholar]
- 4.Abbasi AA, Prasad AS, Rabbani PR. Experimental zinc deficiency in man: effect on spermatogenesis. Trans Assoc Am Physicians. 1979;92:292–302 [PubMed] [Google Scholar]
- 5.Merrells KJ, Blewett H, Jamieson JA, Taylor CG, Suh M. Relationship between abnormal sperm morphology induced by dietary zinc deficiency and lipid composition in testes of growing rats. Br J Nutr. 2009;102:226–32 [DOI] [PubMed] [Google Scholar]
- 6.Oteiza PL, Olin KL, Fraga CG, Keen CL. Oxidant defense systems in testes from zinc-deficient rats. Proc Soc Exp Biol Med. 1996;213:85–91 [DOI] [PubMed] [Google Scholar]
- 7.Oteiza PI, Clegg MS, Keen CL. Short-term zinc deficiency affects nuclear factor-{{kappa}}B nuclear binding activity in rat testes. J Nutr. 2001;131:21–6 [DOI] [PubMed] [Google Scholar]
- 8.Song Y, Elias V, Loban A, Scrimgeour AG, Ho E. Marginal zinc deficiency increases oxidative DNA damage in the prostate after chronic exercise. Free Radic Biol Med. 2010;48:82–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y, Elias V, Wong C, Scrimgeour A, Ho E. Zinc transporter expression profiles in the rat prostate following alterations in dietary zinc. Biometals. 2010;23:51–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitt JA, Zoellner MJ, Carney EW. Developmental toxicity of dietary zinc deficiency in New Zealand white rabbits. Reprod Toxicol. 1997;11:781–9 [DOI] [PubMed] [Google Scholar]
- 11.Eltohamy MM, Younis M. Response of testes, epididymis, and seminal vesicle of rabbits to zinc deficiency. Arch Exp Veterinarmed. 1991;45:155–60 [PubMed] [Google Scholar]
- 12.Pitts WJ, Miller WJ, Fosgate OT, Morton JD, Clifton CM. Effect of zinc deficiency and restricted feeding from two to five months of age on reproduction in Holstein bulls. J Dairy Sci. 1966;49:995–1000 [DOI] [PubMed] [Google Scholar]
- 13.Mason KE, Burns WA, Smith JC., Jr Testicular damage associated with zinc deficiency in pre- and postpubertal rats: response to zinc repletion. J Nutr. 1982;112:1019–28 [DOI] [PubMed] [Google Scholar]
- 14.Wuehler SE, Peerson JM, Brown KH. Use of national food balance data to estimate the adequacy of zinc in national food supplies: methodology and regional estimates. Public Health Nutr. 2005;8:812–9 [DOI] [PubMed] [Google Scholar]
- 15.McClain CJ, Gavaler JS, Van Thiel DH. Hypogonadism in the zinc-deficient rat: localization of the functional abnormalities. J Lab Clin Med. 1984;104:1007–15 [PubMed] [Google Scholar]
- 16.Prasad AS. Clinical and biochemical manifestation zinc deficiency in human subjects. J Pharmacol. 1985;16:344–52 [PubMed] [Google Scholar]
- 17.Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol. 2010;205:117–31 [DOI] [PubMed] [Google Scholar]
- 18.Kellokumpu S, Rajaniemi H. Effect of zinc on the uptake of human chorionic gonadotropin (hCG) in rat testis and testosterone response in vivo. Biol Reprod. 1981;24:298–305 [DOI] [PubMed] [Google Scholar]
- 19.Hesketh JE. Effects of dietary zinc deficiency on Leydig cell ultrastructure in the boar. J Comp Pathol. 1982;92:239–47 [DOI] [PubMed] [Google Scholar]
- 20.Kundu TK, Rao MRS. Zinc dependent recognition of a human CpG island sequence by the mammalian spermatidal protein TP2. Biochemistry. 1996;35:15626–32 [DOI] [PubMed] [Google Scholar]
- 21.Pradeepa MMRM. Chromatin remodeling during mammalian spermatogenesis: role of testis specific histone variants and transition proteins. Soc Reprod Fertil Suppl. 2007;63:1–10 [PubMed] [Google Scholar]
- 22.Liu D-Y, Sie B-S, Liu M-L, Agresta F, Baker HWG. Relationship between seminal plasma zinc concentration and spermatozoa-zona pellucida binding and the ZP-induced acrosome reaction in subfertile men. Asian J Androl. 2009;11:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafique M, Khan N, Perveen K, Naqvi A. The effects of lead and zinc on the quality of semen of albino rats. J Coll Physicians Surg Pak. 2009;19:510–3 [PubMed] [Google Scholar]
- 24.Colagar AH, Marzony ET, Chaichi MJ. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr Res. 2009;29:82–8 [DOI] [PubMed] [Google Scholar]
- 25.Mert H, Karakus K, Yılmaz A, Aygun T, Mert N, Apaydın B, Seyhan E. Effects of genotype on testis, semen quality, and mineral composition of semen in various ram breeds. Biol Trace Elem Res. Epub 2009 May 9 [DOI] [PubMed] [Google Scholar]
- 26.Himeno S, Yanagiya T, Fujishiro H. The role of zinc transporters in cadmium and manganese transport in mammalian cells. Biochimie. 2009;91:1218–22 [DOI] [PubMed] [Google Scholar]
- 27.Clegg MS, Keen CL, Lonnerdal B, Hurley LS. Influence of ashing techniques on the concentration of trace elements in animals tissues. 1: wet ashing. Biol Trace Elem Res. 1981;3:107–15 [DOI] [PubMed] [Google Scholar]
- 28.Kelleher SL, Lonnerdal B. Zinc transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. J Nutr. 2003;133:3378–85 [DOI] [PubMed] [Google Scholar]
- 29.Eide DJ. The SLC39 family of metal ion transporters. : Hediger MA, editor The ABC of solute carriers. New York: Springer-Verlag; 2003 [Google Scholar]
- 30.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–22 [DOI] [PubMed] [Google Scholar]
- 31.Connolly MP, Ledger W, Postma MJ. Economics of assisted reproduction: access to fertility treatments and valuing live births in economic terms. Hum Fertil. 2010;13:13–8 [DOI] [PubMed] [Google Scholar]
- 32.Stallard L, Reeves PG. Zinc deficiency in adult rats reduces the relative abundance of testis-specific angiotensin-converting enzyme mRNA. J Nutr. 1997;127:25–9 [DOI] [PubMed] [Google Scholar]
- 33.Oteiza PI, Olin KL, Fraga CG, Keen CL. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J Nutr. 1995;125:823–9 [DOI] [PubMed] [Google Scholar]
- 34.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc-regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem. 2004;279:49082–90 [DOI] [PubMed] [Google Scholar]
- 35.Augustine LM, Markelewicz RJ, Boekelheide K, Cherrington NJ. Xenobitotic and endobiotic transporter mRNA expression in the blood-testis barrier. Drug Metab Dispos. 2005;33:182–9 [DOI] [PubMed] [Google Scholar]
- 36.Elgazar V, Razanov V, Stoltenberg M, Hershfinkel M, Huleihel M, Nitzan YB, Lunenfeld E, Sekler I, Silverman WF. Zinc-regulating proteins, ZnT-1, and metallothionein I/II are present in different cell populations in the mouse testis. J Histochem Cytochem. 2005;53:905–12 [DOI] [PubMed] [Google Scholar]
- 37.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol. 2008;73:1413–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monsefi M, Sanaz A, Ali M, Lahya R. Cadmium-induced infertility in male mice. Environ Toxicol. 2010;25:94–102 [DOI] [PubMed] [Google Scholar]
- 39.Sadik NA. Effects of diallyl sulfide and zinc on testicular steroidogenesis in cadmium-treated male rats. J Biochem Mol Toxicol. 2008;22:345–53 [DOI] [PubMed] [Google Scholar]
- 40.Sorensen MB, Stoltenberg M, Henriksen K, Ernst E, Danscher G, Parvinen M. Histochemical tracing of zinc ions in the rat testis. Mol Hum Reprod. 1998;4:423–8 [DOI] [PubMed] [Google Scholar]
- 41.Kehr S, Malinouski M, Finney L, Vogt S, Labunskyy VM, Kasaikina MV, Carlson BA, Zhou Y, Hatfield DL, et al. X-ray fluorescence microscopy reveals the role of selenium in spermatogenesis. J Mol Biol. 2009;389:808–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henkel R, Bittner J, Weber R, Hüther F, Miska W. Relevance of zinc in human sperm flagella and its relation to motility. Fertil Steril. 1999;71:1138–43 [DOI] [PubMed] [Google Scholar]
- 43.Martin GB, White CL, Markey CM, Blackberry MA. Effects of dietary zinc deficiency on the reproductive system of young male sheep: testicular growth and the secretion of inhibin and testosterone. J Reprod Fertil. 1994;101:87–96 [DOI] [PubMed] [Google Scholar]
- 44.Taylor KM. A distinct role in breast cancer for two LIV-1 family zinc transporters. Biochem Soc Trans. 2008;36:1247–51 [DOI] [PubMed] [Google Scholar]
- 45.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.