Abstract
Somatic evolution, which underlies tumor progression, is driven by two essential components: (1) diversification of phenotypes through heritable mutations and epigenetic changes and (2) selection for mutant clones which possess higher fitness. Exposure to ionizing radiation (IR) is highly associated with increased risk of carcinogenesis. This link is traditionally attributed to causation of oncogenic mutations through the mutagenic effects of irradiation. On the other hand, potential effects of irradiation on altering fitness and increasing selection for mutant clones are frequently ignored. Recent studies bring the effects of irradiation on fitness and selection into focus, demonstrating that IR exposure results in stable reductions in the fitness of hematopoietic stem and progenitor cell populations. These reductions of fitness are associated with alteration of the adaptive landscape, increasing the selective advantages conferred by certain oncogenic mutations. Therefore, the link between irradiation and carcinogenesis might be more complex than traditionally appreciated: while mutagenic effects of irradiation should increase the probability of occurrence of oncogenic mutations, IR can also work as a tumor promoter, increasing the selective expansion of clones bearing mutations which become advantageous in the irradiation-altered environment, such as activated mutations in Notch1 or disrupting mutations in p53.
Key words: Notch, p53, fitness, irradiation, hematopoietic, evolution
IR and Cancer
While exposure to IR, including γ- or X-ray irradiation, has been known to be associated with an increased risk of cancer (predominantly leukemias) for over 100 years,1–3 the exact mechanisms underlying IR-induced malignancy are still poorly understood. IR is extensively used in modern medical procedures, such as CT scans and radiotherapy. Indeed, radiotherapy is used to treat 60% of solid tumors in the US, and is highly associated with secondary leukemias and other malignancies.1,4,5 In mice, γ-irradiation has been shown to lead to T-cell lineage lymphomas of the thymus.6 Historically, the carcinogenic properties of IR have been attributed to the capacity to induce oncogenic mutations,2 albeit without evidence indicating that oncogenic events found in IR-associated cancers were directly caused by IR exposure. Causation of oncogenic mutations is not the only feasible mechanism to explain the link between IR and cancers, as the effects of IR on tissues are quite complex. Beyond the direct induction of DNA damage, IR exposure leads to persistent increases in the production of reactive oxygen species (ROS), alterations in cell signaling and augmented levels of particular cytokines and growth factors.7,8 Irradiation can also result in cell cycle arrest, senescence or apoptosis, with different responses for different cell types. Global perturbations of the cellular microenvironment and both direct and indirect DNA damage as a result of IR exposure, likely exert effects on the fitness of stem and progenitor cell populations.
The concept of fitness can be applied toward populations of stem and progenitor cells.
Progression of cancers represents a process of somatic evolution that follows Darwinian logic: genetic mutations or heritable epigenetic changes [together referred to as “(epi)genetic”] that improve competitive advantage are selected for, leading to increased frequencies of tumor cells with selectively advantageous (epi)genotypes over time. The competitive advantage is best described in terms of fitness: a measure of the ability of individuals of a certain genotype to pass on this genotype to subsequent generations. Tumor cell clones with higher fitness values compared to the average fitness of cells competing for the same niches will be subjected to positive selection (increase in frequency), while those with lower fitness will be subjected to negative selection. Thus, the competitive success or failure of any given clone is dependent on the fitness of competing clones. Importantly, the relationship between an (epi)genotype and fitness is not invariant and instead depends on the environment, as the same genotypes can provide dramatically different fitness in different environments.9
But can the concept of fitness be applied toward initiating stages of tumorigenesis in populations of stem and progenitor cells? The meaningful applicability of the concept of fitness is contingent on the existence of competition between clones that differ in heritable traits. Phenomena of competition between wild-type (WT) stem cells in multicellular organisms have been known for a long time.10 In fact, the competitive nature of the hematopoietic system has long been exploited in competitive bone marrow (BM) transplantation experiments, a standard tool in hematopoietic research. Were competing stem cells (epi) genetically identical, competition between different cells would have no evolutionary consequences. However, in real populations of stem cells, heritable diversity should inevitably arise as the result of the accumulation of spontaneously occurring (epi) mutations as well as mutagen exposure. Therefore, the concept of fitness should be applicable toward populations of stem cells, as both competition with peers and differences in heritable features should lead to the selection of clones with higher fitness and the success of this competition would be determined both by the fitness of a given clone and by the fitness of competing cells.
Similarly, the concept of fitness can be applied to evolutionary processes which occur in populations of short-term progenitor cells. Although mutational changes in progenitors mostly represent evolutionary dead ends, as these cells possess limited self-renewal capacity, (epi)mutations occurring in progenitors have a non-zero chance of being fixed as a result of additional mutations that confer self-renewal. Moreover, (epi)mutations that are fixed within stem cell populations will impact the fitness of downstream progenitor cells. Recent publications have highlighted the relevance of competition between WT stem and progenitor cells in normal hematopoiesis, where the competitive success or failure is dependent on prior damage and the relative activity of p53.11–13,23
IR Exposure Impacts Cellular Fitness
Multiple studies of species ranging from bacteria to humans have shown that radiation exposure results in a decrease in cellular fitness. For example, exposure of E. coli to components of solar radiation has been shown to result in reduced fitness,14 and IR exposure has been shown to decrease the fitness of murine hematopoietic stem cells,12,13 coinciding with an increase in multipotent progenitors displaying hallmarks of senescence.13 This reduction in fitness can be at least partially caused by accumulation of random mutations, as previous studies have shown that the genetic disruption of DNA repair pathways in mice result in a competitive disadvantage for HSC.15–17
In humans, long-term studies following atomic bomb survivors indicate that up to 50 years after IR exposure, individuals have reduced hemoglobin levels, increased peripheral blood cytokine levels and decreased T-cell-mediated immunity.18,19 Individuals with occupational exposure to IR show decreased humoral immunity as well as peripheral blood CD4+ T-cell numbers,20 and pediatric leukemia survivors who undergo radiation therapy experience multiple growth and developmental defects as well as increased risk of secondary malignancies.5
Decreased Fitness Generates Selective Pressure for Adaptive Mutations
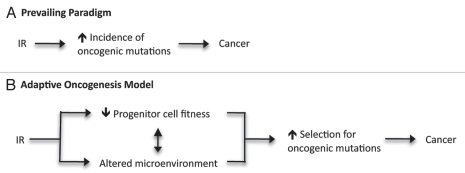
Prevailing paradigms attribute the tumorigenic effects of ionizing radiation to its direct mutagenic action on genetic loci encoding oncogenes and tumor suppressor genes (Fig. 1A). We have previously proposed that stem and progenitor cells are evolutionarily highly adapted to their niches in young, healthy individuals, minimizing the selective pressure for trait-altering (epi) mutations.21 However, when cellular fitness is reduced as a result of damage accumulating in both the stem cells themselves and in their microenvironment, a WT (epi)genotype will no longer possess optimal fitness and certain oncogenic (epi)mutations will have an increased chance of being adaptive and hence advantageous (Fig. 1B).
Figure 1.
Prevailing paradigm and adaptive oncogenesis models of cancer development. (A) Conventional Model: IR exposure increases the production of oncogenic mutations and the accumulation of these mutations leads to cancer development. (B) Adaptive Oncogenesis: The promotion of cancer development by IR exposure acts through decreasing progenitor cell fitness and altering the microenvironment, which increase selection for adaptive oncogenic mutations, promoting the initiation of cancer.
Thus, analogous to Natural Selection observed at the organismal level, reductions in the fitness of stem cell pools should increase selective pressure for adaptive (epi)mutations, which provide a cell with an increase in fitness relative to neighboring cells competing for the same niches. Indeed, we have shown that oncogenic mutations can be such adaptive mutations.12,22 Notably, a mutation does not need to completely restore the fitness of a cell to pre-insult levels to be adaptive, but simply must improve fitness above the average fitness of the population (which was reduced by an insult like IR). Finally, it is important to emphasize that models A and B depicted in Figure 1 likely act in concert: IR-induced genomic damage should augment the genetic diversity within cellular populations upon which IR-mediated selective pressures can act.
Prior exposure to IR leads to persistent decreases in HSC fitness, promoting selection for activating Notch1 mutations.
We and others have shown that exposure to IR results in persistent decreases in the fitness of both HSC and committed hematopoietic progenitors.11–13 Thus, even after hematopoiesis has fully recovered months after sublethal irradiation, with HSC and progenitor numbers restored to pre-irradiation levels (as determined by cell surface marker expression), the fitness of these stem and progenitor cells is severely reduced. Limiting dilution assays reveal that the number of functional HSCs in previously irradiated but homeostatically restored (“IRp”) mice is decreased nearly 10-fold as compared to control mice.12,13,23 Since designation as an HSC requires contributions to hematopoiesis for at least 3 months, these data are consistent with poor function for most IRp HSC. Additional studies have shown that IRp HSC form smaller colonies in vitro,13 and IRp BM cells compete poorly with non-irradiated competitors to reconstitute hematopoiesis in competitive BM transplantation assays.12,13,23 One potential explanation for decreased fitness of HSC and progenitor cells as the result of IR exposure may be an induction of senescence in affected cells, as indicated by the persistent upregulation of senescence markers in HSC after IR exposure,11,13 although IRp HSC still possess some replication and reconstitution capacity as shown by their ability to maintain hematopoiesis in mice in the absence of competition.11–13 These results suggest that exposure to IR results in partial but not complete loss of HSC function, potentially by decreased self-renewal, survival, differentiation or through other mechanisms that affect stem cell fitness.
The reduction of fitness of HSC and progenitor cells resulting from prior IR exposure, together with the known increases in hematopoietic malignancies following IR, represent a context compatible with the Adaptive Oncogenesis hypothesis (Fig. 1B). Therefore, we asked whether prior irradiation might alter selection for oncogenic mutations. Surprisingly, we found that prior IR exposure selects against expression of Bcr-Abl or activated N-Ras, inhibiting clonal expansion and leukemogenesis driven by these oncogenes.12 Notably, Bcr-Abl is clearly adaptive in other contexts of impaired hematopoietic fitness, such as due to impaired DNA replication.22 In contrast, previous IR substantially enhanced selection for and leukemogenesis driven by the activated Notch1 mutant, ICN1. Notch1, a transmembrane receptor, has been implicated in stem cell maintenance by inhibiting differentiation as well as increasing self-renewal.24–26 Activating Notch1 mutations that generate N-terminally truncated proteins that are predicted to functionally mimic the intracellular Notch1 domain (ICN1), are found in more than half of human T-cell acute lymphoblastic leukemia (T-ALL) patients.27 Additionally, leukemias in mice exposed to IR are often associated with Notch1 mutations predicted to produce truncated and activated proteins.28
Selection for ICN1 occurs within IRp HSC-enriched pools.
Clonal expansion of ICN1 expressing cells only occurs if competitor cells are also IRp, but does not occur if the competitor cells are non-irradiated, indicating a non-cell autonomous component to selection for ICN1 expression: non-irradiated competitor cells can suppress the expansion of IRp HSC expressing ICN1.12 These results support the hypothesis that the selective advantage provided by oncogenic events is specific to particular contexts: a cellular insult alters HSC fitness in such a way that particular oncogenic events, but not all, provide the cell with a selective advantage relative to compromised competitor cells. The fitness of cell populations may need to decline before certain oncogenic mutations become advantageous, allowing for clonal expansion of the mutant cells. These conclusions may appear counterintuitive, as we propose that random changes induced by IR exposure lead to consistent and recurrent defects that contribute to selective adaptation by specific oncogenic mutations (whether IR-induced or not). We argue that although the damage is untargeted, cells are capable of mounting specific responses, which could affect the adaptive landscape in predictable ways. Additionally, certain signaling pathways or cellular processes may be more sensitive to global perturbations.
At present, we do not understand why ICN1 becomes adaptive within IRp HSC pools. One speculative possibility is that IRp HSC lose self-renewal capacity, which is reversed by ICN1 expression. Irrespective of the mechanisms that underlie increased selection of ICN1 expressing cells in previously irradiated BM, the ability of unirradiated BM competitors to reverse both the competitive advantage and increased leukemogenesis of ICN1 transduced irradiated progenitors suggests that normal hematopoiesis can be tumor-suppressive. The tumor suppressive effect of normal hematopoietic progenitors is also consistent with our previous studies which showed that fit competitors potently limit the expansion of Bcr-Abl transduced and p53 mutant hematopoietic progenitors under a context of genetically impaired DNA replication caused by E2f mutations.22 Moreover, chemical impairment of the proliferation of rat hepatocytes has been shown to promote the expansion of normal or initiated liver cells transplanted into these livers, resulting in substantially increased carcinoma development from the initiated cells, while a normal liver environment suppressed expansion and carcinogenesis.29 An analogy can be drawn from ecology: the restoration of native species in grasslands has been shown to limit the invasion of a non-native grass species, and native species that occupy similar niches as the invasive species optimally prevent invasion.30
IR-Dependent Selection for Radioprotective Mutations
The study described above focused on stable effects of carcinogenic contexts on progenitor fitness,12 which lead to selection for adaptive oncogenic events, which is mechanistically very different from the immediate selection for mutations conferring resistance following an acute insult. Cytotoxicity associated with carcinogenic treatments has been proposed to increase selection for oncogenic mutations that confer resistance to the insult, thereby initiating tumorigenesis.21,31–34 Despite the persuasive logic of this idea, its validity has had limited experimental support in vivo.
Perhaps the best potential candidate for an oncogenic mutation providing increased selection under cytotoxic stress is the tumor suppressor p53, which is an essential regulator of apoptosis in response to many forms of cellular stress, such as DNA damage or oncogenic signaling.35,36 The role of p53 in cancer initiation and progression has been explored by many groups and is recognized as a critical tumor suppressor whose mutation or loss in about 50% of human cancers appears to be a pivotal step in cancer development.37 Recent studies from our lab and the Medzhitov lab using mouse models have shown that p53 loss protects hematopoietic stem and progenitor cells from IR exposure, both by preventing the immediate loss of cells as well as by promoting the long-term maintenance of progenitor fitness.11,23 Thus, it's not just the IR-induced damage per se that reduces cell fitness, but the level of p53 activation in the cell. These studies indicate that in a normal environment with negligible external stress, there is minimal to no selective pressure for decreased p53 activity. In contrast, loss or reductions in p53 activity are strongly selected for in mouse HSC and progenitor cells following acute irradiation. p53 loss in the context of acute IR exposure provides radioprotection that results in a long-term increase in fitness, expansion of p53 disrupted progenitor clones,11,23 and increased lymphomagenesis.23 In contrast, p53 disruption does not provide an obvious selective advantage within previously irradiated BM allowed to recover from irradiation23 (but does prove advantageous when inactivated 7 days post-IR11). Thus, p53 deficiency must be present during or soon after IR exposure in order to confer a selective advantage.
Gene expression analyses of HSC expressing WT or mutant p53 during hematopoietic reconstitution under competitive and non-competitive contexts have provided important insight into the mechanism of IR-dependent selection for p53 inhibition. While during non-competitive reconstitution post-IR, WT HSC exhibit increased expression of genes important for cell cycle progression, the expression of these same genes was inhibited and the expression of pro-senescent genes was increased in WT competitors in the presence of p53 mutant competitors.11 Inhibiting p53 prevented the induction of this senescence program, both in competitive and non-competitive contexts of hematopoietic reconstitution. By competing for niches or by other non-cell autonomous means, following IR the p53 mutant “winners” suppress the ability of WT “losers” to be maintained in the self-renewing HSC pools.11,38 Still, it is not entirely clear why p53 loss promotes long-term radioprotection. Certainly, the failure to activate p53 dependent apoptotic programs would be expected to prevent the immediate elimination of progenitors following irradiation. Reductions of p53 activity during IR may also prevent responses to IR-induced damage that result in more stable reductions in fitness, such as the activation of senescence-like programs (even if stem and progenitor cells with these programs activated do not completely lose clonogenic potential).
Relating p53-dependent radioprotection and tumor suppression.
In mice, null mutation of p53 dramatically predisposes to the development of T-cell lymphomas,39–41 and irradiation of p53 heterozygotes leads to development of p53 mutant lymphomas.42 The tumor suppressive function of p53 in the context of irradiation has been long attributed to the p53 dependence for elimination of cells with excessive DNA damage by apoptosis. This attribution was strongly challenged by studies that used murine models of inducible p53 restoration or genetic deletion to examine chronologic specificity of p53 activity in lymphomagenesis. In the Evan lab study, conditional activation of p53 during irradiation had no effect on its tumor suppressor function, while a 6 day period of p53 activation starting 8 days post IR (after the acute damage is resolved) was sufficient to delay tumor onset.43 The Donehower lab showed that induction of p53 deletion for up to 4 weeks post-IR resulted in similar lymphoma rates compared to disrupting p53 prior to IR.44 Therefore, the authors proposed that p53 activity during IR exposure is not tumor suppressive whereas p53 activity post-IR is essential in prevention of oncogenesis. These studies concluded that the essential tumor suppressor function of p53 during IR-induced lymphomagenesis is to eliminate cells with activated oncogenes, while the p53-dependent elimination of cells with radiation-induced DNA damage is dispensable. The recent studies from our lab and the Medzhitov lab extend this conclusion: since irradiation leads to elimination and functional arrest of progenitors with intact p53 function, it selects for p53 deficient clones. Thus, under conditions of total body irradiation (TBI), p53 may actually be tumor promoting by increasing selection for clones with radioprotective mutations.
Still, all of the models used to study irradiation and carcinogenesis described above rely on TBI. While clinically relevant, TBI is not a stress that we (or other animals) evolved to endure, as TBI doubtfully limited the fitness of individuals during our evolutionary history. But the evolution of mechanisms to eliminate the occasional damaged cell likely did contribute to the fitness of long-lived multicellular organisms. Such limited exposure to radiation (and other DNA damaging agents) results in damage to a small population of cells rather than to the majority of cells in the body, in which case p53-dependent deletion of the rare damaged cell(s) would be expected to be tumor suppressive (contrasting with the situation for TBI). We would argue that the damage-induced p53 response serves an important function in an organism by reducing the fitness of a cell (0 < fitness < 1; where the fitness of the WT genotype is 1), leading to the loss of the damaged cell due to competition from less damaged or undamaged cells. This p53-modulated competitive elimination of damaged cells could complement p53-dependent apoptosis and senescence, which can lead to the complete and immediate elimination of a damaged cell from the replicative progenitor pool (fitness = 0). We would speculate that at high levels of damage, cell autonomous mechanisms that lead to apoptosis and senescence may dominate. But at low levels of damage, which are probably more common, mechanisms that reduce the fitness of a damaged cell may predominate.
Irradiation-Induced Alterations of Cellular Microenvironments
IR-induced changes in the microenvironment have been proposed to contribute to IR-dependent tumor promotion. Carcinogens, such as IR exposure, are able to induce changes in the microenvironment milieu, including alterations in cytokines, chemokines, ROS, growth factors, cell-to-cell signaling and rearrangement of the ECM, and these changes are expected to contribute to cancer development.7,45 Recent studies from the Campisi lab suggest that senescent cells induced by persistent DNA damage may contribute to these IR-mediated changes, by secretion of tumor-promoting pro-inflammatory cytokines, such as IL6.45
It should be noted that unlike the studies described earlier, microenvironmental models have generally focused on alterations of the extra-cellular matrix and supporting stroma (rather than on the normal stem/progenitor cells that compete with the oncogenically initiated stem/progenitor cells), postulating that these alterations improve the proliferation and survival of pre-cancer and cancer cells. In addition to positive impacts on pre-cancer cells, many of the IR-induced microenvironmental changes would be expected to alter the adaptive landscape by decreasing the fitness of stem cells, even for stem cells not directly exposed to irradiation. Particular oncogenic mutations could provide an adaptation to such microenvironment-mediated changes in stem cell fitness, leading to clonal expansion of oncogenically initiated cells. Even beyond initiation, cancer progression is associated with numerous barriers imposed by the microenvironment, such as nutrient limitation, providing pressure for the selection of specific oncogenic mutations that overcome these barriers.46 IR exposure either before tumor initiation or as a therapeutic for an established cancer would certainly be expected to alter barriers (decreasing some while enacting others), and thus influence the evolutionary path of the cancer.
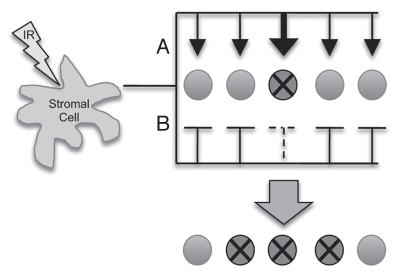
Given the complexity of the microenvironment and the effects of IR, it is likely that an irradiated microenvironment influences cancer initiation and progression by mediating both positive and negative influences on progenitor cells (with and without oncogenic mutations). If IR-induced effects on the microenvironment are to promote cancer initiation, then these influences should be selective for oncogenically initiated cells, and may do so in one of two ways: (1) stromal cells produce positive cell proliferation/survival signals which have a greater positive impact on the oncogene-bearing stem cells relative to WT (Fig. 2A). (2) Stromal cells produce inhibitory signals that are less inhibitory for mutant stem cells (Fig. 2B). As the tumor grows, the relevant cellular competition influenced by the IR-exposed microenvironment may shift from being between normal and initiated cells to between different malignant clones.
Figure 2.
IR-induced alteration of the stromal compartment can influence selection for mutant progenitor cells. The cell marked with an X depicts a mutant progenitor cell; arrows depict positive influences on progenitor cells; bars represent inhibitory influences on progenitor cells; the weight of the arrow or bar represents the predicted magnitude of influence the stromal cell exerts on the progenitor cell.
IR Exposure and the Adaptive Landscape
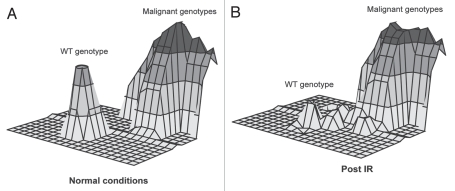
Exposure to IR results in both an increase in genetic diversity as well as alterations of selective pressure, each important for Natural Selection (whether at the species level or at the somatic cell level). We have argued that long-lived multicellular organisms (like us) have evolved stem cell populations with high fitness, not only as a means of efficiently maintaining a tissue, but also because high fitness in a cell population will oppose somatic evolution.21 Like animal populations well adapted to their environments, Stabilizing Selection should limit changes that improve fitness in a population of stem cells with high fitness. Highly effective competition in a young healthy stem cell population should serve to maintain the status quo, preventing somatic evolution. The transition to pre-cancer or cancer genotypes, indicated by the malignant genotype fitness peak in Figure 3A, is a considerable leap, both difficult and unlikely (due to intermediates with low fitness). Intrinsic tumor suppressor mechanisms (e.g., apoptosis) may create an additional pit in the landscape around the WT fitness peak, eliminating cells with potentially oncogenic mutations. 47 But in progenitor pools damaged by irradiation (or aging, etc.), the fitness landscape will be dramatically altered (Fig. 3B). The fitness of the stem cell pool will be reduced, promoting selection for mutations and epigenetic events that improve fitness. IR exposure will of course also increase genetic diversity in the population, providing fuel for selection. Finally, IR effects on the microenvironment will change the adaptive landscape, such as by altering niches, creating new opportunities for adaptation (new fitness peaks that may facilitate transitions to malignancy).Thus, IR-induced perturbations in the fitness landscape allow for easier transitions to malignant genotypes with higher fitness. Effects of IR or other insults on fitness will most likely need to be in long-lived stem cell populations, in order to exert stable effects on the adaptive landscape. Altered selection could still occur in a short-term progenitor population, the fitness of which could be affected by changes incurred by the relevant stem cells.
Figure 3.
Adaptive landscape of stem cell populations under normal conditions and in irradiated or aging environments. The x- and y-axes represent the potential genetic and epigenetic diversity. Adjacent points on the X-Y landscape correspond to more similar (epi)genotypes. The z-axis (vertical axis with peaks above the plane) represents fitness.
Conclusions
We propose that long-lived multicellular organisms have evolved fitness landscapes for their stem cells that highly favor the WT (epi)genotype, preventing somatic evolution. In addition to potentially increasing the frequency of oncogenic mutations, ionizing irradiation can impact the landscape in multiple ways, such as by stably reducing the fitness of stem cell pools, leading to selection for oncogenic mutations such as in Notch1. The selection for oncogenic mutations such as in Notch1 adaptive within IRp HSC pools with persistently reduced fitness is mechanistically different than the selection for radioprotective mutations (such as in p53). Studies described above also indicate that the effects of IR on the fitness of stem cells is in part genetically controlled, with loss of p53 activity associated with better maintenance of fitness post-IR. Thus, under contexts of the rare damaged cell surrounded by competing undamaged or less damaged cells, the p53 tumor suppressor pathway normally acts to eliminate cells with damage by decreasing their competitive edge. But under contexts of damage to the majority of a stem cell population (such as with TBI), p53-mediated elimination of such a massive number of cells leads to selection and expansion of radioresistant cells, including p53-mutant cells, in that the mass deletion and impairment of stem cells after TBI reduces the ability of these cells to act as effective competitors.
Generally speaking, insults like IR (or more unavoidably, aging) create stem cell pools with “room for improvement.” (Epi) mutations that improve a cell's phenotype are much less likely to be fixed or selected for within a cell population with high fitness, whereas if the mean fitness of a population declines due to cellular insults such as IR, there is an increased probability of selection for mutations that provide a fitness advantage to cells relative to the impaired competition. We think that this logic can also be applied to solid malignancies, though the competition in stem and progenitor cell pools might be more limited by the spatial constrains of niches. It will be no trivial task to tease out the mechanisms by which IR induced cell-intrinsic and -extrinsic alterations impact on the adaptive landscape, but understanding these mechanisms is essential for adequate comprehension of the links between IR and carcinogenesis.
Acknowledgements
We would like to thank Drs. Bob Gatenby, Larry Donehower, Ruslan Medzhitov and Tanya Bondar for their critical review of this manuscript. J.D. is supported by the National Cancer Institute (RO1 CA109657) and A.M. by a Department of Defense Breast Cancer Research Program Postdoctoral Fellowship: BC087579.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12311
References
- 1.Finch SC. Radiation-induced leukemia: Lessons from history. Best Pract Res Clin Haematol. 2007;20:109–118. doi: 10.1016/j.beha.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 3.Ron E. Ionizing radiation and cancer risk: evidence from epidemiology. Radiat Res. 1998;150:30–41. [PubMed] [Google Scholar]
- 4.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 5.Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111:5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kominami R, Niwa O. Radiation carcinogenesis in mouse thymic lymphomas. Cancer Sci. 2006;97:575–581. doi: 10.1111/j.1349-7006.2006.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment—tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 8.Rzeszowska-Wolny J, Przybyszewski WM, Widel M. Ionizing radiation-induced bystander effects, potential targets for modulation of radiotherapy. Eur J Pharmacol. 2009;625:156–164. doi: 10.1016/j.ejphar.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Sieber OM, Tomlinson SR, Tomlinson IP. Tissue, cell and stage specificity of (epi)mutations in cancers. Nat Rev Cancer. 2005;5:649–655. doi: 10.1038/nrc1674. [DOI] [PubMed] [Google Scholar]
- 10.Khare A, Shaulsky G. First among equals: competition between genetically identical cells. Nat Rev Genet. 2006;7:577–583. doi: 10.1038/nrg1875. [DOI] [PubMed] [Google Scholar]
- 11.Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 6:309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marusyk A, Casas-Selves M, Henry CJ, Zaberezhnyy V, Klawitter J, Christians U, et al. Irradiation alters selection for oncogenic mutations in hematopoietic progenitors. Cancer Res. 2009;69:7262–7269. doi: 10.1158/0008-5472.CAN-09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muela A, Garcia-Bringas JM, Arana II, Barcina II. The Effect of Simulated Solar Radiation on Escherichia coli: The Relative Roles of UV-B, UV-A and Photosynthetically Active Radiation. Microb Ecol. 2000;39:65–71. doi: 10.1007/s002489900181. [DOI] [PubMed] [Google Scholar]
- 15.Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 16.Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood. 2003;102:1626–1633. doi: 10.1182/blood-2002-10-3035. [DOI] [PubMed] [Google Scholar]
- 17.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 18.Kusunoki Y, Hayashi T. Long-lasting alterations of the immune system by ionizing radiation exposure: implications for disease development among atomic bomb survivors. Int J Radiat Biol. 2008;84:1–14. doi: 10.1080/09553000701616106. [DOI] [PubMed] [Google Scholar]
- 19.Wong FL, Yamada M, Tominaga T, Fujiwara S, Suzuki G. Effects of radiation on the longitudinal trends of hemoglobin levels in the Japanese atomic bomb survivors. Radiat Res. 2005;164:820–827. doi: 10.1667/rr3470.1. [DOI] [PubMed] [Google Scholar]
- 20.Godekmerdan A, Ozden M, Ayar A, Gursu MF, Ozan AT, Serhatlioglu S. Diminished cellular and humoral immunity in workers occupationally exposed to low levels of ionizing radiation. Arch Med Res. 2004;35:324–328. doi: 10.1016/j.arcmed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Marusyk A, DeGregori J. Declining cellular fitness with age promotes cancer initiation by selecting for adaptive oncogenic mutations. Biochim Biophys Acta. 2008;1785:1–11. doi: 10.1016/j.bbcan.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilousova G, Marusyk A, Porter CC, Cardiff RD, DeGregori J. Impaired DNA replication within progenitor cell pools promotes leukemogenesis. PLoS Biol. 2005;3:401. doi: 10.1371/journal.pbio.0030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8:1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 25.Stier S, Cheng T, Dombkowski D, Carlesso N, Scadden DT. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood. 2002;99:2369–2378. doi: 10.1182/blood.v99.7.2369. [DOI] [PubMed] [Google Scholar]
- 26.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 27.Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 28.Tsuji H, Ishii-Ohba H, Ukai H, Katsube T, Ogiu T. Radiation-induced deletions in the 5′ end region of Notch1 lead to the formation of truncated proteins and are involved in the development of mouse thymic lymphomas. Carcinogenesis. 2003;24:1257–1268. doi: 10.1093/carcin/bgg071. [DOI] [PubMed] [Google Scholar]
- 29.Laconi S, Pani P, Pillai S, Pasciu D, Sarma DS, Laconi E. A growth-constrained environment drives tumor progression invivo. Proc Natl Acad Sci USA. 2001;98:7806–7811. doi: 10.1073/pnas.131210498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakker J, Wilson S. Using ecological restoration to constrain biological invasion. Journal of Applied Ecology. 2004;41:1058–1064. [Google Scholar]
- 31.Blagosklonny MV. Oncogenic resistance to growth-limiting conditions. Nat Rev Cancer. 2002;2:221–225. doi: 10.1038/nrc743. [DOI] [PubMed] [Google Scholar]
- 32.Laconi E, Doratiotto S, Vineis P. The microenvironments of multistage carcinogenesis. Semin Cancer Biol. 2008;18:322–329. doi: 10.1016/j.semcancer.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 34.Thilly WG. Have environmental mutagens caused oncomutations in people? Nat Genet. 2003;34:255–259. doi: 10.1038/ng1205. [DOI] [PubMed] [Google Scholar]
- 35.Junttila MR, Evan GI. p53—a Jack of all trades but master of none. Nat Rev Cancer. 2009;9:821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 36.Wallace-Brodeur RR, Lowe SW. Clinical implications of p53 mutations. Cell Mol Life Sci. 1999;55:64–75. doi: 10.1007/s000180050270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soussi T, Wiman KG. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell. 2007;12:303–312. doi: 10.1016/j.ccr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Green DR. Cell competition: pirates on the tangled bank. Cell Stem Cell. 6:287–288. doi: 10.1016/j.stem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 40.Harvey M, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A, Donehower LA. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet. 1993;5:225–229. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- 41.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 42.Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet. 1994;8:66–69. doi: 10.1038/ng0994-66. [DOI] [PubMed] [Google Scholar]
- 43.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 44.Hinkal G, Parikh N, Donehower LA. Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression. PLoS One. 2009;4:6654. doi: 10.1371/journal.pone.0006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatenby RA, Gillies RJ. A microenvironmental model of carcinogenesis. Nat Rev Cancer. 2008;8:56–61. doi: 10.1038/nrc2255. [DOI] [PubMed] [Google Scholar]
- 47.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]