Abstract
Hyperforin (HF) is a phloroglucinol compound obtained from St. John's Wort (Hypericum perforatum). Recent studies have shown that Hyperforin can be used to improve psychopathologic symptoms of Alzheimer's disease but the mechanism is not clear. This may be partly due to the difficult in studying Hyperforin, since this chemical is unstable and is sensitive to light, oxygen, and heat. In this study, we explored the effects of acetylate hyperforin (ace-HF), a stable derivative of hyperforin, on the processing of amyloid precursor protein (APP). HEK293 cells transfected with pcDNA3.1APP695sw and SH-SY5Y cells were treated with ace-HF, followed by measuring the levels of APP and sAPPα. Twelve hours of treatment led to an increase in extracellular sAPPα, but APP mRNA and protein levels were unchanged. Further studies with α-secretase and a pan PKC inhibitor, Calphostin C, indicated that ace-HF's effect on extracellular sAPPα was closely related to PKC activities and α-secretase activities. Our findings suggest that ace-HF can modulate α-secretase-mediated APP processing via a PKC signaling pathway.
Keywords: Soluble amyloid precursor protein α (sAPPα), protein kinase C (PKC), Alzheimer's disease (AD), hyperforin
Introduction
One of the hallmarks of Alzheimer's disease (AD) is the neuronal senile plagues, which are composed of β-amyloid (Aβ) peptides with 39–43 amino acids (Mw-4kDa) [1]. β-amyloid is derived from amyloid precursor protein (APP) after sequential cleavages of APP by β- and γ-secretases. In addition to the amyloidgenic pathway, there is a non-amyloidgenic pathway, which involves the activation of α-secretase. The activated α-secretase cleaves the Aβ sequence of APP, therefore precluding Aβ production. The α-secretase-mediated APP processing results in generating sAPPα (soluble amyloid precursor protein α) [2,3]. In vitro studies have suggested multiple neuron-protective and neurontrophic functions of sAPPα, which can promote neurite outgrowth [4] and protect neurons from metabolic and excitotoxic insults [5,6]. It has also been shown that in vivo administration of sAPPα to severe diffuse traumatic brain injury in rats can improve motor function and reduced neuronal cell loss and axonal injury of the animals [7]. These findings suggest that promoting the non-amyloidgenic pathway by pharmaceutical modulation is likely beneficial for AD treatment [8].
Hyperforin (HF) is one of the major active constituents of the extracts of St. John's Wort (Hypericum perforatum), which have been used for a long time to treat depressive episodes [9,10]. Published evidence indicates that HF has a broad range of activities, including inhibition of synaptosomal uptake of norepinephire, dopamine, serotonin, GABA and L-glutamate, modulation of neuronal membranes, and inhibition of cyclooxygenase-1 and ion channels [11]. Froestl and coworkers reported that HF is able to enhance the production of sAPPα [12]. Their studies reveal that HF can be used as a potential drug for AD treatment. However, the mechanism and signal pathway associated with this functional role are not clear. This may be due to the instability of HF, which represents the major drawback for clinical use of HF in AD treatment. In practice, HF is extremely sensitive to light and oxygen, and its activity declines quickly even when the fresh plant is dried [13]. To facilitate the study of HF, chemical modifications have been introduced to stabilize this chemical [14]. Acetylate hyperforin (ace-HF) is one of the derivatives of HF with improved stability [15], which is also helpful in passing through the blood brain barrier due to its increased lipid solubility. In this study, we have examined the effect of ace-HF on the cleavage of overexpressed and endogenous APP in HEK293 and SH-SY5Y cells. Our results reveal a role of the PKC signal pathway in mediating the effects of ace-HF on APP processing.
Materials and Methods
Drug
Ace-HF was produced in the Laboratory of Pharmacognosy and Natural Medicinal Chemistry, School of Pharmaceutical Sciences, Sun Yat-Sen University.
Vector
pcDNA-APP695sw plasmid DNA was kindly provided by Dr. I. Lefterov [16] (University of Pittsburgh, USA), which contains the APP Swedish mutant (K595M596→N595L596).
Antibodies
The monoclonal anti-human APP antibody 22C11 was purchased from Chemicon (Temecula, CA, USA). Human APP ELISA kit was purchased from Biosource International (Camerillo, CA, USA). Fluorometric α-Secretase Activity Kit is the product of R&D Systems.
Reagents
Electrophoresis reagents were obtained from Bio-Rad (Hercules, CA, USA). PKC inhibitor Calphostin C was purchased from Alexis Biochemicals Co. (San Diego, CA, USA). All other reagents were of highest grade available and purchased from Sigma Chemical Co. unless otherwise indicated.
Methods
Cell culture
Human Embryonic Kidney 293 (HEK293) cells and Human neuroblastoma SH-SY5Y cells were cultured in DMEM (GIBCO Life Technologies, USA) supplemented with 10% FBS (GIBCO Life Technologies, USA), 1% antibiotic (100 U/mL penicillin / streptomycin) at 37°C in an incubator containing 5% CO2.
MTT
Cell viability was measured by MTT (Methylthiazolyldiphenyl-tetrazolium bromide, MTT) assay, which was based on the conversion of MTT to form crystals by mitochondrial dehydrogenase. Cells were plated at a density of 1×104 cells/well in 96-well plates for 12 h before treating with ace-HF or DMSO (control) for 24 h. Four hours before the desired end point, 20 μL MTT (5 mg/mL in PBS) was added to each well to dissolve formazan. Absorbance (OD value) was measured at 570 nm in a 96-well plate reader (Bio-Rad Model 550).
Cell transfection and drug treatment
HEK293 cells were plated at a density of 2×105 cells per well in 6-well plates. When the cells reached 60–70% confluence, they were transfected with pcDNA-APP695sw plasmid with the Calcium Phosphate Transfection System. In brief, 20 μg plasmid DNA were mixed with 125 μL CaCl2 (1 M) and brought to 500 μL with distilled water, to which 500 μL 2×BBS Buffer (50 mM BES, pH6.95, 280 mM NaCl, 1.5 mM Na2HPO4) were added in a drop-by-drop manner. The mixture was kept at room temperature for 15 min before it was added to cell cultures. The cultures were incubated in a 5% CO2 incubator at 37°C for 10 h. The medium was then changed with regular medium containing 10% FBS. For drug treatment, the HEK293 APP Swedish cells (12 h after transfection) were treated with ace-HF at different concentrations (0.1, 1, 10, 100 and 200 μmol/L) for 12 h. DMSO was used as a vehicle control.
RT-PCR
Cells in 6-well plates were collected and subjected to RNA isolation with TRIZOL reagent (Invitrogen, CA, USA) according to the manufacturer's instructions. Semi-quantitative RT-PCR was performed to determine human APP mRNA expression, with MLV Reverse transcriptase (Promega) and the following primers: APPforw (5'CACCACAGAGTCTGTGGAA-GA) and APPrev (5'AGGTGTCTGA-GATACTTGT). The PCR primers for transfected APP were T7/APPrev. Control PCR was performed with primers specific to human β-actin (Forward: 5'-GCAATGC CTGGGTACAT GGTGG-3', Reverse: 5'-GTCGTACCACAGGCATT GTGATGG-3'). PCR was carried out on the GeneAmp PCR System 9700 (Applied Biosystems), with a denaturation step at 94°C for 5 min followed by 30 reaction cycles composed of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec. A final extension step was performed at 72°C for 10 min. The RT-PCR products were analyzed in agarose gel electrophoresis.
Western blot analysis
Cells were washed with cold PBS and protein was extracted with a lysis buffer (50 mM Tris-HCl, pH 7.6; 150 mM NaCl; 2 mM EDTA; 2 mM DTT; 1% NP-40; protease inhibitor cocktail) at 4°C for 20 min. The lysate was centrifuged at 10,000 xg for 20 min at 4°C and the supernatant was collected. Protein concentra-tions were measured using the BCA protein assay kit (Pierce Biochemicals, USA) before electrophoresis. Thirty μg of protein were resolved in 8% SDS-PAGE gels, and transferred to 0.22 μm PVDF membranes (Millipore). The membranes were blocked with 5% fat-free milk in TBST (Tris-buffered saline with 0.1% Tween 20) at room temperature for 2 h. The membranes were incubated with polyclonal antibody 22C11 (1:1000 dilution) at 4°C overnight. After washing, the membranes were incubated with secondary goat-anti-mouse horseradish peroxidase conjugated antibody (1:1000) at room temperature for 1 h. The signal was detected with enhanced chemiluminescence system (ECL kit, Pierce), and analyzed with a GDS-8000 imaging system (UVP, USA). Expression β-actin was analyzed in the same way to serve as a loading control.
ELISA assay
SH-SY5Y cells were treated with 0.5 μmol/L pan PKC inhibitor (Calphostin C) and 50 μmol/L ace-HF for 12 h. sAPPα in the media was measured with the human APP ELISA kit (Biosource International, Camerillo, CA) according to the manufacturer's instructions. The OD at 450 nm was recorded using a 96-well plate reader (Bio-Rad model 550).
α-secretase activity
The α-secretase activity was determined using a Fluorometric α-Secretase Activity Kit. Following treatment with 50 μmol/L ace-HF for 12 h, SH-SY5Y cells were lysed as above. Fifty μg of proteins were transferred to each well of a 96-well microplate, followed by addition of 50 μL 2×reaction buffer and 5 μL substrate, obtaining a final volume of 105 μL. The plate was covered, gently mixed by tapping and incubated in the dark at 37°C for 2 h. The fluorescent emission from EDANS was measured at λ = 355 nm (excitation) and λ = 510 nm (emission) and corrected against the background. The results were expressed as percentage of control, and high fluorescence correlated with high enzymatic activity.
Results
Effect of ace-HF on cell viability
In order to evaluate the effect and cytotoxicity of ace-HF, MTT assays were performed on HEK293 and SH-SY5Y cells. All cells were treated with ace-HF at 0.1, 1, 10, 100 and 200 μmol/L for 12 h. We did not observe a significant decrease in the cell number at 0.1∼100 μmol/L ace-HF (Figure 1), although a small decrease in cell number was detected at 200 μmol/L ace-HF (*P<0.05). Therefore, we performed further experiments with ace-HF at 0.1∼100 μmol/L.
Figure 1.
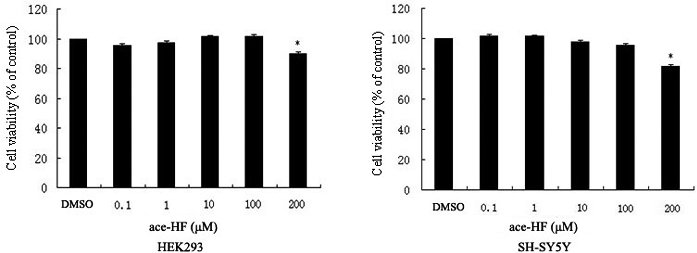
Effects of ace-HF on HEK293 and SH-SY5Y cell viability. Cells were treated with ace-HF at different concentrations (0, 0.1, 1, 10, 100 and 200 μmol/L) for 24 h. MTT assay was performed to detect cell viability. Statistical analysis was performed using the software SPSS 13. (*P<,0.05, n=3)
Effect of ace-HF on APP expression
HEK293 APP Swedish cells were transfected with pcDNA-APP695sw. To examine the effect of ace-HF on the expression of transfected APP695sw, we performed RT-PCR and Western blot analysis 12 h after ace-HF treatment to measure APP mRNA and protein levels. As shown in Figure 2, ace-HF (100 μmol/L) produced little effects on APP expression at the mRNA and protein levels compared with the control (Figure 2A, 2B).
Figure 2.
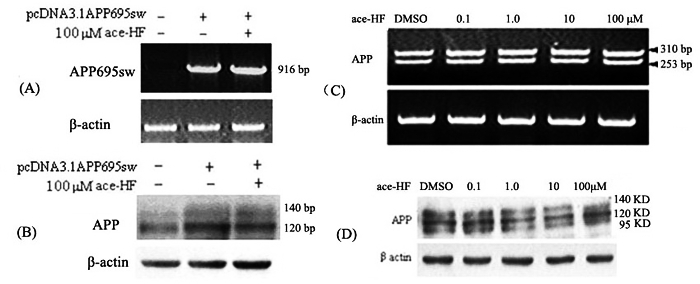
Effects of ace-HF on APP mRNA and protein levels. (A), RT-PCR analysis of transfected APP695sw mRNA expression in HEK293 cells. HEK293 cells were transfected with the plasmid DNA pcDNA-APP695sw using the Calcium Phosphate Transfection System. Twelve hours after the transfection, cells were treated with 100 μol/L ace-HF; (B), Western blot analysis of APP protein in HEK293 APP Swedish cells probed with antibody 22C11. The cells were treated in the same way as (A); (C), RT-PCR analysis of endogenous APP mRNA expression in SH-SY5Y cells treated with different ace-HF concentration; (D), Western blot analysis of endogenous APP protein level in SH-SY5Y cells probed with antibody 22C11.Cells were treated with ace-HF at concentrations of 0, 0.1, 1, 10 and 100 μol/L for 12 h.
We next investigated the effect of ace-HF on endogenous APP transcription in SH-SY5Y cells; we chose SH-SY5Y instead of HEK 293 cells because APP expression in SH-SY5Y is higher. We performed RT-PCR 12 h after ace-HF (0.1, 1.0, 10, 100 μmol/L) treatment. Two PCR products of 310 bp and 253 bp were detected, which probably represent two different APP mRNA splicing isoforms. Under these conditions (0.1∼100 μmol/L ace-HF for 12 h), ace-HF did not change the levels of APP mRNA (Figure 2C).
In order to confirm the RT-PCR results, we carried out western blot analysis to determine holo-APP protein expression with antibody 22C11. As shown in Figure 2D, intracellular holo-APP was not altered by treatment with ace-HF for 12 h.
Ace-HF induces significant increase of extracellular sAPPα
We next investigated whether ace-HF treatment affected production of sAPPα, a product of APP by α-secretase hydrolyzation. We performed ELISA assays to measure extracellular sAPPα synthesized by HEK293 APP Swedish cells. As shown in Figure 3A, sAPPα level significantly increased to 900% in the medium as compared with the untransfected controls. In addition, ace-HF treatment at 100 μmol/L increased to 10% even more sAPPα secretion than the untreated controls (P<0.05, n=3).
Figure 3.
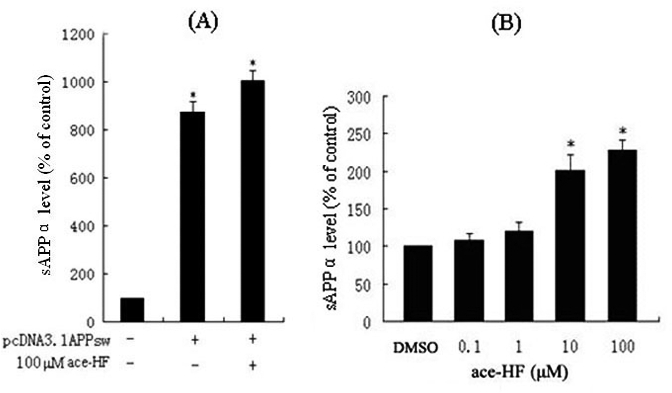
ELISA assay of extracellular sAPPα. Data are presented as a percentage of control (DMSO) (*P< 0.05, n=4). (A), Transfected experiment for sAPPα level in HEK293 APP Swedish cells. HEK293 cells were transfected with the plasmid DNA pcDNA-APP695sw using the Calcium Phosphate Transfection System. Twelve hours after the transfection, cells were treated with 100 μmol/L ace-HF for 12 h. (B), sAPPα level of endogenous APP processing in SH-SY5Y cells treated with ace-HF at concentrations of 0, 0.1, 1, 10 and 100 μmol/L ace-HF for 12 h.
In order to explore whether or not ace-HF stimulated endogenous sAPPα release, we performed ELISA to measure extracellular sAPPα from SH-SY5Y cells. As shown in Figure 3B, 12 h after ace-HF treatments at 10 μmol/L and 100 μmol/L, extracellular sAPPα significantly increased to 100% and 125% respectively (*P<0.05, n=3).
Involvement of PKC signal pathway in the ace-HF-induced enhancement of sAPPα release
Previous studies have shown that PKC is involved in the pathogenesis of AD [12,17]. We therefore performed experiments to determine the potential involvement of PKC in the ace-HF-induced increase of sAPPα secretion. To block the activities of different PKC isoforms, we treated SH-SY5Y cells with a pan PKC inhibitor Calphostin C. Compared with DMSO vehicle, treatment with 0.5 μmol/L Calphostin C decreased extracellular sAPPα by 40% in the culture media. Interestingly, Calphostin C also abolished ace-HF (50 μmol/L) -stimulated sAPPα secretion with the rates of decrease at about 35% compared to only 50 μmol/L ace-HF treated cells (*P<0.05, ** P<0.001, n=5) (Figure 4A). In supporting these results, we analyzed α-secretase activity following ace-HF (50 μmol/L) treatment. Our experiments indicated that that the activities of α-secretase increased significantly compared with the controls (P<0.01, n=3) (Figure 4B). These results suggest that ace-HF can modulate APP processing through promotion of α-secretase pathway via PKC signaling.
Figure 4.
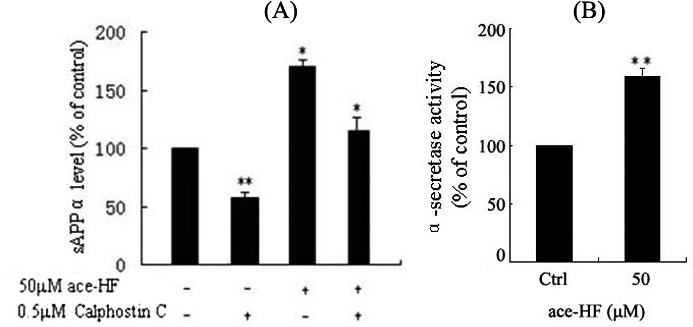
(A), Effects of the PKC inhibitor Calphostin C on ace-HF-stimulated sAPPα secretion from SH-SY5Y cells. Cells were co-treated with 50 αmol/L ace-HF and 0.5 αmol/L Calphostin C for 12 h. Then ELLISA assay was performed to detect sAPPα level. Data are presented as a percentage of control (DMSO) (*P< 0.05, ** P< 0.001,n=5). (B), Effects of ace-HF on α-secretase activity in SH-SY5Y cells. Cells were treated with 50 μmol/L ace-HF for 12 h. Then ELLISA assay was performed to detect α-secretase activities (** P< 0.001, n=3).
Discussion
sAPPα is a soluble secretive protein from α-secretase-mediated cleavages of APP [2,3]. Previous studies have shown that sAPPα is a trophic factor that can protect neurons from Aβ toxicity [18]. Thus, APP processing via the non-Aβ pathway may have a protective effect on AD pathogenesis. Pharmaceutical controls of APPs processing to increase sAPPα secretion have been a focal point of the current research of AD therapy. In this report, we show that ace-HF is able to stimulate sAPPα production from both endogenous and transfected Swedish APP (Figure 3). At the same time, we observed that treatment with ace-HF did not cause significant changes in APP mRNA and protein expression 12 h after treatment (Figure 2). These findings suggest that the increase in sAPPα secretion was not due to an increase in APP protein expression, but may have resulted from a direct or indirect activation of α-secretase. In this way, ace-HF may stimulate APP processing via the α / γ-secretase pathway.
It is known PKC signaling plays a critical role in regulating APP processing [19,20]. We found that the enhancement of sAPPα secretion by ace-HF required PKC activation (Figure 4A). At the same time, we also observed that ace-HF induced α-secretase activity (Figure 4B). It is possible that ace-HF functions as an agonist to activate PKC, which then increases the activity of α-secretase to stimulate sAPPα production. The activation of the non-amyoidgenic pathway of APP processing by ace-HF would not only generate more neurotrophic sAPPα, but also preclude Aβ production.
In conclusion, our studies have demonstrated that ace-HF treatment steers APP processing toward the α-secretase cleavage via a PKC pathway. The up-regulation of neural-protective sAPPα by ace-HF may reduce the toxicity of Aβ, which is expected to be beneficial to AD therapy. These findings suggest that ace-HF is likely an effective drug candidate for further development to treat AD.
Acknowledgments
We thank Dr. Iliyam Lefterov (University of Pittsburgh, USA) for kindly providing pcDNA-APP695sw. We also thank Dr. Burton Yang (University of Toronto, Canada) for kindly suggestions to this paper's composition.
This work was supported by grants No. 30873457 from the National Natural Science Foundation of China and No.2007B031406001, No.2008A060202010 from the Scientific Technology Project of Guangdong Province of China.
References
- 1.Selkoe DJ. Alzheimer's disease: genotypes, phenotypes, and treatments, Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 2.Strooper BD, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113:1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 3.Postina R, Schroeder A, Dewachter J, Bohl J, Schmitt U, Kojro E, et al. A disintegrin-metallo proteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin L, Ninomiya H, Roch J, Schubert D, Masliah E, Otero D, et al. Pepteides containing the RERMS sequence of amyloid (beta)/A4 protein precursor bind cell surface and promote neurite extension. J Neurosci. 1994;14:5461–5470. doi: 10.1523/JNEUROSCI.14-09-05461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodovitz S, L.Klein W. Cholesterol modulates alpha-secretase cleavage of amyloid precursor. J Biol Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- 7.Thornton E, Vink R, Blumbergs PC, Van Den Heuvel C. Soluble amyloid precursor protein α reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res. 2006;1094:38–46. doi: 10.1016/j.brainres.2006.03.107. [DOI] [PubMed] [Google Scholar]
- 8.Famer D, Meaney S, Mousavi M, Norberg A, Bjorkhem I, Crisby M. Regulation of α- and β-seccretase activity by oxysterols: cerebrosterol stimulates processing of APP via the α-secretase pathway. Biochem Biophys Res Commun. 2007;359:46–50. doi: 10.1016/j.bbrc.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Zanli P. Role of hyperforin in the pharmacological activities of St. John's Wort. CNS Drug Rev. 2004;10:203–218. doi: 10.1111/j.1527-3458.2004.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiskey E, Wernecke U, Taylor D. A systermatic review and meta-analysis of Hypericum perforatum in depression: a comprehensive clinical review. Int Clin Psychopharmacol. 2001;16:239–252. doi: 10.1097/00004850-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Medina MA, Martinez-Poveda B, Amores-Sanchez MI, Quesada AR. HyperforMore than an antidepressant bioactive compound? Life Sci. 2006;79:105–111. doi: 10.1016/j.lfs.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Froestl B, Steiner B, Muller WE. Enhancement of proteolytic processing of the beta-amyloid precursor protein by hyperforin. Biochem Pharmacol. 2003;66:2177–2184. doi: 10.1016/j.bcp.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Ang CY, Hu L, Heinze TM, Cui Y, Freeman JP, Kozak K, et al. Instability of St. John's wort (Hypericum perforatum L.) and degradation of hyperforin in aqueous solutions and functional beverage. J Agric Food Chem. 2004;52:6156–6164. doi: 10.1021/jf0490596. [DOI] [PubMed] [Google Scholar]
- 14.Bombardelli E, Morazzoni P, Riva A, Fuzzati N. Hyperforin derivatives, the use there of and formulations containing them. 2003. WO Patent: 2003091194.
- 15.Liang YH, Wang DM, Yang DP, Guo ZM, Li WM. Advance in chemical and neuropharmacological studies on hyperforin and its derivatives. Chinese Traditional and Herbal Drugs. 2007;38:11–13. [Google Scholar]
- 16.Lefterov I, Koldamova RP, Lazo JS. Human bleomycin hydrolase regulates the secretion of a myloid precursor protein. FASEB J. 2000;14:1837–1847. doi: 10.1096/fj.99-0938com. [DOI] [PubMed] [Google Scholar]
- 17.Lanni C, Mazzucchelli M, Porrello E, Govoni S, Racchi M. Differential involvement of protein kinase C alpha and epsilon in the regulated secretion of soluble amyloid precursor protein. Eur J Biochem. 2004;271:3068–3075. doi: 10.1111/j.1432-1033.2004.04240.x. [DOI] [PubMed] [Google Scholar]
- 18.Stein TD, Anders NJ, DeCarli C, Chan SL, Mattson MP, Johnson JA. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPsw mice resulting in tau phosphorylation and loss of hippocampal neurons: support for the amyloid hypothesis. J Neurosci. 2004;24:7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buxbaum JD, Liu KN, Luo Y, Slack JL, Stockin KL, Peschon JJ, et al. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloidprotein precursor. J Biol Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 20.Cedazo-Minguez A, Bonecchi L, Winblad B, Post C, Wong EH, Cowburn RF, Benatti L. Nicergoline stimulates protein kinase C mediated α-secretase processing of the amyloid precursor protein in cultured human neuroblastoma SH-SY5Y cells. Neurochem Int. 1999;35:307–315. doi: 10.1016/s0197-0186(99)00074-1. [DOI] [PubMed] [Google Scholar]


