Abstract
The tumor suppressor ARF plays an essential role in the cellular response to oncogenic stress mainly through activation of p53. Nucleophosmin (NPM), a multifunctional protein, forms a stable protein complex with ARF in the nucleolus and protects ARF from the proteasome-mediated degradation. Notably, NPM is mutated in about one third of acute myeloid leukaemia (AML) patients and these mutations lead to aberrant cytoplasmic dislocation of nucleophosmin (NPM-c). Cytoplasmic NPM mutants lose their abilities to retain ARF in the nucleolus and fail to stabilize ARF. Thus, activation of the ARF-p53 axis is significantly compromised in these AML cells. We have recently identified the ubiquitin ligase of ARF (ULF) as a key factor that controls ARF turnover in human cells. Here, we found that the steady levels of both ARF and p53 are very low in human acute myeloid leukaemia OCI-AML3 cells expressing cytoplamsic dislocated nucleophosmin (NPM-c). As expected, ARF is very unstable and rapidly degraded by proteasome. Nevertheless, ULF knockdown stabilizes ARF and reactivates p53 responses in these AML cells. These results further demonstrate that ULF is a bona fide E3 ligase for ARF and also suggest that ULF is an important target for activating the ARF-p53 axis in human AML cells.
Key words: ARF, ubiquitination, ULF, p53, NPM, B23, NPM-c
Introduction
The p53 tumor suppressor acts as the major sensor for a regulatory circuit that monitors signaling pathways from diverse sources, including DNA damage, oncogenic events and others abnormal cellular processes.1–3 While p53 mutations have been documented in more than half of human tumors, defects in other key components of the p53 pathway, such as the ARF tumor suppressor, are frequently observed in tumor cells that retain wild type p53.4–7 Thus, inactivation of either ARF or p53 appears to be a common, if not universal, feature of human cancer. ARF (known as p14 in humans and p19 in mice) was originally identified as an alternative transcript of the Ink4a/ARF tumor suppressor locus, a gene that encodes the Ink4a/p16 inhibitor of cyclin-dependent kinases.8 While ARF suppresses aberrant cell growth in response to oncogenic stress mainly by activating the p53 pathway, ARF-mediated growth repression can also occur in a p53-independent manner under certain conditions.4 The mechanisms by which ARF activates p53 function are complex. On one hand, ARF is predominately a nucleolar protein and the work by Sherr et al. showed that ARF can stabilize nucleoplasmic p53 by binding Mdm2, a major ubiquitin ligase of p53, and sequestering it in the nucleolus.9 On the other hand, nucleoplasmic forms of ARF can still activate p53 by directly inhibiting the ubiquitin ligase activity of Mdm2.10–12 Moreover, in addition to suppressing Mdm2-mediated effects on p53, ARF may modulate the activity of other E3 ligases such as ARF-BP1, and our recent studies demonstrate that the ARF/ARF-BP1 interaction is involved in both p53-dependent and p53-independent functions of ARF.13 Although the precise molecular mechanisms by which ARF conducts its biological activities need further elucidation, ARF is a bona fide tumor suppressor with an essential role in p53 regulation.
The low steady-state levels of ARF in normal cells are dramatically induced upon oncogenic stress, which suppresses abnormal cell proliferation by triggering p53-dependent growth arrest or apoptosis.4–7 The molecular mechanism of ARF induction has been solely focused on its transcriptional regulation. For example, transcription of ARF is induced upon overexpression of oncogenes such as Myc, E1A, E2F1 and Ras but is repressed by Rb-E2F complexes, Bmi-1, Twist and certain T-box factors.4,14 Although recent studies indicated that ARF polypeptides are degraded by the ubiquitination pathway,15 the ubiquitin ligase(s) responsible for ARF turnover has not been identified and the role of ubiquitin-mediated ARF stability control in oncogenic stress responses remains unclear. We recently showed that while ARF protein is rapidly turned over in normal human cells, it becomes stabilized in most cancer cells.16 Using biochemical purification methods, we identify ULF as a specific ubiquitin ligase for ARF, and show that ULF inactivation in normal cells induces ARF stabilization and ARF-dependent, p53-mediated cell growth arrest. By uncovering a novel mechanism for ARF induction, these findings significantly alter the current view about how oncogenic stress modulates the ARF/p53 pathway.16
Results
The binding between ULF and NPM is required for NPM-mediated effects on ULF activity.
Several studies indicate that ARF stability is tightly regulated by NPM expression.17–22 Since ULF was identified as a NPM-associated factor,16 ULF-mediated control of ARF expression is likely modulated by NPM. To validate a role for NPM in regulating ULF function in vivo, we first tested the interaction between endogenous NPM and ULF proteins. Thus, cell extracts from human osterosarcoma U2OS cells were immunoprecipitated with α-ULF or with control IgG. As seen in Figure 1A, NPM was detected in the immunoprecipitates obtained with the α-ULF antiserum but not with control IgG. Conversely, endogenous ULF was readily immunoprecipitated with a NPM-specific monoclonal antibody but not with control antibody (Fig. 1B). We also examined whether NPM can bind ULF in vitro. As shown in Figure 1C, 35S-labeled ULF strongly bound immobilized GST-NPM but not GST alone. These data demonstrate that ULF interacts with NPM both in vitro and in vivo.
Figure 1.

NPM interacts with ULF in vivo and in vitro. (A) Co-immunoprecipitation of NPM with ULF from U2OS cells. Western blot analysis of whole-cell extract (WCE) (lane 1) or immunoprecipitates with anti-ULF specific antibody (lane 3) or control IgG (lane 2) by a NPM monoclonal antibody (lower) or ULF specific antibody (upper). (B) Co-immunoprecipitation of ULF with NPM from U2OS cells. Western blot analysis of WCE (lane 1) or immunoprecipitates with anti-NPM monoclonal antibody (lane 3) or control IgG (lane 2) by an ULF specific antibody (lower) or a NPM polyclonal antibody (upper). (C) NPM interacts with ULF in vitro. The GST-NPM protein (lane 3) or GST alone (lane 2) were used in a GST pull-down assay with in vitro translated 35S-labeled ULF. The presence of 35S-labeled ULF was detected by autoradiography (upper). The recombinant proteins for GST pull-down assay were stained with Coomassic blue (lower).
To further map the specific domain of NPM critical for ULF interactions, we performed the GST-pull down assays with different deletion mutants of NPM. As shown in Figure 2A, 35S-labeled ULF strongly bound immobilized GST-NPM (187–294) but not GST-NPM (1–117) or GST-NPM (118–186) (lane 5 vs. lane 3 and lane 4). These data suggest that ULF directly interacts with the C-terminus of NPM (187–294). Moreover, by using co-expression of these NPM mutants in cells, we found that the NPM mutant (1–186), lacking the C-terminal domain for binding ULF, failed to inhibit ULF-mediated ubiquitination of ARF (lane 5, Fig. 2B) although the wild type NPM effectively suppressed ARF ubiquitination. These results suggest that the binding between ULF and NPM is required for NPM-mediated effects on ULF activity. Nevertheless, since the nucleolus localization signal (NrLS) sequence is located in the C-terminus of NPM, the same NPM mutant (1–186) also loses its ability to relocate ARF to the nucleolus.
Figure 2.
The binding between ULF and NPM is necessary for NPM-mediated effects on ULF function. (A) ULF interacts with NPM (187–294). The GST-NPM (187–294) (lane 5), GST-NPM (118–186) (lane 4), GST-NPM (1–117) (lane 3) or GST alone (lane 2) were used in a GST pull-down assay with in vitro translated 35S-labeled ULF. 35S-labeled proteins were detected by autoradiography (upper). Levels of the GST alone and different mutant GST NPM proteins were shown (lower). (B) ARF ubiquitination mediated by ULF is inhibited by NPM but not NPM-c and NPM (1–186). NPM-/-p53-/-MEF cells were cotransfected with vectors encoding HA-tagged ubiquitin, p14ARF, ULF, NPM, NPM-c or NPM1–186 as indicated. Lysates were immunoprecipitated with anti-ARF antibody (ab-4, Labvision), and separated proteins were blotted with antibodies to the HA-tag (upper), polyclonal ARF (middle) or monoclonal NPM antibodies (lower). (C) NPM-c as well as NPM interacts with ULF in vitro. The GST-NPM-c protein (GST-NPM-c) (lane 4), GST-NPM (lane 3) or GST alone (lane 2) were used in a GST pull-down assay with in vitro translated 35S-labeled ULF. The presence of 35S-labeled protein was detected by autoradiography (upper). The proteins of GST-NPM and GST alone were stained with Coomassic blue (lower).
The coding sequences of the NPM gene are mutated in ∼35% of primary acute myeloid leukemias (AMLs).23,24 In contrast to the predominant nucleolar localization of wild type NPM, tumor-derived NPM mutants usually reside in the cytoplasm and are thus described as “cytoplasmic NPM mutant” (NPM-c).22–24 Unlike wild type NPM, which promotes ARF retention in the nucleoli, the nucleolar localization of ARF is disrupted by NPM-c.22–24 Notably, the tumor-derived mutant NPM-c cannot relocate ARF to the nucleolus but binds equally well with ULF as the wild type NPM (Fig. 2C). This NPM mutant (NPM-c) fails to inhibit ULF-mediated ubiquitination. Taken together, although the binding between ULF and NPM is required for NPM-mediated effects on ULF activity, these data favor the model that NPM stabilizes ARF most likely, through sequestering ARF away from ULF-mediated ubiquitination in the nucleoplasm.
Inactivation of ULF stabilizes ARF and activates p53 in NPM mutant AML cells.
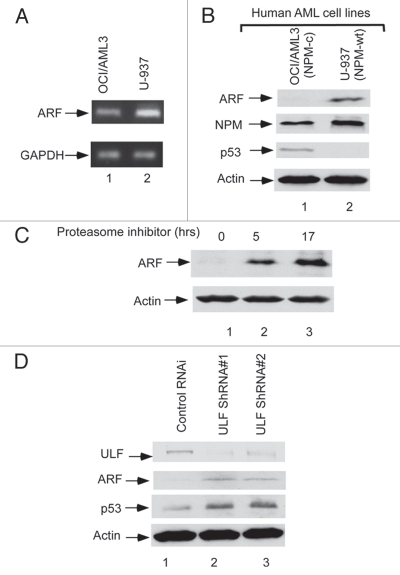
To further investigate the function of NPM-c in modulating ARF stability in tumor cells, we examine the role of ULF in ARF degradation in NPM mutant AML cells. Although mutations of NPMc are high frequent in human AML tumor samples, the NPM-c AML cell line is extremely rare.24 One previous study showed that NPM is mutated in a human acute myeloid leukemia OCI-AML3 cell line and that the mutation of NPM (NPM-c) leads to both the cytoplamsic and nucleoplasmic localization of NPM in these cells.24 We first examined the expression levels of ARF, NPM and p53 in a NPM-c mutant OCI-AML3 cell line. Although ARF was clearly expressed in OCI-AML3 cells (lane 1, Fig. 3A), the protein levels of ARF were extremely low in these cells (lane 1, Fig. 3B). On the contrary, high levels of ARF were detected in a human AML cell line U-937, where NPM is expressed as a wild type form but p53 is mutated (lane 2).24 Notably, the levels of ARF were dramatically increased in OCI-AML3 cells upon the treatment of proteasome inhibitors (Fig. 3C), suggesting that the low steady state levels of ARF protein in OCI-AML3 cells are indeed caused by proteasome mediated degradation. Moreover, as shown in Figure 3D, upon RNAi-mediated knockdown of endogenous ULF in these cells, the protein levels of ARF were significantly increased and p53 was also stabilized. Thus, these new data demonstrated that ULF is responsible for the low stability of ARF observed in NPM-c AML cells and further validate the critical role of NPM in modulating ULF-mediated degradation of ARF under physiological settings.
Figure 3.
Inactivation of ULF reactivates the ARF-p53 axis in AML cells. (A) ARF mRNA expressions by RT-PCR from the cells in human AML NPM cytoplasmic mutant cell line (OCI/AML3) as well as wild type NPM cell line (U-937). (B) ARF protein level is low in OCI/AML3 (NPM-c) cells. Western blot analysis of cell extracts from OCI/AML3 (NPM-c) as well as U-937 (NPM-wt) with the antibodies against ARF, NPM, p53 and actin. (C) ARF expression levels are significantly increased in OCI/AML3 cells after proteasome inhibitor treatment. Western blot analysis of cell extracts from OCI/AML3, harvested at indicated time points (hr) after proteasome inhibitor treatment with an anti-ARF antibody. (D) Endogenous ULF was knocked down by pGPIZ lentiviral ULF ShRNAmir in OCI/AML3 cells. Western blot analysis of cell extracts of OCI/AML3 treated with a control ShRNAmir (lane 1), ULF ShRNA#1 (lane 2), ULF ShRNA#2 (lane 3) with the antibodies against ULF, p53, ARF and actin.
Discussion
The ARF/p53 pathway plays a central role in mediating cellular responses to oncogene activation and other abnormal cellular processes.4–7,25 Since abrogation of this pathway occurs in most, if not all, human tumors, the molecular events that induce ARF in response to oncogene activation are critically important. Our studies highlight a novel mechanism that controls ARF-mediated p53 activation through differential stability of the ARF protein in normal and cancer cells. In particular, we identified that ULF protein acts as an E3 ligase to ubiquitinate ARF and target it for proteasomal degradation in normal cells. Notably, ULF (also called Trip12) has been recently shown involved in the ubiquitin fusion degradation (UFD) pathway.26 It will be interesting to know whether the UFD pathway plays a role in ULF-mediated ARF degradation as N-terminal ubiquitination is especially critical for ARF degradation.15 Moreover, at least two factors commonly associated with oncogenic stress, NPM and c-Myc, bind ULF and inhibit its enzymatic function, resulting in a marked induction of ARF steady-state levels and eliciting the tumor suppression activities of p53. Thus, ULF serves as an important regulator of ARF in oncogenic stress that represses ARF/p53 function in unstressed cells, but allows for robust, transcription-independent, induction of the ARF/p53 pathway in cells at risk of malignant transformation (Fig. 4).
Figure 4.
A model of ARF regulation in a proteasome-dependent pathway.
On the other hand, if ULF acts in normal cells as a key factor of oncogenic stress, then abrogation of the ULF/ARF branch of the p53 pathway should be observed in at least some human malignancies. Obviously, downstream deregulations in the p53 pathway, such as p53 mutation and MDM2 amplification, would impair p53 activation by either DNA damage or oncogene stress.1–3 In contrast, upstream lesions in ARF should specifically disable p53 responses to oncogenic stress. Since ARF mutations are indeed found in human tumors, future studies may also uncover tumor-associated deregulation in the ULF gene. In this regard, it is noteworthy that NPM is one of the most frequent targets of genetic alterations in hematopoietic tumors.23–25,27–31 For example, NPM is rearranged to form an oncogenic fusion gene in anaplastic large-cell lymphomas and mutated in 35% of acute myeloid leukemias to encode potential oncogenic NPM-c polypeptides. Moreover, NPM is commonly overexpressed in many types of human cancer.25,27 Although several studies have shown that NPM is crucial for ARF stability,27–31 the molecular basis for this effect is still not well understood. Based on our findings, ARF stabilization occurs through a mechanism whereby NPM binds and sequesters ARF in the nucleolus, resulting in the segregation of ARF from its cognate E3 ligase (ULF) in the nucleoplasm. This notion is further supported by the fact that tumor-derived cytoplasmic NPM-c mutants, which lack the ability to retain ARF in the nucleolus, also fail to inhibit ULF function and stabilize ARF. Indeed, activation of the ARF-p53 axis is significantly compromised in these AML cells expressing NPM-c proteins. Nevertheless, ULF knockdown stabilizes ARF and reactivates p53 responses in these AML cells. Thus, our studies suggest that ULF is a potential target for reactivating the ARF/p53 axis in human AML cancer cells.
Materials and Methods
Plasmids, antibodies and cell culture.
The full-length ULF, NPM, cytoplasmic NPM mutant (NPM-c), GST-NPM and GST-NPM-c expression vectors were used and have been described previously.16 For pcDNA3.1/v5-His-Topo NPM (1–186), pGEX NPM (1–117), pGEX NPM (118–186) and pGEX NPM (187–294), DNA sequences corresponding to different regions were amplified by PCR from the full length NPM construct and subcloned into respective expression vectors. Rabbit polyclonal p14ARF (ab-4) and mouse monoclonal (ab-3) p14ARF antibodies were purchased from Labvision. Rabbit polyclonal p14ARF (NB 200-111) was from Novus Biologicals. Rabbit polyclonal ULF and polyclonal p14ARF (A300-340A and A300-342A) were purchased from Bethyl. p53-specific monoclonal (DO-1), anti-NPM (H106) polyclonal antibodies were purchased from Santa Cruz Biotechnology. Anti-NPM monoclonal antibody (clone 322) was generous gift from Brunangelo Falini's lab. Rat anti-HA monoclonal antibody was purchased from Roche.
U2OS, 293 and U-937 cells were maintained in DMEM medium, OCI-AML3 cells in MEM medium. All media were supplemented with 10% fetal bovine serum except 20% for OCI/AML3 cells.
GST-pull-down.
GST-NPM and its mutants were induced in Rosetta (DE3) pLys (Novagen) cells at 25°C, extracted with buffer BC500 (20 mM Tris-HCl, pH 7.3, 0.2 mM EDTA, 500 mM NaCl, 10% glycerol, 0.5 mM PMSF and 1 mM DTT) containing 1% NP-40 and purified on glutathione-Sepharose (Pharmacia). pcDNA3.1-ULF were labeled by incorporation of 35S-Methionine during in vitro translation following the manufacture's protocol (Promega). 5 µl of 35S-labeled protein was incubated with 3 µg of the purified GST-NPM, GST-NPM-c or GST alone as indicated in BC200 (same as BC500 except 200 mM NaCl) on a rotator overnight at 4°C. The GST beads were washed three times with binding buffer and two times BC100. 40 µl 1x SDS sample buffer was added to the beads and boiled for 5 min. The presence of 35S-labeled protein was detected by autoradiography.
Lentiviral infections.
GIPZ Lentiviral shRNAmir vectors for ULF and control were purchased from Open biosystems. Lentiviral supernatants were produced by transiently co-transfecting the lentiviral vectors, the packaging vector delta 8.9 and the pMD.G plasmid as previously described.32 For infection, OCI/AML3 cells (3 × 105 cells/ml) were mixed with viral supernatants, supplemented with 5 µg/ml polybrene and centrifuged for 60 min at 450 g, then replaced with fresh viral supernants plus polybrene for overnight. The same procedure for infection was repeated in the second day. The lentiviral infected OCI/AML3 cells were selected with puromycin (0.5 µg/ml) for 5–7 days and collected for analysis.
In vivo ubiquitination assays.
The in vivo ubiquitination assay was performed as reported previously with some modifications.13 NPM-/-p53-/-MEF cells were co-transfected with expression vectors of ARF, HA-ubiquitin, ULF, NPM, NPM-c and NPM (1–186) as indicated. Cells were lysed in RIPA buffer with 0.05% SDS containing protease inhibitors. Cell lysates were precleared with rabbit IgG and protein A/G beads for 1 hr, and precleared supernatants were precipitated with α-ARF antibody (NB200-111, Novus Biologicals). The protein A/G beads were washed four times with RIPA buffer and two times BC100. The beads were boiled in 2x sample buffer for 5 min. Denatured immune complexes were resolved on SDS gel. Western blots were used to detect the proteins with antibodies against HA, ARF and NPM.
RT-PCR analysis.
Total RNAs were isolated from U937 and OCI/AML-3 cells by using TRIzol Reagent (Invitrogen), and cDNA was synthesized from 1 µg of total RNA by using the SuperScript First-Strand synthesis kit (Invitrogen) with the oligo-dT primer. The following primers were used to amplify the prepared cDNA samples in the PCR reaction. Human ARF (forward): 5′-GTG CGC AGG TTC TTG GTG ACC; Human ARF (reverse): 5′-CTG CCC GTG GAC CTG GCT GA. Human GAPDH (forward): 5′-GAA GGT GAA GGT CGG AGT; Human GAPDH (reverse): 5′-GAA GAT GGT GAT GGG ATT TC.
Acknowledgements
We thank James Lee for carefully reading the manuscript and B. Falini for providing the human AML cell lines. This study was supported by grants from NIH and the Leukemia and Lymphoma Society. W.G. is an Ellison Medical Foundation Senior Scholar in aging.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12355
References
- 1.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 2.Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin in Cancer Biol. 2003;13:49–58. doi: 10.1016/s1044-579x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 3.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer. 2006;6:663–673. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- 5.Saporita AJ, Maggi LB, Apicelli AJ, Weber JD. Therapeutic targets in the ARF tumor suppressor pathway. Curr Med Chem. 2007;14:1815–1827. doi: 10.2174/092986707781058869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matheu A, Maraver A, Serrano M. The Arf/p53 pathway in cancer and aging. Cancer Res. 2008;68:6031–6034. doi: 10.1158/0008-5472.CAN-07-6851. [DOI] [PubMed] [Google Scholar]
- 7.Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–1334. doi: 10.1016/j.cell.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative Reading Frames Of The Ink4a Tumor-Suppressor Gene Encode 2 Unrelated Proteins Capable Of Inducing Cell-Cycle Arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 9.Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 10.Llanos S, Clark PA, Rowe J, Peters G. Stabilization of p53 by p14(ARF) without relocation of MDM2 to the nucleolus. Nat Cell Biol. 2001;3:445–452. doi: 10.1038/35074506. [DOI] [PubMed] [Google Scholar]
- 11.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Midgley CA, Desterro JM, Saville MK, Howard S, Sparks A, Hay RT, Lane DP. An N-terminal p14(ARF) peptide blocks Mdm2-dependent ubiquitination in vitro and can activate p53 in vivo. Oncogene. 2000;19:2312–2323. doi: 10.1038/sj.onc.1203593. [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Kon N, Li M, Zhang W, Qin J, Gu W. ARF-BP1/mule is a critical mediator of the ARF tumor suppressor. Cell. 2005;121:1071–1083. doi: 10.1016/j.cell.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 14.Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. doi: 10.1016/s0959-437x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 15.Kuo ML, den Besten W, Bertwistle D, Roussel MF, Sherr CJ. N-terminal polyubiquitination and degradation of the Arf tumor suppressor. Genes Dev. 2004;18:1862–1874. doi: 10.1101/gad.1213904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D, Shan J, Zhu WG, Qin J, Gu W. Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses. Nature. 2010;464:624–627. doi: 10.1038/nature08820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertwistle D, Sugimoto M, Sherr CJ. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol Cell Biol. 2004;24:985–996. doi: 10.1128/MCB.24.3.985-996.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moulin S, Llanos S, Kim SH, Peters G. Binding to nucleophosmin determines the localization of human and chicken ARF but not its impact on p53. Oncogene. 2008;27:2382–2389. doi: 10.1038/sj.onc.1210887. [DOI] [PubMed] [Google Scholar]
- 19.Colombo E, Bonetti P, Lazzerini Denchi E, Martinelli P, Zamponi R, Marine JC, et al. Nucleophosmin is required for DNA integrity and p19(Arf) protein stability. Mol Cell Biol. 2005;25:8874–98886. doi: 10.1128/MCB.25.20.8874-8886.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colombo E, Martinelli P, Zamponi R, Shing DC, Bonetti P, Luzi L, et al. Delocalization and destabilization of the Arf tumor suppressor by the leukemia-associated NPM mutant. Cancer Res. 2006;66:3044–3050. doi: 10.1158/0008-5472.CAN-05-2378. [DOI] [PubMed] [Google Scholar]
- 21.den Besten W, Kuo ML, Williams RT, Sherr CJ. Myeloid leukemia-associated nucleophosmin mutants perturb p53-dependent and independent activities of the Arf tumour suppressor protein. Cell Cycle. 2005;4:1593–1598. doi: 10.4161/cc.4.11.2174. [DOI] [PubMed] [Google Scholar]
- 22.Cheng K, Grisendi S, Clohessy JG, Majid S, Bernardi R, Sportoletti P, et al. The leukemia-associated cytoplasmic nucleophosmin mutant is an oncogene with paradoxical functions: Arf inactivation and induction of cellular senescence. Oncogene. 2007;26:7391–7400. doi: 10.1038/sj.onc.1210549. [DOI] [PubMed] [Google Scholar]
- 23.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. NEJM. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 24.Quentmeier H, Martelli MP, Dirks WG, Bolli N, Liso A, Macleod RA, et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia. 2005;19:1760–1767. doi: 10.1038/sj.leu.2403899. [DOI] [PubMed] [Google Scholar]
- 25.Lindstrom MS, Zhang YP. B23 and ARF—Friends or foes? Cell Biochem Biophy. 2006;46:79–90. doi: 10.1385/CBB:46:1:79. [DOI] [PubMed] [Google Scholar]
- 26.Park Y, Yoon SK, Yoon JB. The HECT domain of Trip12 ubiquitinates substrates of the ubiquitin fusion degradation pathway. J Biol Chem. 2009;284:1540–1549. doi: 10.1074/jbc.M807554200. [DOI] [PubMed] [Google Scholar]
- 27.Feuerstein N, Mond JJ. “Numatrin,” A Nuclear Matrix Protein Associated With Induction Of Proliferation In Lymphocytes-B. J Biol Chem. 1987;262:11389–11397. [PubMed] [Google Scholar]
- 28.Nozawa Y, vanBelzen N, vanderMade ACJ, Dinjens WNM, Bosman FT. Expression of nucleophosmin/B23 in normal and neoplastic colorectal mucosa. J Pathol. 1996;178:48–52. doi: 10.1002/(SICI)1096-9896(199601)178:1<48::AID-PATH432>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Zindy F, Eischen CM, Randle DH, Kamijo T, Cleveland JL, Sherr CJ, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493–505. doi: 10.1038/nrc1885. [DOI] [PubMed] [Google Scholar]
- 31.Rego EM, Ruggero D, Tribioli C, Cattoretti G, Kogan S, Redner RL, et al. Leukemia with distinct phenotypes in transgenic mice expressing PML/RAR alpha, PLZF/RAR alpha or NPM/RAR alpha. Oncogene. 2006;25:1974–1979. doi: 10.1038/sj.onc.1209216. [DOI] [PubMed] [Google Scholar]
- 32.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissure-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]