Abstract
In 2006, the AHA released diet and lifestyle recommendations (AHA-DLR) for cardiovascular disease (CVD) risk reduction. The effect of adherence to these recommendations on CVD risk is unknown. Our objective was to develop a unique diet and lifestyle score based on the AHA-DLR and to evaluate this score in relation to available CVD risk factors. In a cross-sectional study of Puerto Rican adults aged 45–75 y living in the greater Boston area, information was available for the following variables: diet (semiquantitative FFQ), blood pressure, waist circumference (WC), 10-y risk of coronary heart disease (CHD) (Framingham risk score), and fasting plasma lipids, serum glucose, insulin, and C-reactive protein (CRP) concentrations. We developed a diet and lifestyle score (AHA-DLS) based on the AHA-DLR. The AHA-DLS had both internal consistency and content validity. It was associated with plasma HDL cholesterol (P = 0.001), serum insulin (P = 0.0003), and CRP concentrations (P = 0.02), WC (P < 0.0001), and 10-y risk of CHD score (P = 0.01 in women). The AHA-DLS was inversely associated with serum glucose among those with a BMI < 25 (P = 0.01). Women and men in the highest quartile of the AHA-DLS had lower serum insulin (P-trend = 0.0003) and CRP concentrations (P-trend = 0.002), WC (P-trend = 0.0003), and higher HDL cholesterol (P-trend = 0.008). The AHA-DLS is a useful tool to measure adherence to the AHA-DLR and may be used to examine associations between diet and lifestyle behaviors and CVD risk.
Introduction
Cardiovascular disease (CVD)7 is the leading cause of death among Hispanics residing in the US, contributing to nearly 1 in every 4 deaths (1). Hispanics report more multiple comorbidities and CVD risk factors than non-Hispanic whites (2). The “Hispanic Paradox,” an observation that Hispanics have lower all-cause and cardiovascular mortality despite greater prevalence of risk factors and socioeconomic disadvantage (3), recently has been challenged (4). The paradox concept was primarily based on data from Mexican Americans (5, 6). Other Hispanic groups, such as Puerto Ricans, differ in ancestral genetic history and exposures to known risk factors.
Puerto Ricans have unique dietary intake patterns, as well as social, cultural, and environmental exposures that may contribute to CVD risk. For example, data from the Hispanic Health and Nutrition Examination Survey (HHANES; 1986–89) showed that Puerto Ricans living on the U.S. mainland reported lower consumption of vegetables, cereals, and protein-rich foods than other Hispanic groups (7). Data from the Massachusetts Hispanic Elders Study showed that Puerto Rican elders consumed diets high in refined carbohydrates and low in fiber and that diets had greater variety with higher level of acculturation (8). Little is known about how the Puerto Rican diet is associated with CVD risk.
Whereas the relationship between diet and disease traditionally has been studied using single foods or nutrients as the exposure, individuals consume meals consisting of a variety of foods, with complex combinations of nutrients that are likely to be interactive or synergistic (9, 10). Pattern analysis provides an additional dimension to analyses of diet and disease risk and provides a more realistic approach to disease prevention or treatment, because the focus is on the entire diet rather than a single food or nutrient (11). Dietary pattern analysis using score-based approaches is an a priori approach based on published dietary recommendations. Diet indexes have been constructed based on national recommendations to evaluate their effect on disease risk (12, 13). In 2006, the AHA released Diet and Lifestyle Recommendations (AHA-DLR) for CVD risk reduction (14). Although these are aimed at decreasing CVD risk in the general population, we know of no studies that assessed the effect of adherence to AHA-DLR on CVD risk factors.
Our aim was to characterize dietary patterns of the Puerto Rican population by creating a unique diet and lifestyle score based on the principles of the AHA-DLR. We tested both the content and predictive validity of the AHA-Diet and Lifestyle score (AHA-DLS) by assessing cross-sectional associations between the AHA-DLS, nutrient intakes, available CVD risk factors, and a risk assessment tool, the Framingham risk score (FRS).
Methods
Study participants.
The Boston Puerto Rican Health Study is an ongoing population-based longitudinal cohort study of 1500 Puerto Rican adults, aged 45–75 y, living in the greater Boston area. The study design and methods of the Boston Puerto Rican Health Study have been described in detail elsewhere (15). Briefly, self-identified Puerto Ricans were recruited primarily through door-to-door enumeration from high Hispanic density blocks based on year 2000 Census data. Other forms of recruitment included participation in community events/fairs, referrals from participants, and calls to the study office from flyers distributed at community locations. At baseline, bilingual interviewers visited the participant’s home to complete a comprehensive set of questionnaires. In addition, fasting blood samples were collected by a certified phlebotomist on the day following the home interview or soon thereafter. Only those participants who were unable to answer questions due to serious health conditions, those who planned to move away from the greater Boston area within 2 y and those with a low mini-mental state examination score (<10) were excluded from the study.
For the current analyses, we excluded participants reporting implausible energy intakes (<2510 or >20,083 kJ) (n = 67) at baseline. We also excluded participants with missing information on variables needed for computing the AHA-DLS (n = 67). There were no significant differences in baseline sociodemographic characteristics between those with complete and incomplete information. However, participants with missing data had higher fat intake (92.2 vs. 77.3 g/d; P = 0.02) and greater percent of energy from total fat (35.2 vs. 31.9%; P = 0.0002) compared with those with complete data. The present study includes 1203 participants with complete baseline data available at the time of analysis. All study protocols were approved by the Institutional Review Board of Tufts University/Tufts Medical Center.
Dietary assessment.
Habitual food consumption and nutrient intakes were captured using a semiquantitative FFQ designed and validated for the Puerto Rican population (16). The FFQ was based on the format of the National Cancer Institute/Block FFQ. Foods that contributed to nutrient intake of Puerto Rican adults in the HHANES were ranked to identify foods to be added to the food list. These included plantains, avocado, mango, cassava, empanadas, and custard. Compared with the National Cancer Institute/Block FFQ, the revised FFQ captured intakes reported in the 24-h recalls more accurately.
Reported food intakes were converted into gram amounts. To reflect the food groups in the AHA-DLR, we created 4 food groups (fruit, vegetables, fish, and alcohol) based on the USDA food grouping system. Self-reported mixed dishes were disaggregated and intake amounts were added to the appropriate food group. Nutrient intakes were calculated using the Nutrition Data System for Research software (version 2007, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN).
AHA-DLS score components and scoring.
We constructed a new score based on AHA-DLR for Americans (14) (Table 1). Foods, nutrients, and lifestyle variables were used to calculate the AHA-DLS, adapting from approaches previously used in the development of the USDA Healthy Eating Index (17) but based directly on 8 of 9 AHA-DLR recommendations for CVD risk reduction. A total possible score of 110 is calculated from scores for each of the subcomponents (Table 1).
TABLE 1.
Components and scoring system of the AHA-DLS
| Component | Scores for subcomponents (possible values/range) | Maximum points (range) | Scoring system | Score |
| Balance energy intake and physical activity to achieve or maintain a healthy body weight | 20 (0–20) | |||
| BMI, 2 | 10 (0, 5, or 10) | BMI < 18.5 | 5 | |
| BMI ≥18.5 and < 25 | 10 | |||
| BMI 25–29.9 | 5 | |||
| BMI > 30 | 0 | |||
| Physical Activity | 10 (0 or 10) | Moderate/vigorous | 10 | |
| Sedentary | 0 | |||
| Consume a diet rich in fruits and vegetables | 20 (0–20) | |||
| Fruit and vegetable intake,12servings/d | 10 (0–10) | ≥5 | 10 | |
| <2–5 | 0–10 | |||
| Fruit and vegetable variety, percentile of distribution | 10 (0, 5, or 10) | <25th | 0 | |
| 25th–75th | 5 | |||
| >75th | 10 | |||
| Choose whole-grain, high-fiber foods | 10 (0–10) | |||
| % of total grain that is whole grain1 | ≥50 | 10 | ||
| <50 | 0–10 | |||
| Consume fish, especially oily fish, at least twice/wk | 10 (0–10) | |||
| Total fish intake (excluding fried),12servings/wk | ≥2 | 10 | ||
| 0 – ≤2 | 0–10 | |||
| Limit your intake of saturated and trans fat and cholesterol | 20 (0–20) | |||
| Saturated fat,1% energy | 6 (0–6) | ≤3.5 | 6 | |
| >3.5 – ≤7 | 3–6 | |||
| ≥7 – ≥15 | 0–3 | |||
| > 15 | 0 | |||
| Trans fat,1% energy | 6 (0–6) | ≤0.5 | 6 | |
| >0.5 – ≤1 | 3–6 | |||
| >1 – ≤3 | 0–3 | |||
| > 3 | 0 | |||
| Dietary cholesterol,1mg/d | 4 (0–4) | ≤150 | 4 | |
| >150 – ≤300 | 0–4 | |||
| >300 | 0 | |||
| Total fat, % energy | 4 (0, 2, 4) | 25–35 | 4 | |
| <25 | 2 | |||
| >35 | 0 | |||
| Minimize your intake of beverages and foods with added sugars3 | 10 (0–10) | |||
| Added sugars,1g/d | > UL of discretionary energy | 0 | ||
| UL | 5 | |||
| 0 – ≤ UL | 5–10 | |||
| Choose and prepare foods with little or no salt | 10 (0–10) | |||
| Sodium,1g/d | ≤1.5 | 10 | ||
| >1.5 – ≤2.3 | 5–10 | |||
| >2.3 | 0 | |||
| If you consume alcohol, do so in moderation | 10 (0–10) | |||
| Alcohol,1servings/d | >0 to ≤2 drinks/d for men and >0 to ≤1 drink/d for women | 0–10 | ||
| Nondrinkers | 0 | |||
| >2 drinks/d for men and >1 drink/d for women | 0 |
Scores were prorated linearly for intakes between ranges.
1 serving of fruit = ½ cup (66 g) of dried fruit, 1 cup (138 g) of fruit, 1 cup (177 mL) of 100% fruit juice. 1 serving of vegetable = 1 cup (164 g) of non-leafy vegetables, 1 cup (177 mL) of vegetable juice, or 2 cups 9220 g) of raw leafy vegetables. 1 serving of fish = ~227 g.
For participants who consume alcohol, the UL for added sugars is one-third the discretionary energy. For participants who do not consume alcohol, the UL is one-half the discretionary energy.
We used 2 components to represent adherence to the recommendation for balancing energy intake and physical activity to achieve or maintain a healthy body weight. Given potential misreporting of energy intake using FFQ (18) and because energy imbalance is reflected in body weight, we assigned scores to participants based on their BMI status. A second component for physical activity was dichotomous. Participants engaging in moderate or vigorous activity were allotted 10 points and those engaging in sedentary or light activity were not assigned any points.
Both quantity and variety in fruit and vegetable intake were used to measure adherence to the recommendation to consume a diet rich in these components. For each participant, we calculated the total servings of fruit and vegetables (excluding starchy vegetables) consumed per day. Because the AHA-DLR do not consider fruit juice as equivalent to whole fruit, we did not count servings of fruit juice. Because a quantitative guideline for adequacy in fruit and vegetable intake was not provided by the AHA-DLR, we used the CDC recommendation of at least 5 servings/d of fruit and vegetables (19). One serving of fruit equals 1/2 cup (66 g) of dried fruit or 1 cup (138 g) of fruit or 100% fruit juice (177 mL). A serving of vegetable equals 1 cup (164 g) of non-leafy vegetables, 1 cup (177 mL) of vegetable juice, or 2 cups (220 g) of raw leafy vegetables. Variety in fruit and vegetable intake was defined as the number of unique types of fruit and vegetables consumed at least once per month. Based on the category assigned, participants received a score of 0, 5, or 10.
The AHA recommends that at least one-half of grain intake come from whole grains. Detailed methodology for creation of a whole-grain database is described elsewhere (20). The percentage of whole-grain intake was determined by dividing grams of whole grain by grams of total grain intake. Participants consuming at least one-half of total grain intake as whole grains were assigned 10 points. Because many Americans do not meet this recommendation (21), scores were prorated linearly between 0 and 10 for intakes between 0 and 50%. Because cereal, but not fruit, fiber has been reported to be associated with reduced CVD risk (22, 23), we did not assign a score for total fiber intake. However, by measuring whole-grain intake, we were able to capture cereal fiber intake.
One component measured adherence to fish intake. Because the recommendation is based on both oily and non-oily fish, we measured servings of total fish intake per week but excluded intake of deep-fried fish (24, 25). One serving of fish is ~227 g.
The AHA-DLR include consuming <7% of energy as saturated fat, <1% of energy as trans fat, and <300 mg cholesterol/d. In addition, the AHA-DLR also state that a range of 25–35% of energy from total fat is appropriate in a healthy dietary pattern. Therefore, we created 4 components, one each for saturated fat, trans fat, dietary cholesterol, and percentage of energy from total fat. Dietary cholesterol is known to raise blood cholesterol in only approximately one-third of people. However, intakes of saturated and trans fatty acids are known to result in dyslipidemia (26). Thus, intakes of both saturated and trans fat received greater weight (6 points) than did dietary cholesterol or percent of energy from total fat (4 points). Intakes below the cutpoints provided by the AHA-DLR were given maximum credit. Intakes at the recommendation level received one-half the total points. Intakes between these ranges were linearly prorated. No points were awarded for intakes over the recommendations. Sensitivity analyses included repeating analyses by providing greater weight to saturated and trans fat (8 points each) and lower weight to dietary cholesterol and percent of energy from fat (2 points each).
Scores for added sugars were based on the most recent scientific statement issued by the AHA (27). This statement proposes a specific upper limit (UL) for added sugars. Accordingly, a prudent UL is one-half of the discretionary energy allowance for each individual. However, if an individual consumes alcohol, this is reduced to accommodate the additional energy from alcohol intake. We first determined the suggested energy intake for each age/sex group using tables provided by Britten et al. (28) for development of food intake patterns for the MyPyramid system. To prevent overestimation of discretionary energy, we assumed each participant to be sedentary. Based on the suggested energy intake, we then determined the discretionary energy allowance for each energy level using the MyPyramid food intake patterns. The UL was set as one-half the discretionary energy for nondrinkers and one-third the discretionary energy for alcohol consumers. Those exceeding the UL were awarded no points. Participants with intakes at the UL received only partial credit. Those with added sugar intake below the UL received higher scores prorated linearly for intakes between the UL and no added sugar.
The salt recommendation is represented by one component. Participants with intakes less than the desirable standard of 1.5 g/d were awarded 10 points. The AHA recognizes that reducing sodium intake to 1.5 g/d may not be easily achievable due to the high-sodium food supply. Thus, they propose an achievable recommendation of 2.3 g/d. Participants meeting this recommendation received a score of 5 with scores prorated linearly for intakes between 1.5 and 2.3 g/d.
The AHA-DLR for alcohol consumption provides cutoff points of ≤2 drinks/d for men and ≤1 drink/d for women. One drink equals 355 mL of regular beer, 148 mL of wine, or 44 mL of spirits. Scores were linearly prorated for intakes between 0–2 drinks/d for men and 0–1 drink/d for women. Those consuming more than this received no points. Based on documented protective effects of moderate alcohol consumption (29, 30), no points were awarded for nondrinkers as well.
Lifestyle assessment.
Standing height and weight were measured in duplicate. Weight was measured using a quality clinical scale (Toledo Weight Plate, Model I5S, Bay State and Systems), which was regularly calibrated with known weights. Height was measured using a Harpenden pocket stadiometer. BMI was calculated as weight (kg) divided by height (m2). Physical activity was assessed using a modified Paffenbarger questionnaire from the Harvard Alumni Activity Survey (31, 32).
Biologic measures.
A 12-h fasting blood sample was drawn by a certified phlebotomist on the day following the home interview, or as soon as possible thereafter, in the participant’s home. For plasma, blood was collected into vacutainers containing EDTA, inverted gently prior to processing and centrifuged at 3421 × g at 4°C for 15 min and kept cold. For serum, blood was collected into vacutainers containing no anticoagulant, allowed to clot at room temperature for ∼15 min, centrifuged at 3421 × g at 4°C for 15 min and placed upright in a cooler but not directly on ice. Whole blood was collected into a separate vacutainer and kept on a rocker at room temperature until analyzed for hematology measures. All vacutainers were shielded from light during specimen collection, processing, and handling. All samples were kept cold and brought back to the Nutrition Evaluation Laboratory at the Jean Mayer USDA Human Nutrition Research Center for further processing and storage. Aliquots were stored in cryogenic tubes at −80°C prior to analysis.
Other covariates.
Information on age, education, household income, and family size were collected using a questionnaire based on questions from NHANES III, the HHANES, and the National Health Interview Survey Supplement on Aging. Information on health behaviors includes smoking and frequency, history, and type of alcohol consumption. Diabetes status was defined as fasting serum glucose ≥ 6.99 mmol/L or use of diabetes medication (33). Hypertension was defined as blood pressure ≥ 140/90 mm Hg or use of antihypertensive medication (34). Detailed information on prescription and over-the-counter medication use was collected. Acculturation was captured using the bidimensional Acculturation Scale for Hispanics. The scale yields 2 scores that rank acculturation in the Hispanic and the non-Hispanic domains (35). A score of 100 indicates full acculturation with fluent English language use. We also administered the Spanish version of the Perceived Stress Scale (36).
Outcome measures.
We used the Framingham risk equations (37) to calculate the estimated FRS for each participant free of heart attack, heart disease, and stroke (self report) at baseline (n = 254 men and 688 women). Risk factors considered include sex, age, diabetes, smoking, systolic and diastolic blood pressure, total cholesterol, and LDL and HDL cholesterol. C-reactive protein (CRP) was measured in serum using a solid-phase, 2-site chemiluminescent immunometric assay with a commercial kit (IMMULITE 1000, Diagnostics Products). The intra- and inter-assay CV% for this assay are 4.2–6.4 and 4.8–10.0%, respectively. Plasma cholesterol, TG, and HDL cholesterol concentrations were analyzed with an enzymatic endpoint reaction in an Olympus AU400, using standard operating procedures. The intra- and inter-assay CV% for plasma cholesterol, TG, and HDL cholesterol concentrations were 1.8 and 2.2%, 2.8 and 2.7%, and 3.0 and 7.0%, respectively. LDL cholesterol was calculated using the Friedewald formula, unless TG concentrations exceeded 4.52 mmol/L (38). Serum glucose was measured using an enzymatic kinetic reaction on the Olympus AU400 with Olympus Glucose reagents (OSCR6121). Serum insulin was measured using a solid-phase, 2-site chemiluminescent immunometric assay using a commercial kit (IMMULITE 1000, Diagnostic Products). The intra- and inter-assay CV% for serum glucose and insulin were 2.0 and 3.4%, 5.2–6.4% and 5.9–8.0%, respectively. Blood pressure was measured using an electronic sphygmomanometer (Model HEM-71, OMRON Healthcare) at 3 different time points during the interview. An average of the second and 3rd readings was used to obtain systolic and diastolic blood pressure. Waist circumference (WC) was measured using a nonelastic tape on the smallest area of the waist and was recorded to the nearest one-tenth of a centimeter.
Statistical analyses.
Statistical analyses were conducted using SAS 9.2. The AHA-DLS was used as a continuous measure and was also divided into quartile categories. We calculated the age- and sex-adjusted means for sociodemographic characteristics, health behaviors, and biological measures across quartiles of AHA-DLS with ANCOVA. We tested the content validity of the AHA-DLS by calculating age-, sex-, and energy-adjusted intakes of nutrients known to be associated with a diet based on the AHA-DLR across quartiles of the AHA-DLS. We assessed the significance across quartiles of AHA-DLS using linear (for continuous variables) or logistic regression (for categorical variables). All analyses were adjusted for multiple comparisons using Tukey’s HSD. Internal consistency was determined using inter-item correlation matrixes. Spearman rank correlations were used to examine associations between individual component scores and the total AHA-DLS, as well as among the individual component scores. Bonferroni adjustment was applied for multiple testing. For all other analyses, a P-value of 0.05 was considered significant.
Because the AHA-DLR were formulated for CVD risk reduction, we tested the association of the AHA-DLS with plasma lipoprotein measures, the FRS, and CVD risk factors, including systolic and diastolic blood pressure, serum glucose, insulin, and CRP, and WC. A logarithmic transformation was applied to plasma TG, serum glucose, insulin, and CRP to improve normality. Log-transformed values were back transformed and results were expressed as geometric means. To test the association between the AHA-DLS and plasma lipoproteins, we adjusted for age, sex, smoking status, diabetes, hypertension, and WC. Models with LDL cholesterol as the outcome were adjusted for lipid-lowering medication use and HDL cholesterol (model 2). Models with HDL cholesterol as the outcome variable were adjusted for LDL cholesterol, TG, and cardiovascular medication use. Models with TG as the outcome were adjusted for LDL cholesterol, cardiovascular medication use, and total carbohydrate intake (model 2). In our final model (model 3), we further adjusted for acculturation and perceived stress score (PSS). Because risk equations for calculating the FRS differ for men and women, we constructed sex-specific models to test the associations between FRS and AHA-DLS with the following adjustments: 1) age, supplement use, and cardiovascular medication use; 2) model 1 + WC, and income; 3) model 2 + acculturation and PSS. We used ANCOVA to test associations between the AHA-DLS, systolic and diastolic blood pressure, serum glucose, and serum insulin. In our base model, we adjusted for age, sex, and smoking status. Because diabetes is known to affect blood pressure (39), models with systolic and diastolic blood pressure were also adjusted for diabetes status. Models 2 and 3 were further adjusted for supplement use, medication use, WC, income, acculturation, and PSS. Models with WC as the outcome variable were sequentially adjusted for the following covariates: 1) age, sex, smoking status, diabetes, hypertension, and BMI; 2) model 1 + CRP, insulin medication use, and income; and 3) model 2 + acculturation and PSS. We used a similar approach to test associations between AHA-DLS and log CRP. Model 1 was adjusted for age, sex, smoking status, diabetes, and hypertension. Model 2 further adjusted for WC, white blood cell count, and income. In our final model, we adjusted for acculturation and PSS. We tested for effect modification by sex, BMI, and diabetes status by including a cross-product term in the regression model. Tests for linear trend were conducted across quartile categories by including the median score for each quartile as a continuous measure in the regression model.
Results
The AHA-DLS.
The AHA-DLS was normally distributed. The mean AHA-DLS for participants in our cohort was 32.1 (range 5.1–72.2) out of a total possible score of 110. Fewer than 3% of the population scored more than one-half of the maximum possible score (Table 2). Median intake of fruit and vegetables was below the CDC recommendation. Nearly 75% of participants did not meet recommendations for added sugars and sodium intake. Nearly all (~98%) had intake of whole grains below the recommendation. Spearman rank correlation coefficients between subcomponents and the total AHA-DLS were all positive and significant (P < 0.0001) and ranged from 0.13 for dietary cholesterol to 0.56 for variety in fruit and vegetable intake (Table 2). Adjustment for age, sex, and energy intake slightly strengthened but did not significantly change these correlations (data not shown). Correlation coefficients between the subcomponents ranged from −0.32 for fish and dietary cholesterol to 0.51 for saturated fat and trans fat intakes (Supplemental Table 1).
TABLE 2.
Component values, score distributions, and correlations of the AHA-DLS in older Puerto Ricans1
| Component | % | Intake distribution | Score distribution3 | % with minimum score | % with maximum score | Spearman rank r |
| Balance energy intake and physical activity to achieve or maintain a healthy body weight | ||||||
| BMI,2kg/m2 | 57.7 | 12.6 | 0.29 | |||
| BMI < 18.5 | 0.3 | NA3 | 0 | |||
| BMI ≥18.5 and < 25 | 12.6 | NA | 10 | |||
| BMI 25–29.9 | 29.7 | NA | 5 | |||
| BMI ≥ 30 | 57.4 | NA | 0 | |||
| Physical activity2 | 95.6 | 4.4 | 0.21 | |||
| Moderate/vigorous | 4.4 | NA | 10 | |||
| Sedentary | 95.6 | NA | 0 | |||
| Consume a diet rich in fruits and vegetables | ||||||
| Fruit and vegetable intake, servings/d | NA | 2.70 (0.83–6.46) | 2.32 (0–10) | 32.8 | 12.2 | 0.46 |
| Fruit and vegetable variety,24percentile of distribution | 25.0 | 25.0 | 0.56 | |||
| <25th | 25.0 | 17.0 (8–21) | 0 | |||
| 25th–75th | 50.0 | 27.0 (22–31) | 5 | |||
| >75th | 25.0 | 36.0 (32–43) | 10 | |||
| Choose whole-grain, high-fiber foods, % of total grain that is whole grain | NA | 10.3 (0.6–36.4) | 2.05 (0.12–7.3) | 0 | 1.7 | 0.39 |
| Consume fish, especially oily fish, at least twice/wk, servings/d | NA | 0.83 (0.04–3.72) | 4.15 (0.21–10) | 0 | 18.1 | 0.41 |
| Limit your intake of saturated and trans fat and cholesterol | ||||||
| Saturated fat, % energy | NA | 9.49 (5.9–13.3) | 2.1 (0.6–3.9) | 0 | 0 | 0.34 |
| Trans fat, % energy | NA | 1.2 (0.6–1.8) | 2.8 (1.7–5.2) | 0 | 1.5 | 0.28 |
| Dietary cholesterol, mg/d | NA | 271 (96–634) | 0.8 (0–4) | 43.1 | 17.5 | 0.13 |
| Total fat,2% energy | 32.2 (22.6–40.5) | 29.6 | 59.6 | 0.24 | ||
| 25–35 | 29.6 | 4 | ||||
| <25 | 10.8 | 2 | ||||
| >35 | 59.6 | 0 | ||||
| Minimize your intake of beverages and foods with added sugars, g/d of added sugars | NA | 47.8 (9.9–147) | 0 (0–7.3) | 84.2 | 0 | 0.17 |
| Choose and prepare foods with little or no salt, mg/d of sodium | NA | 4410 (1950–9390) | 0 (0–7.2) | 89.5 | 1.3 | 0.25 |
| If you consume alcohol, do so in moderation,5servings/d | NA | 0.2 (0.02–3.1) | 5.5 (0–9.3) | 67.5 | <0.01 | 0.31 |
| Total score | 31.4 (14.9–50.5) | |||||
Values are median (5th–95th percentile) or percentage of participants falling under each categorical component. Overall n = 1203.
Points for each categorical component.
NA, Not applicable.
Variety defined as the total number of unique fruits and vegetables consumed at least once per month in the last 12 mo.
Values only for current drinkers, n = 447.
Participant characteristics and content validity.
There was more than a 2-fold difference in the median scores of the extreme quartiles of the AHA-DLS (Table 3). Those in the highest quartile, compared with the lowest, were more likely to be physically active, alcohol consumers, acculturated, supplement users, to report less perceived stress, and to have lower BMI and greater education and household income.
TABLE 3.
Participant characteristics across quartiles of the AHA-DLS in the Boston Puerto Rican Health Study1
| Quartiles of AHA-DLS |
|||||
| Characteristic2 | Q1 19.0 (4.8–23.1) | Q2 26.7 (23.1–30.4) | Q3 33.8 (30.4–38.5) | Q4 43.0 (38.6–72.2) | P-trend |
| n | 300 | 301 | 301 | 301 | |
| Age, y | 56.4 ± 0.5 | 57.5 ± 0.5 | 57.5 ± 0.5 | 57.7 ± 0.5 | 0.18 |
| Female, % | 75.1 | 70.4 | 73.4 | 70.0 | 0.28 |
| BMI, kg/m2 | 33.2 ± 0.4 | 31.6 ± 0.4* | 30.9 ± 0.4† | 29.2 ± 0.4† | <0.0001 |
| Physical activity, % | |||||
| Sedentary/light | 98.7 | 96.0 | 95.8 | 86.3† | <0.0001 |
| Moderate/vigorous | 1.3 | 4.0 | 4.2 | 13.7† | <0.0001 |
| Smoking, % | |||||
| Never | 39.9 | 46.0 | 34.7 | 43.3 | 0.63 |
| Past | 30.4 | 29.9 | 33.9 | 31.1 | 0.99 |
| Current | 28.8 | 22.6 | 30.3 | 24.7 | 0.63 |
| Alcohol, % | |||||
| Nondrinker | 76.1 | 65.5** | 54.1† | 36.1† | <0.0001 |
| Moderate | 15.7 | 25.2** | 35.9† | 52.9† | <0.0001 |
| Heavy | 5.5 | 7.3 | 8.2 | 9.3 | 0.03 |
| Diabetes (y/n), % yes | 43.6 | 40.0 | 41.0 | 38.0 | 0.20 |
| Hypertension (y/n), % yes | 69.7 | 70.9 | 71.6 | 67.8 | 0.62 |
| Total household income, $/y | 14,518 ± 2054 | 16,723 ± 2017 | 21389 ± 2032 | 23,535 ± 1994** | 0.0003 |
| Education, % | |||||
| ≤8th grade | 51.5 | 50.3 | 46.8 | 39.0** | 0.0007 |
| 9th–12th grade or GED | 39.9 | 36.3 | 38.2 | 38.5 | 0.84 |
| College/some graduate school | 8.4 | 13.4 | 14.6** | 22.5† | <0.0001 |
| Supplement use (y/n), % yes | 53.5 | 53.5 | 59.5 | 63.1* | 0.006 |
| Acculturation, % | 22.0 ± 1.2 | 24.4 ± 1.2 | 25.2 ± 1.2 | 30.1 ± 1.2† | <0.0001 |
| PSS | 25.2 ± 0.6 | 23.0 ± 0.5 | 22.3 ± 0.6*** | 21.3 ± 0.5† | <0.0001 |
Values are median (range), mean ± SEM, or %, adjusted for age/sex and calculated using ANCOVA. Symbols indicate different from Q1: * < 0.05, **P < 0.01, ***P < 0.001.
P < 0.0001, adjusting for age/sex using ANCOVA for linear variables and logistic regression for categorical variables. Adjustments were made for multiple comparisons using Tukey's honestly significant difference.
There was no significant difference in energy intake across quartiles of the AHA-DLS (Table 4). The AHA-DLS was positively associated with intakes of protein, total carbohydrate, fiber, (n-3) fatty acids, alcohol, β-carotene, lycopene, folate, vitamin C, potassium, and magnesium across quartiles (P-trend < 0.0001). Conversely, participants in the highest quartile of the AHA-DLS had lower intakes of added sugar, total fat, and percent of energy from total fat (P-trend < 0.0001).
TABLE 4.
Selected daily intake of nutrients known to be available in food groups that constitute the AHA-DLS1
| Quartiles of AHA-DLS |
|||||
| Nutrient intake2 | Q1 19.0 (4.8–23.1) | Q2 26.7 (23.1–30.4) | Q3 33.8 (30.4–38.5) | Q4 43.0 (38.6–72.2) | P-trend |
| n | 300 | 301 | 301 | 301 | |
| Energy,2kJ/d | 9425 ± 217 | 9166 ± 213 | 9852 ± 215 | 9174 ± 213 | 0.85 |
| Protein, g/d | 87.8 ± 1.1 | 89.7 ± 1.1 | 91.6 ± 1.1* | 93.8 ± 1.0*** | <0.0001 |
| Total carbohydrate, g/d | 265 ± 3 | 267 ± 3 | 271 ± 3 | 275 ± 3 | 0.003 |
| Added sugar, g/d | 64.1 ± 2.4 | 58.0 ± 2.3 | 54.1 ± 2.3** | 52.9 ± 2.3** | 0.0002 |
| Total fiber, g/d | 16.9 ± 0.3 | 18.1 ± 0.3* | 19.8 ± 0.3† | 21.2 ± 0.3† | <0.0001 |
| Total fat, g/d | 81.9 ± 0.8 | 79.1 ± 0.8* | 76.4 ± 0.8† | 72.8 ± 0.8† | <0.0001 |
| Total fat, % energy | 33.9 ± 0.3 | 32.7 ± 0.3* | 31.6 ± 0.3† | 29.8 ± 0.3† | <0.0001 |
| Total (n-3) fatty acids, g/d | 1.58 ± 0.03 | 1.66 ± 0.03 | 1.74 ± 0.03*** | 1.75 ± 0.03*** | <0.0001 |
| Trans fatty acids, g/d | 3.19 ± 0.06 | 2.97 ± 0.06** | 2.75 ± 0.06† | 2.58 ± 0.06† | <0.0001 |
| Alcohol, g/d | 2.9 ± 0.8 | 4.7 ± 0.8 | 5.3 ± 0.8 | 7.0 ± 0.8** | 0.0003 |
| β-Carotene, μg/d | 2519 ± 176 | 2768 ± 173 | 3079 ± 176 | 3750 ± 173† | <0.0001 |
| Lycopene, μg/d | 6383 ± 236 | 6440 ± 232 | 7532 ± 235** | 7734 ± 232*** | <0.0001 |
| Folic acid, μg/d | 465 ± 10 | 487 ± 10 | 535 ± 10† | 557 ± 10† | <0.0001 |
| Vitamin C, mg/d | 113 ± 5 | 124 ± 5 | 143 ± 5† | 165 ± 5† | <0.0001 |
| Potassium, mg/d | 2899 ± 36 | 3111 ± 36*** | 3240 ± 36† | 3465 ± 35† | <0.0001 |
| Magnesium, mg/d | 301 ± 6 | 332 ± 5*** | 350 ± 6† | 380 ± 5† | <0.0001 |
Values are median (range) or mean ± SEM, adjusted for age, sex, and energy intake using ANCOVA unless otherwise noted. Symbols indicate different from Q1: < 0.05, **P < 0.01, ***P < 0.001, †P < 0.0001 using ANCOVA. Adjustments were made for multiple comparisons using Tukey's honestly significant difference.
Values are mean ± SEM, adjusted for age and sex using ANCOVA.
CVD risk factors.
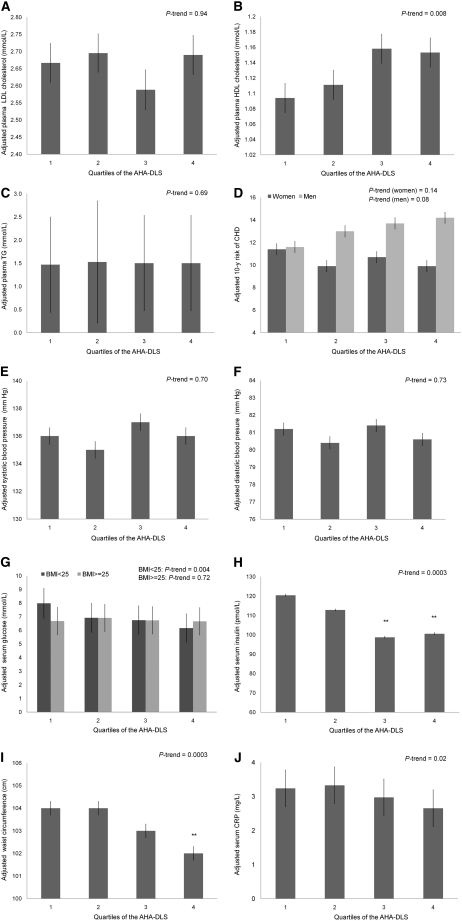
The AHA-DLS was positively associated with HDL cholesterol, but no significant associations were noted with LDL cholesterol or TG concentrations (Table 5). Likewise, no interactions were noted with sex, BMI, or diabetes status (P > 0.10). The AHA-DLS was inversely associated with the FRS in women (P = 0.01) but not men (P = 0.32). There was no evidence for effect modification by BMI or diabetes (P > 0.10). However, there was an interaction between BMI and log glucose (P = 0.002). The AHA-DLS was inversely associated with log glucose among those with a BMI < 25 (P = 0.01) but not in those participants who were overweight or obese (P = 0.58). Serum insulin, WC, and CRP were each inversely associated with the AHA-DLS after multivariate adjustment (P = 0.0003, P < 0.0001, and P = 0.02, respectively). No significant associations were noted with blood pressure (Table 5). HDL cholesterol increased across quartiles of the AHA-DLS (P-trend = 0.008). There were decreasing trends in adjusted mean insulin (P-trend = 0.0003) and CRP (P-trend = 0.002) concentrations and WC (P-trend = 0.0003) across quartiles of the AHA-DLS. Among those with BMI < 25, there was an inverse trend in geometric mean glucose concentration across AHA-DLS quartiles (P-trend = 0.004) (Fig. 1). There were no substantial differences in results when saturated and trans fat received greater weight (8 vs. 6 points) compared with dietary cholesterol and percent of energy from fat (4 vs. 2 points) (data not shown).
TABLE 5.
Cross-sectional associations between the AHA-DLS and CVD Risk Factors in older Puerto Ricans1
| CVD risk factor | n | β ± SE | P-value |
| Plasma lipids | 1069 | ||
| LDL cholesterol,2mmol/L | |||
| Model 1 | 0.014 ± 0.024 | 0.56 | |
| Model 2 | −0.012 ± 0.026 | 0.63 | |
| Model 3 | −0.011 ± 0.026 | 0.67 | |
| HDL cholesterol,3mmol/L | |||
| Model 1 | 0.034 ± 0.009 | <0.0001 | |
| Model 2 | 0.031 ± 0.009 | 0.0004 | |
| Model 3 | 0.029 ± 0.009 | 0.001 | |
| Log TG,4mmol/L | |||
| Model 1 | 0.003 ± 0.006 | 0.59 | |
| Model 2 | 0.005 ± 0.006 | 0.41 | |
| Model 3 | 0.003 ± 0.006 | 0.57 | |
| FRS5 | |||
| Men | 219 | ||
| Model 1 | 0.092 ± 0.450 | 0.84 | |
| Model 2 | 0.423 ± 0.491 | 0.39 | |
| Model 3 | 0.495 ± 0.499 | 0.32 | |
| Women | 628 | ||
| Model 1 | −0.804 ± 0.250 | 0.001 | |
| Model 2 | −0.679 ± 0.272 | 0.01 | |
| Model 3 | −0.717 ± 0.279 | 0.01 | |
| Other CVD risk factors | |||
| Systolic blood pressure,6mm Hg | 1078 | ||
| Model 1 | −0.402 ± 0.519 | 0.43 | |
| Model 2 | −0.035 ± 0.272 | 0.95 | |
| Model 3 | −0.184 ± 0.559 | 0.74 | |
| Diastolic blood pressure,6mm Hg | 1077 | ||
| Model 1 | −0.585 ± 0.291 | 0.05 | |
| Model 2 | −0.315 ± 0.308 | 0.31 | |
| Model 3 | −0.278 ± 0.315 | 0.37 | |
| Log serum glucose,7mmol/L | |||
| BMI < 25, 2 | 138 | ||
| Model 1 | −0.014 ± 0.010 | 0.16 | |
| Model 2 | −0.025 ± 0.011 | 0.02 | |
| Model 3 | −0.029 ± 0.011 | 0.01 | |
| BMI ≥ 25, 2 | 958 | ||
| Model 1 | −0.008 ± 0.004 | 0.07 | |
| Model 2 | −0.002 ± 0.004 | 0.60 | |
| Model 3 | −0.002 ± 0.004 | 0.58 | |
| Log serum insulin,7pmol/L | 1093 | ||
| Model 1 | −0.059 ± 0.008 | <0.0001 | |
| Model 2 | −0.030 ± 0.008 | 0.0003 | |
| Model 3 | −0.030 ± 0.008 | 0.0003 | |
| WC,8cm | 1083 | ||
| Model 1 | −0.10 ± 0.03 | <0.0001 | |
| Model 2 | −0.10 ± 0.03 | <0.0001 | |
| Model 3 | −0.10 ± 0.03 | <0.0001 | |
| Inflammatory marker | 1083 | ||
| CRP,9mg/L | |||
| Model 1 | −0.008 ± 0.001 | <0.0001 | |
| Model 2 | −0.003 ± 0.001 | 0.02 | |
| Model 3 | −0.003 ± 0.001 | 0.02 | |
β-Coefficients ± SE for every 10-unit increase in the AHA-DLS were calculated using ANCOVA.
Model 1: adjusted for age (y), sex, smoking status (former, current, never), diabetes (y/n), hypertension (y/n). Model 2: Model 1 + lipid medication use (y/n), HDL cholesterol (mmol/L), income ($/y), WC (cm). Model 3: Model 2 + acculturation (%), PSS.
Model 1: adjusted for age (y), sex, smoking status (former, current, never), diabetes (y/n), hypertension (y/n). Model 2: Model 1 + LDL cholesterol (mmol/L), TG (mmol/L), cardiovascular medication use (y/n), income ($/y), WC (cm). Model 3: Model 2 + acculturation (%), PSS.
Model 1: adjusted for age (y), sex, smoking status (former, current, never), diabetes (y/n), hypertension (y/n). Model 2: Model 1 + LDL cholesterol (mmol/L), HDL cholesterol (mmol/L), total carbohydrate intake (g/d), cardiovascular medication use (y/n), income ($/y), WC (cm). Model 3: Model 2 + acculturation (%), PSS.
Model 1: adjusted for age (y), supplement use (y/n), cardiovascular medication use (y/n). Model 2: Model 1 + WC (cm), income ($/y). Model 3 adjusted for model 2 + acculturation (%), PSS.
Model 1: adjusted for age (y), sex, smoking status (former, current, never), diabetes (y/n). Model 2: Model 1 + supplement use (y/n), hypertension medication use (y/n), WC (cm), income ($/y). Model 3: Model 2 + acculturation (%), PSS.
Model 1: adjusted for age (y), sex, smoking status (former, current, never). Model 2: Model 1 + supplement use (y/n), diabetes medication use (y/n), WC (cm), income ($/y). Model 3: Model 2 + acculturation (%), PSS.
Model 1: adjusted for age (y), sex, smoking status (former, current, never), diabetes (y/n), hypertension (y/n), BMI (kg/m2). Model 2: Model 1 + CRP (mg/L), income ($/y), insulin medication use (y/n). Model 3: Model 2 + acculturation (%), PSS.
Model 1: adjusted for age (y), sex, smoking status (former, current, never), diabetes (y/n), hypertension (y/n). Model 2: Model 1 + white blood cell count (mm3), income ($/y), WC (cm). Model 3: Model 2 + acculturation (%), PSS.
FIGURE 1.
Adjusted means for select CVD risk markers across quartiles of the AHA-DLS in older Puerto Ricans. Values are adjusted means ± SE, n = 300 or 301. **Different from Q1 (P < 0.01) using ANCOVA. Adjustments for multiple comparisons were made using Tukey’s honestly significant difference. (A) Adjusted for age (y), sex, smoking status (current, former, never), diabetes (y/n), hypertension (y/n), lipid medication use (y/n), HDL cholesterol (mmol/L), income ($/y), WC (cm), acculturation (%), PSS. (B) Adjusted for age (y), sex, smoking status (current, former, never), diabetes (y/n), hypertension (y/n), LDL cholesterol (mmol/L), TG (mmol/L), cardiovascular medication use (y/n), income ($/y), WC (cm), acculturation (%), PSS. (C) Adjusted for age (y), sex, smoking status (current, former, never), diabetes (y/n), hypertension (y/n), LDL cholesterol (mmol/L), HDL cholesterol (mmol/L), total carbohydrate intake (g/d), cardiovascular medication use (y/n), income ($/y), WC (cm), acculturation (%), PSS. (D) Adjusted for age (y), supplement use (y/n), cardiovascular medication use (y/n), income ($/y), WC (cm), acculturation (%), PSS. (E,F) Adjusted for age (y), sex, smoking status (current, former, never), diabetes (y/n), supplement use (y/n), hypertension medication use (y/n), income ($/y), WC (cm), acculturation (%), PSS. (G,H) Adjusted for age (y), sex, smoking status (current, former, never), supplement use (y/n), diabetes medication use (y/n), income ($/y), WC (cm), acculturation (%), PSS. (I) Adjusted for age (y), sex, smoking status (current, former, never), diabetes (y/n), hypertension (y/n), BMI (kg/m2), CRP (mg/L), insulin medication use (y/n), income ($/y), acculturation (%), PSS. (J) Adjusted for age (y), sex, smoking status (current, former, never), diabetes (y/n), hypertension (y/n), white blood cell count (mm3), income ($/y), WC (cm), acculturation (%), PSS.
Discussion
We developed the AHA-DLS to assess the relationship between adherence to the AHA-DLR and CVD risk. During the process of development, we attempted to limit subjectivity in the creation of food groups and interpretation of the recommendations. The decision to include lifestyle factors in the AHA-DLS was based on the premise that the AHA-DLR were intended to provide a foundation for a public health approach to CVD risk reduction through both diet and lifestyle modifications. Thus, a unique feature of the AHA-DLS is that it includes foods, nutrients, health, and lifestyle factors. To our knowledge, this is the first diet and lifestyle score developed from the AHA-DLR.
Due to the nature of the recommendations, scales for scoring are both categorical and continual. For foods and nutrients, scoring was on a continuous scale. This is advantageous, because it does not assume a linear relationship but allows for U-shaped correlations with health outcomes, when appropriate (13). We interpreted ideal body weight as within the recommended range for BMI. Because BMI is used to estimate healthy body weight based on a person’s height (40), this variable represents the recommendation to balance energy intake and expenditure. The AHA-DLR restrict intakes of saturated fat, trans fat, and cholesterol, which tend to be highly inter-correlated and thus contribute a large proportion to the total score (41). Further, increased consumption of foods such as red meat, which is high in both saturated fat and dietary cholesterol, may be associated with reduced consumption of foods such as fish, further contributing to the cumulative effect of scoring components (12).
Two examples of dietary patterns that appear to be generally consistent with the AHA-DLR are the Dietary Approaches to Stop Hypertension (DASH) diet (42) and Therapeutic Lifestyle Changes provided by the National Heart Lung and Blood Institute (43). Previous studies have shown that a score based on the DASH diet was associated with CVD endpoints, such as nonfatal and fatal coronary heart disease (CHD) (44), incident heart failure (45, 46), and CHD and CVD mortality (47). Our results are consistent with those of Nettleton et al. (48), who observed that a Comprehensive Healthy Dietary Pattern score was associated with lower WC and lower fasting CRP and insulin, but not with fasting glucose or blood pressure, in a multi-ethnic population aged 45–84 y. Our finding of a positive association between the AHA-DLS and HDL cholesterol is important in the Puerto Rican population, where a high prevalence of low HDL cholesterol concentration has been identified, both previously (49) and in this study. Unlike the study populations of Dauchet et al. (50) and Schulze et al. (51) who noted that a diet consistent with the DASH principles was associated with lower blood pressure, participants in our study were older and nearly 70% had hypertension at study enrollment. This may have limited our ability to observe an association between the AHA-DLS and blood pressure.
Our findings need to be interpreted in the context of a few limitations. First, we characterized diet using a semiquantitative FFQ, which by its nature has limited precision and some misclassification. However, while FFQ cannot measure absolute intakes, they have been shown to rank usual intakes well (52). A second limitation is that we categorized the BMI, physical activity, and variety components. This categorization may limit the range of possible scores. However, we did not find substantial evidence that, e.g., a BMI of 18.5 should receive a different score than a BMI of 24.9. Similarly, there were no national guidelines or recommendations for variety in fruit and vegetable intake. We thus based our scoring criteria on the distribution of our data.
A 3rd limitation is the subjectivity involved in the differential scoring of the fat components. While it is known that saturated and trans fats contribute to greater CVD risk than dietary cholesterol or percent of energy intake from fat (26), our decision to assign 6 compared with 4 points was ultimately subjective. However, when we repeated our analyses providing greater weight to saturated and trans fat and lower weight to dietary cholesterol and percent of energy from fat, results did not change substantially. Additionally, our associations between the AHA-DLS and CVD risk factors are cross-sectional in nature. It is possible that those with these CVD risk factors may have modified their diet to a healthier pattern, thus attenuating relationships between the AHA-DLS and CVD risk factors.
Last, we used the FRS to estimate the 10-y risk of CHD among participants in our study. The FRS was developed primarily from the experience of the Framingham Heart Study, a predominantly Caucasian population (37). Portability of the FRS to the Puerto Rican population has been previously determined and the score was found to systematically overestimate CHD events in much older data with Puerto Ricans living in Puerto Rico (53). However, risk levels have changed considerably since the Puerto Rico Heart Health Program, conducted in 1965 (54). By using the FRS as a continuous measure, participants in our study will be ranked appropriately according to their risk estimates and the linear associations between the AHA-DLS and FRS are, thus, valid.
In the present study, we observed that only ∼3% of the population had a score ≥ 50%, indicating poor adherence to the AHA-DLR. An important observation is that even relatively modest adherence to the AHA-DLR was associated with significantly lower FRS, WC, and serum insulin and CRP concentrations, and a higher HDL cholesterol concentration in this high-risk minority group. In conclusion, the AHA-DLS appears to be a useful instrument for assessing adherence to the AHA-DLR in this group of Puerto Rican adults, and to assess relationships with diet and lifestyle behaviors and health outcomes. Although this study is limited to Puerto Ricans living in the Boston area, there is no reason to expect that the AHA-DLS cannot be generalized to Puerto Ricans elsewhere and to other ethnic groups. Results from our study provide important information to public policy for an understudied population with documented health disparities. The Puerto Rican population appears to be at high risk for chronic disease and diet and lifestyle interventions based on the AHA-DLR may provide substantial benefits.
Supplementary Material
Acknowledgments
S.N.B. formulated the study concept, analyzed data, and wrote the paper; A.H.L., B.D.H., and K.L.T. critically revised the manuscript for important intellectual content; S.N.B. and K.L.T. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Footnotes
Supported by grants from the NIH (P01 AG023394 and P50 HL105185).
Supplemental Table 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AHA-DLR, AHA Diet and Lifestyle Recommendations; AHA-DLS, AHA Diet and Lifestyle Score; CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; FRS, Framingham risk score; HHANES, Hispanic Health and Nutrition Examination Survey; PSS, perceived stress score; UL, upper limit; WC, waist circumference.
Literature Cited
- 1.CDC Health of Hispanic or Latino population. National Center for Health Statistics; 2009 [Google Scholar]
- 2.Tucker KL, Falcon LM, Bianchi LA, Cacho E, Bermudez OI. Self-reported prevalence and health correlates of functional limitation among Massachusetts elderly Puerto Ricans, Dominicans, and non-Hispanic white neighborhood comparison group. J Gerontol A Biol Sci Med Sci. 2000;55:M90–7 [DOI] [PubMed] [Google Scholar]
- 3.Crimmins EM, Kim JK, Alley DE, Karlamangla A, Seeman T. Hispanic paradox in biological risk profiles. Am J Public Health. 2007;97:1305–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DP, Bradshaw BS. Rethinking the Hispanic paradox: death rates and life expectancy for US non-Hispanic White and Hispanic populations. Am J Public Health. 2006;96:1686–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey DK, Labarthe DR, Goff DC, Chan W, Nichaman MZ. Community-wide coronary heart disease mortality in Mexican Americans equals or exceeds that in non-Hispanic whites: the Corpus Christi Heart Project. Am J Med. 2001;110:81–7 [DOI] [PubMed] [Google Scholar]
- 6.Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, Hazuda HP. All-cause and cardiovascular mortality among Mexican-American and non-Hispanic White older participants in the San Antonio Heart Study: evidence against the "Hispanic paradox". Am J Epidemiol. 2003;158:1048–57 [DOI] [PubMed] [Google Scholar]
- 7.Kuczmarski MF, Kuczmarski RJ, Najjar M. Food usage among Mexican-American, Cuban, and Puerto Rican Adults: findings from the Hispanic HANES. Nutr Today. 1995;30:30–7 [Google Scholar]
- 8.Bermudez OI, Falcon LM, Tucker KL. Intake and food sources of macronutrients among older Hispanic adults: association with ethnicity, acculturation, and length of residence in the United States. J Am Diet Assoc. 2000;100:665–73 [DOI] [PubMed] [Google Scholar]
- 9.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9 [DOI] [PubMed] [Google Scholar]
- 10.Sacks FM, Obarzanek E, Windhauser MM, Svetkey LP, Vollmer WM, McCullough M, Karanja N, Lin PH, Steele P, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5:108–18 [DOI] [PubMed] [Google Scholar]
- 11.Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–7 [DOI] [PubMed] [Google Scholar]
- 12.Kourlaba G, Panagiotakos DB. Dietary quality indices and human health: a review. Maturitas. 2009;62:1–8 [DOI] [PubMed] [Google Scholar]
- 13.Waijers PM, Feskens EJ, Ocke MC. A critical review of predefined diet quality scores. Br J Nutr. 2007;97:219–31 [DOI] [PubMed] [Google Scholar]
- 14.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96 [DOI] [PubMed] [Google Scholar]
- 15.Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18 [DOI] [PubMed] [Google Scholar]
- 17.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–901 [DOI] [PubMed] [Google Scholar]
- 18.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158:1–13 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. 5 A Day. 2008 July 10th, 2008 [cited 2009 December 26th]; Availabel from: http://www.cdc.gov/nccdphp/dnpa/5aday/ [Google Scholar]
- 20.Maras JE, Newby PK, Bakun PJ, Ferrucci L, Tucker KL. Whole grain intake: The Baltimore Longitudinal Study of Aging. J Food Compost Anal. 2009;22:53–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleveland LE, Moshfegh AJ, Albertson AM, Goldman JD. Dietary intake of whole grains. J Am Coll Nutr. 2000;19:S331–8 [DOI] [PubMed] [Google Scholar]
- 22.McKeown NM, Yoshida M, Shea MK, Jacques PF, Lichtenstein AH, Rogers G, Booth SL, Saltzman E. Whole-grain intake and cereal fiber are associated with lower abdominal adiposity in older adults. J Nutr. 2009;139:1950–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996;275:447–51 [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Gottdiener JS, Siscovick DS. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol. 2006;97:216–22 [DOI] [PubMed] [Google Scholar]
- 25.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–7 [DOI] [PubMed] [Google Scholar]
- 26.Constance C. The good and the bad: what researchers have learned about dietary cholesterol, lipid management and cardiovascular disease risk since the Harvard Egg Study. Int J Clin Pract Suppl. 2009;163:9-14:27–43 [DOI] [PubMed] [Google Scholar]
- 27.Johnson RK, Appel LJ, Brands M, Howard BV, Lefevre M, Lustig RH, Sacks F, Steffen LM, Wylie-Rosett J. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–20 [DOI] [PubMed] [Google Scholar]
- 28.Britten P, Marcoe K, Yamini S, Davis C. Development of food intake patterns for the MyPyramid Food Guidance System. J Nutr Educ Behav. 2006;38:S78–92 [DOI] [PubMed] [Google Scholar]
- 29.Albert CM, Manson JE, Cook NR, Ajani UA, Gaziano JM, Hennekens CH. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation. 1999;100:944–50 [DOI] [PubMed] [Google Scholar]
- 30.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paffenbarger RS, Wing A. L, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75 [DOI] [PubMed] [Google Scholar]
- 32.Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–45 [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30 Suppl 1:S42–7 [DOI] [PubMed] [Google Scholar]
- 34.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72 [DOI] [PubMed] [Google Scholar]
- 35.Marin G, Gamba RJ. A new measurement of acculturation for Hispanics: The Bidimensional Acculturation Scale for Hispanics (BAS). Hisp J Behav Sci. 1996;18:297–316 [Google Scholar]
- 36.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96 [PubMed] [Google Scholar]
- 37.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47 [DOI] [PubMed] [Google Scholar]
- 38.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502 [PubMed] [Google Scholar]
- 39.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–43 [DOI] [PubMed] [Google Scholar]
- 41.Kant AK. Indexes of overall diet quality: a review. J Am Diet Assoc. 1996;96:785–91 [DOI] [PubMed] [Google Scholar]
- 42.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 43.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 44.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–20 [DOI] [PubMed] [Google Scholar]
- 45.Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch Intern Med. 2009;169:851–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levitan EB, Wolk A, Mittleman MA. Relation of consistency with the dietary approaches to stop hypertension diet and incidence of heart failure in men aged 45 to 79 years. Am J Cardiol. 2009;104:1416–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folsom AR, Parker ED, Harnack LJ. Degree of concordance with DASH diet guidelines and incidence of hypertension and fatal cardiovascular disease. Am J Hypertens. 2007;20:225–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nettleton JA, Schulze MB, Jiang R, Jenny NS, Burke GL, Jacobs DR., Jr A priori-defined dietary patterns and markers of cardiovascular disease risk in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2008;88:185–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bermudez OI, Velez-Carrasco W, Schaefer EJ, Tucker KL. Dietary and plasma lipid, lipoprotein, and apolipoprotein profiles among elderly Hispanics and non-Hispanics and their association with diabetes. Am J Clin Nutr. 2002;76:1214–21 [DOI] [PubMed] [Google Scholar]
- 50.Dauchet L, Kesse-Guyot E, Czernichow S, Bertrais S, Estaquio C, Peneau S, Vergnaud AC, Chat-Yung S, Castetbon K, et al. Dietary patterns and blood pressure change over 5-y follow-up in the SU.VI.MAX cohort. Am J Clin Nutr. 2007;85:1650–6 [DOI] [PubMed] [Google Scholar]
- 51.Schulze MB, Hoffmann K, Kroke A, Boeing H. Risk of hypertension among women in the EPIC-Potsdam Study: comparison of relative risk estimates for exploratory and hypothesis-oriented dietary patterns. Am J Epidemiol. 2003;158:365–73 [DOI] [PubMed] [Google Scholar]
- 52.Willett W. Food frequency methods. Nutritional epidemiology. 2nd ed New York: Oxford University Press; 1998. p. 74–100 [Google Scholar]
- 53.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7 [DOI] [PubMed] [Google Scholar]
- 54.Tucker KL, Bermudez OI, Castaneda C. Type 2 diabetes is prevalent and poorly controlled among Hispanic elders of Caribbean origin. Am J Public Health. 2000;90:1288–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.