Abstract
Nodal is a member of the transforming growth factor β (TGF-β) superfamily that plays critical roles during embyogenesis. Recently, we have demonstrated that Nodal induces apoptosis and inhibits proliferation in human trophoblast and epithelial ovarian cancer cells. To further determine the role of Nodal in controlling cellular activities, we examined the action of Nodal and its type I receptor, activin receptor-like kinase 7 (ALK7), on breast cancer cell lines. Using RT-PCR, we detected Nodal and ALK7 transcripts in MDA-MB-231 and MCF-7 cells. Overexpression of Nodal or activation of constitutively active ALK7 (ALK7-ca) resulted in a significant decrease in the number of live cells and a significant increase in the number of dead cells. This effect was observed for both cell lines; however, Nodal and ALK7-ca had a much stronger effect in MDA-MB-231 cells than in MCF-7 cells. The effect of Nodal was blocked by dominant negative mutants of ALK7, suggesting that Nodal acts through ALK7 to inhibit cell growth/survival. Nodal and ALK7-ca inhibited proliferation in both cell lines; however, while Nodal and ALK7-ca induced apoptosis in MDA-MB-231 cells, they only had a minor effect on MCF-7 cells. In addition, Nodal activated caspase 3 in MDA-MB-231 cells, but had no effect on caspase 3-deficient MCF-7 cells. The effect of Nodal on apoptosis in MDA-MB-231 cells was blocked by a caspase 3 inhibitor. These findings demonstrate that the Nodal-ALK7 pathway exerts anti-proliferative and proapoptotic effects in breast cancer cells and suggest that caspase 3 is important for Nodal-ALK7-induced apoptosis.
Keywords: Nodal, ALK7, caspase 3, apoptosis, breast cancer
Introduction
The transforming growth factor β (TGF-β) superfamily has been shown to regulate a variety of cellular processes ranging from cell proliferation, differentiation, adhesion and migration to apoptosis and plays key roles in development and carcinogenesis [1–3]. The TGF- β superfamily encompasses more than 35 members of structurally related polypeptide growth factors including TGF-βs, activins, bone morphogenetic proteins (BMPs), growth and differentiation factors and other factors such as Nodal and its related proteins [1–3]. The TGF-β superfamily transmits their signals from the cell surface via transmembrane serine/threonine kinase receptor complexes consisting of type I and type II receptors. In mammals, five type II receptors and seven type I receptors (referred to as activin receptor-like kinase, ALK1–7) have been identified [3–6]. Upon ligand binding, the type II receptors recruit and transphosphorylate type I receptors. The latter, in turn, phosphorylate the receptor activated Smad proteins (R-Smads). Activated R-Smads then form a protein complex with the common Smad (Smad4) and translocate into the nucleus where they interact with additional transcription factors and co-activators or co-repressors to regulate target gene expression [1–3].
Nodal is an important regulator of embryonic development. It plays crucial roles in mesoderm formation and left-right axis patterning [7–9]. During early embryogenesis, Nodal signals through type II activin receptors, ActRIIA or ActRIIB [10] and ALK4 or ALK7 [11]. ALK7 was first cloned from adult rat central nervous system as an orphan receptor [12, 13]. Studies have shown that it can bind with Nodal and mediates the effect of Nodal in mesoderm formation [11]. The human ALK7 cDNA has also been cloned [14, 15]. Furthermore, our laboratory has discovered three additional ALK7 transcripts, derived from alternative splicing of the ALK7 gene, that encode for truncated and soluble ALK7 isoforms [14].
Activation of ALK7 has been shown to inhibit cell proliferation and to induce morphological differentiation in the rat neuronal cell line PC12 [16]. Similarly, ALK7 has been reported to inhibit proliferation in human trophoblast cells [17] and ovarian cancer cells [18]. ALK7 also induces apoptosis in a variety of cell types, such as hepatoma [19], trophoblast [17], epithelial ovarian cancer cells [18, 20], pancreatic β cells [21] and ovarian follicles [22]. Nodal has similar growth inhibiting and apoptosis inducing effects as ALK7 in trophoblast [17] and ovarian cancer [18, 20, 23] cells and the effects of Nodal can be blocked by dominant negative ALK7 [17, 18, 20]. These findings suggest that the Nodal-ALK7 pathway plays an important role in regulating cellular activities such as proliferation and apoptosis.
To further determine the role of Nodal in controlling cellular activities and its underlying mechanisms, especially the role of caspase 3 in Nodal-induced apoptosis, we examined the effect of Nodal and ALK7 on proliferation and apoptosis in two breast cancer cell lines, MDAMB-231 and MCF-7.
Materials and Methods
Cell Lines and cell culture
Human breast cancer cell lines, MDA-MB-231 and MCF-7 were obtained from American Type Cell Collection (Rockville, MD, USA). Caspase 3 expressing MCF-7 cells (MCF7/Casp3, 30) and its empty vector-transfected control cell line (MCF7/neo) were kindly provided by Dr. B. Fang (University of Texas M. D. Anderson Cancer Center, Houston, Texas) [24]. Cells were maintained in DMEM (Invitrogen Canada Inc., Burlington, ON) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 100 IU/ml penicillin and 100μg/ml streptomycin (Invitrogen) and 1mM Sodium pyruvate (Sigma-Aldrich, Oakville, ON).
Reagents, plasmids and transient transfection
Recombinant mouse Nodal and caspase 3 inhibitor, Z-DEVD-FMK, were purchased from R & D Systems (Minneapolis, MN) and Calbiochem (San Diego, CA), respectively. Expression constructs for Nodal, constitutively active ALK7 (ALK7-ca), and kinase defective ALK7 (ALK7-kd) have been previously described [17, 18]. Transient transfection was performed as described previously [25]. Briefly, cells were seeded at about 50% cell density onto tissue culture dishes (Sarstedt, Inc., Montreal, Quebec, Canada) and allowed to adhere and grow overnight. After washing the cells, the culture medium was replaced with Opti-MEM (Invitrogen) and the cells were incubated for 1h prior to transfection. Plasmid DNA and 0.18mM 25kDa polyethyleneimine (PEI, Sigma) (0.28μl for each μg of DNA) were diluted into 150mM NaCl. After incubation at room temperature for 5 minutes, these two solutions were mixed and further incubated at room temperature for 12 min. The PEI/DNA mixture was then diluted into Opti-MEM medium and added to the cells. After incubation for 4h at 37°C, the culture medium was changed to DMEM medium supplemented with 10% FBS and cells were recovered for different length of time as specified in each experiment. Transfection efficiency, estimated by transfecting cells with a GFP containing plasimd (pEGFP-N1, BD Biosciences, Mississauga, ON), was approximately 40% for MDA-MB-231 and 60% for MCF-7 cells.
Determination of cell growth and viability
Cell growth was determined by manual cell counting. MDA-MB-231 and MCF7 cells were seeded in 6 well plates at a density of 2×105 cells/well. After overnight culture, cells were transiently transfected with 2.5μg of pcDNA4, ALK7-ca and Nodal plasmid DNA (n=3). At 24h, 48h and 72h after transfection, floating cells were collected by centrifugation and adherent cells were trypsinized. The number of living and dead cells was determined by manual cell counting using trypan blue exclusion. Each experiment was repeated two times.
Cell viability was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded in 96 well plates at a density of 104cells/well. Cells were transiently transfected with different constructs (total amount of DNA was 0.3μg/well) as indicated in the figure legends (n=8 wells). The number of metabolically active cells was measured at 24, 48 and 72h post-transfection, using the MTT cell proliferation kit I (Roche Diagnostics, Laval, QC) following the manufacturer's instruction. To determine if Nodal acts through ALK7, Smad2 and Smad3, MDA-MB-231 cells were transfected with 0.15 μg of Nodal, either alone or in combination with ALK7-kd, DN-Smad2 or DN-Smad3. Vector DNA was used to equalize the total amount of DNA in each well. Fortyeight hours after transfection, MTT assays were performed.
Proliferation assay
Cell proliferation was determined by the measurement of 5-bromo-2-deoxyuridine (BrdU) incorporation in newly synthesized cellular DNA using a cell proliferation ELISA kit (Roche). MDA-MB-231 and MCF-7 cells were seeded onto 96-well plates at a density of 104cells/well and incubated overnight at 37°C prior to transfection. Cells were then transfected with different constructs (0.3μg/well) as indicated in the figures for 4h. The assay was performed as described in the manufacturer's instructions. Briefly, 10μl/well of BrdU labeling solution was added to cells at 24h, 48h or 72h post-transfection. Following overnight incubation, cells were fixed with 100μl/well of fixing solution for 30 min at room temperature and incubated with anti-BrdU antibody conjugated with peroxidase for 90 min. A substrate solution was then added into each well and absorbance was measured using the ELISA plate reader.
RNA extraction and RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen) following the manufacturer's protocols. The RT-PCR was performed as described previously [18, 20]. Primers for ALK7, Nodal and glyceraldehydes-3-phosphate dehydrogenase (GAPDH), p27 and p21 have been previously reported [17, 26]. Primers for BRCA2 are: forward, AAGACACGCTGCAACAAA GC ; reverse, GGACAGGAAACATCATCTGC.
Protein extraction and Western blotting
MDA-MB-231 and MCF-7 cells cultured in 60-mm dishes were transfected with 5μg of plasmid DNA (pcDNA4, Nodal and ALK7-ca). At various time points after transfection, cells were washed twice with ice-cold PBS and lysed with an assay buffer containing 50mM Tris-HCl, 150mMNaCl, 1% Triton X-100, 0.5% deoxycholate, 1% SDS, 1mM dithiothreitol, 1mM Na3VO4, 5mM NaF, 100mM EDTA, 10mg/ml aprotinin and 100mM phenylmethylsulfonyl fluoride. Protein samples were subjected to SDS-PAGE and transferred to a nitrocellulose membrane. After blocking with 5% milk in TBS-T for 1h, the membrane was then incubated overnight at 4°C with a primary antibody: mouse anti-c-Myc(1:1000, Santa Cruz); rabbit anti-cleaved Caspase 3 (Asp 175, 1:1000, Cell Signaling), rabbit anti-human phospho-Smad2(1:1000, Cell Signaling), mouse anti-human Bcl-2 and Bcl-XL (both at 1:1000, Cell Signaling), or mouse anti-human β-actin (1:5000, Sigma). The membranes were subsequently incubated with horseradish peroxidase-conjugated anti-mouse or anti- rabbit secondary antibody (1:5000, Amersham) for 1h at room temperature. Signals were detected using an ECL kit (Amersham) according to the instructions of the manufacturer.
DNA fragmentation assay
MDA-MB-231 and MCF-7 cells were plated on 60-mm dishes and transfected with 5μg of pcDNA4, ALK7-ca or Nodal constructs. 48h after transfection, floating and adherent cells from each dish were combined, pelleted, and washed twice with ice-cold PBS. The pellet was re-suspended in 0.2ml lysis buffer [100mM NaCl, 10mM Tris-HCl(pH8.0), 1mM EDTA, 0.5% Sodium doclecyl sulfate, 0.20mg/ml Proteinase K and 200μg/ml RibonucleaseA]. The cell lysates were incubated at 37°C for 2h. The genomic DNA was extracted and visualized through electrophoresis using a 1.5% agarose gel.
In situ cell death detection (TUNEL assay)
Apoptosis at the single cell level was detected and quantified using an In Situ Cell Death Detection, TMR red kit (Roche Diagnostics) following the manufacturer's protocols. Briefly, at 48h following transfection (as described in DNA fragmentation assay), floating and attached cells were collected and adjusted to the concentration of 2×107 cells/ml. An aliquot (100μl) of cell suspension was transferred into a tube, and an equal amount of a freshly prepared paraformaldehyde (4% in PBS, pH 7.4) was added. Following incubation at room temperature for 1h, the fixative was removed and cells were washed. Cells were incubated in TUNEL reaction mixture for 60 min at 37°C in the dark, washed, re-suspended in PBS and examined by fluorescence microscopy. To quantify apoptotic cells, the number of total cells and TUNEL positive cells in 5 random areas were counted and expressed as % apoptotic cells. To test the caspase 3 inhibitor on Nodal-induced apoptosis, MDA-MB-231 cells were incubated with or without recombinant mouse Nodal (rmNodal, 500ng/ml) in the presence or absence of ZDEVD- FMK (50 μM) for 48h and TUNEL positive cells counted.
Statistical analysis
All data were expressed as mean ± SEM from a representative experiment. Each experiment was repeated 2–3 times. Differences among multiple groups were determined by one-way ANOVA, followed by Student-Newman-Keul's test using GraphPad InStat software. P<0.05 was considered significant.
Results
Nodal and related signaling molecules are expressed in human breast cancer cells
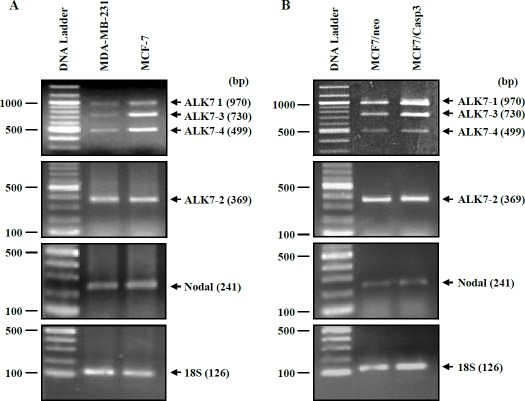
Expression of mRNAs for Nodal and ALK7 was determined by RT-PCR. Using primers spanning exons III and IV, three DNA fragments corresponding to the expected sizes of ALK7-1, ALK7-3 and ALK7-4 were detected in both MDA-MB-231 and MCF7 cells. Expression of ALK7-2 was detected by primers located in exon Ia and exon 2. Nodal mRNA was also observed in both cell lines (Figure 1). Both ALK7 and Nodal mRNAs were also detected in MCF7-caspase 3 expressing cells or its control cell line (Figure 1).
Figure 1.
Expression of four ALK7 transcripts and Nodal mRNA in breast cancer cells. Total RNA was extracted from cells and subjected to RT-PCR using specific primers. 18S (lower panel) was used as an internal control. A representative experiment is shown. bp, base pair.
Nodal and ALK7 inhibit human breast cancer cell growth and survival
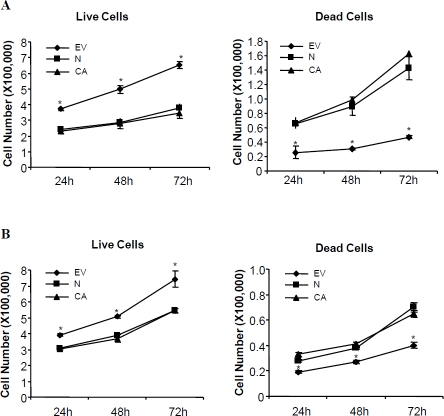
To determine if Nodal and ALK7 exert any effects on breast cancer cell growth and survival, MDA-MB-231 cells and MCF-7 cells were transiently transfected with Nodal, ALK7-ca, or their vector control, pcDNA4. Both live and dead cell numbers were counted at 24, 48 and 72h after transfection. At all time points tested, there was a significantly higher number of living cells in the control group than those in either Nodal or ALK7-ca-transfected group. On the other hand, the number of dead cells was significantly higher in Nodal and ALK7-ca groups than that in the control group (Figure 2). Although an increase in dead cell number and a decrease in living cell number following Nodal and ALK7-ca transfection were observed in both MDA-MB-231 and MCF-7 cells, Nodal and ALK7-ca have a much more potent effect on inducing cell death in MDA MB-231 than in MCF-7 cell lines. Similar results were obtained from MTT assays. Transient transfection with Nodal and ALK7-ca induced a significant reduction in the number of metabolically active cells in both cell lines. The maximal decrease in MDA-MB-231 cells was 35–40% (Figure 3B) while only 10% reduction was observed in MCF-7 cells (Figure 3D).
Figure 2.
Effect of Nodal and constitutively active ALK7 (ALK7-ca) on breast cell growth and viability. MDA-MB-231 (A) and MCF-7 (B) were seeded into 6-cm dishes and transfected with control vector (EV), Nodal (N), or ALK7-ca (CA). The number of live and dead cells was determined by trypan blue exclusion at different time points after transfection. Data represent mean ± SEM (n=3 dishes). * P<0.05 vs other groups at the same time point.
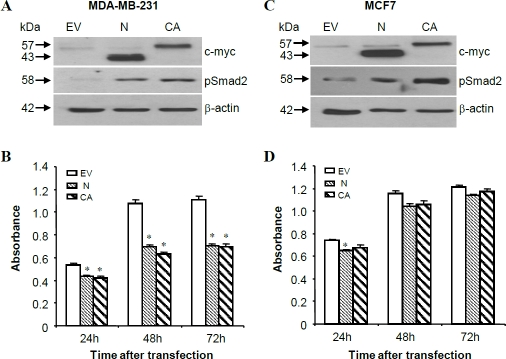
Figure 3.
Effects of Nodal and ALK7-ca on breast cancer cell growth. MDA-MB-231 (A, B) and MCF-7 (C, D) cells were transfected transiently with pcDNA4 (EV), Nodal, or ALK7-ca. At 48h post-transfection, proteins were extracted and probed with anti-c-myc antibody to confirm the expression of transgenes, anti-phosphoSmad2 to confirm the activation of Smad2, and anti-β-actin as a loading control (A, C). The number of metabolically active cells was measured by MTT assays (B, D). Data represent means ± SEM (n=8 wells). Experiments have been repeated twice and similar results obtained. * p<0.05 vs. the respective pcDNA control.
To confirm that the response observed after Nodal transfection was the result of an increase in Nodal expression, Western blot analysis with anti-c-myc antibody was performed using protein samples extracted from EV- Nodal- or ALK7-ca-transfected cells. Expected size bands corresponding to Nodal propeptide and ALK7 were detected in Nodal and ALK7-ca transfected samples (Figure 3A, C). Using an antibody specific for phosphor- Smad2, it was observed that transfection with Nodal and ALK7-ca resulted in activation of Smad2 (Figure 3A, C).
Nodal and ALK7-ca inhibit breast cancer cell proliferation
To determine if the decrease in cell number was in part due to the inhibitory effect of Nodal and ALK7-ca on cell proliferation, BrdU assays were carried out. In both MDA-MB-231 and MCF-7 cells, overexpression of Nodal and ALK7-ca resulted in a significant decrease in cell proliferation when compared to the vector control at 48h after transfection and the effect of Nodal was blocked by co-transfection with ALK7-kd (Figure 4A, B). Nodal and ALK7-ca had similar effects on inhibiting proliferation in both cell lines (Figure 4A, B). Nodal and ALK7-ca also increased mRNA levels of p27, p21 and BRCA2 in both MDA-MB-231 and MCF-7 cells (Figure 4C).
Figure 4.
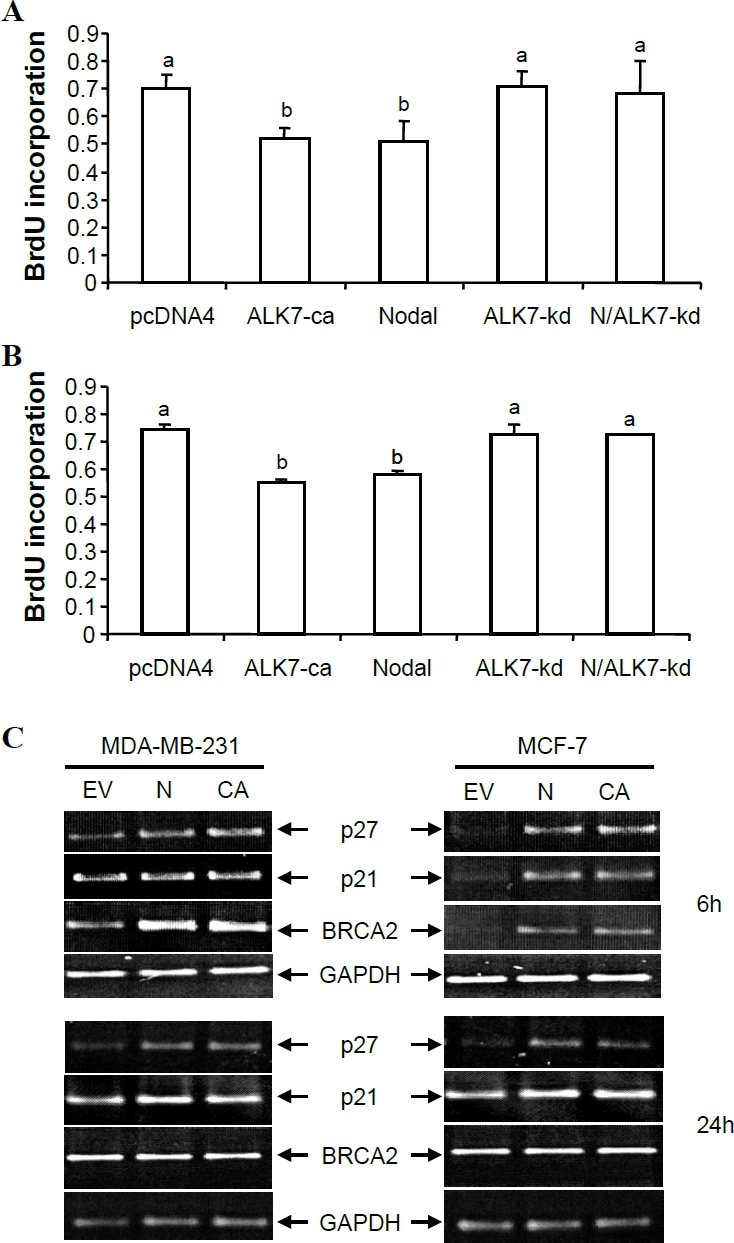
Nodal and ALK7-ca inhibited cell proliferation. A, B) BrdU assays. MDA-MB-231 (A) and MCF-7 (B) cells were transfected with pcDNA4, ALK7-ca, ALK7-kd, Nodal (N) or Nodal plus ALK7-kd. BrdU assays were performed at 48h after transfection. C) RT-PCR measurement of p21, p27, and BRCA2 mRNA levels. Total RNA was extracted from pcDNA4 (EV), Nodal (N) and ALK7-ca (CA) transfected cells at 6 and 24h after transfection and RT-PCR performed. A typical PCR gel picture was shown along with graphs showing quantified data (means ± SEM of three independent transfection and RT-PCR experiments). Different letters denote statistical significance (P<0.05).
Nodal and ALK7-ca induce apoptosis
To determine whether the Nodal-ALK7 pathway also plays a role in regulating apoptosis, MDA-MB-231 and MCF-7 cells were transfected with Nodal, ALK7-ca or vector control. The effect of Nodal and ALK7-ca on cell apoptosis was determined by DNA fragmentation and TUNEL assay. DNA fragmentation results showed that Nodal and ALK7-ca transfection induced a DNA laddering in MDA-MB-231 but not in MCF-7 cells (Figure 5A). TUNEL assays revealed that Nodal and ALK7-ca induced apoptosis in MDA-MB-231 cells. In contrast, MCF-7 cells showed a small response to Nodal and ALK7-ca (Figure 5B).
Figure 5.
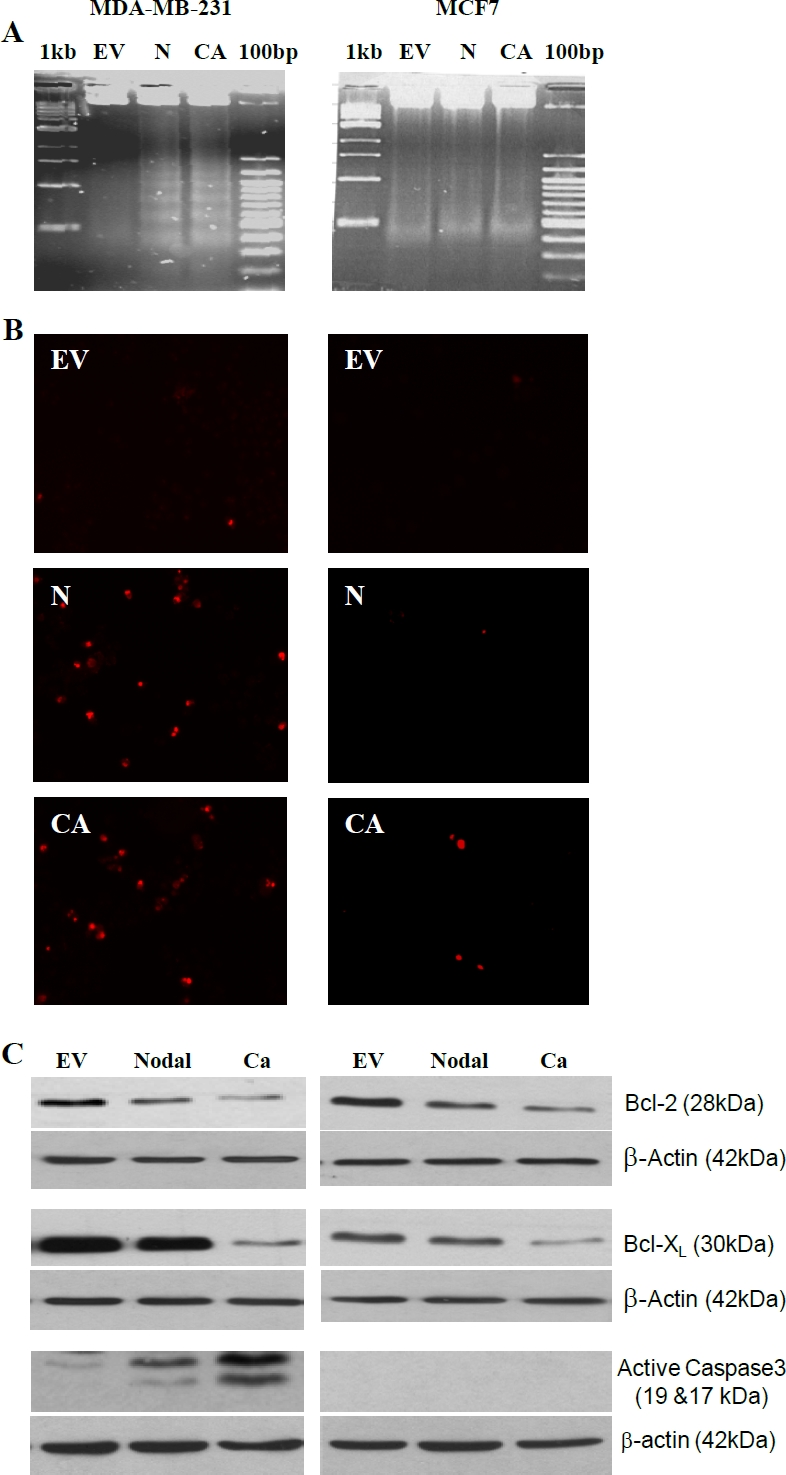
Nodal and ALK7-ca induced apoptosis. A) DNA fragmentation assays. MDA-MB-231 and MCF-7 cells were transfected with control vector (EV), Nodal (N) and ALK7-ca (CA) and 48h later, DNA extracted and analyzed. B) TUNEL assays. Cells were transfected as in A and apoptotic cells were identified by TUNEL staining. C) Western blot analysis of Bcl2, Bcl-XL, and active caspase 3. Bcl2 and Bcl-XL levels were determined at 24h after transfection whereas active caspase 3 was measured at 48h after transfection.
To determine the possible mechanism involved in Nodal-ALK7 induced breast cancer cell apoptosis, Western blot analysis was conducted to examine the effect of Nodal and ALK7-ca on the expression of two anti-apoptotic proteins, Bcl2 and Bcl-XL, as well as caspase3 activation. As shown in Figure 5C, Nodal and ALK7-ca decreased Bcl2 and Bcl-XL levels in both cell lines and activated caspase 3 in MDA-MB-231 cells, however, consistent with previous reports that MCF-7 cells lack caspase 3 [27], no caspase 3 activation was detected in MCF-7 (Figure 5C).
To further examine the involvement of caspase 3 in Nodal/ALK7-induced apoptosis, we tested the effect of Nodal and ALK7-ca on a MCF-7 cell line stably transfected with caspase 3 (MCF-7/Casp3) and its control cell line, MCF-7/neo. Expression of caspase 3 was confirmed in MCF7/Casp3 cells (Figure 6A, B); however, while Nodal and ALK7-ca down-regulated Bcl2, they did not activate caspase 3 (data not shown) and there was no difference in the number of TUNEL positive cells between the MCF-7/neo and MCF-7/Casp3 cells (Figure 6C). We then tested if a caspase 3 inhibitor would block the effect of Nodal. Treatment of MDA-MB-231 cells with rmNodal significantly increased apoptotic cell numbers and the effect was reduced in the presence of Z-DEVD-FMK (Figure 7).
Figure 6.
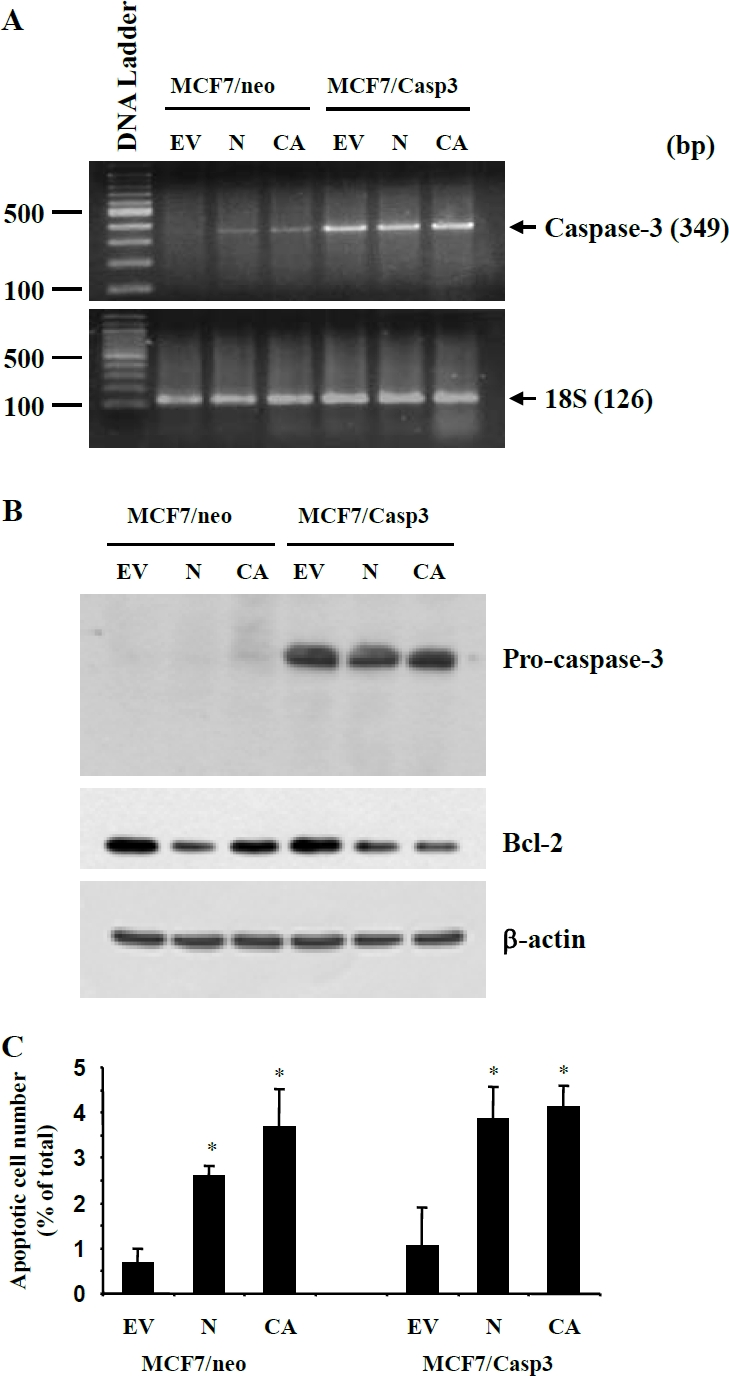
Effects of Nodal and ALK7-ca on apoptosis of caspase 3-expressing MCF-7 cells. Control (MCF7/neo) or caspase 3-expressing MCF-7 (MCF7/Casp3) cells were transfected with Nodal (N), ALK7-ca (CA) or their empty control vector (EV) and 48h later, mRNA and protein extracted and TUNEL assays performed. A) RT-PCR detected the expression of caspase 3 in MCF7/Casp3 cells. B) Western blot showed the expression of caspase 3 in MCF7/Casp3 cells and the down regulation of Bcl-2 by Nodal and ALK7-ca in both cell lines. C) TUNEL assays revealed similar effects of Nodal and ALK7-ca on apoptosis between the control and MCF-7/Casp3 cells. *, p<0,05 vs the respective control.
Figure 7.
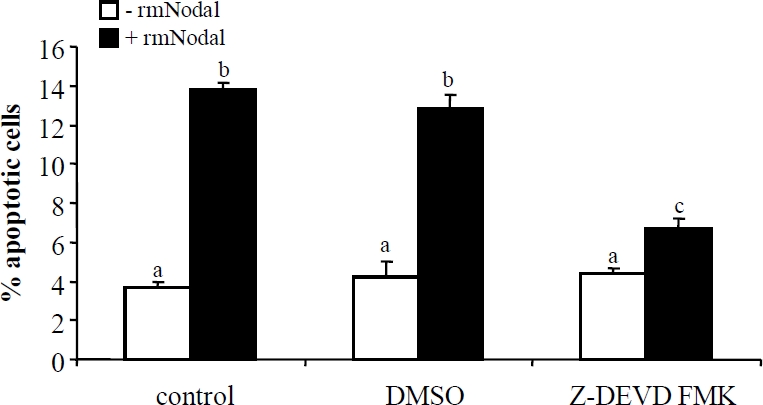
Caspase 3 inhibitor blocked Nodal induced apoptosis. TUNEL assay was performed after MDA-MB-231 cells were incubated with recombinant mouse Nodal (rmNodal, 500 ng/ml) for 48 h with or without 2 hour pretreatment with a caspase 3 inhibitor, Z-DEVD-FMK. Data represent Mean±SEM (n=3). Different letters denote statistical significance.
Discussion
Previously, we have shown that Nodal, acting through ALK7 and Smad2/3, exerts antiproliferative and apoptoic effects in human trophoblast [17] and ovarian cancer cells [18, 20, 23]. The present study extends these findings to breast cancer cells and demonstrates that caspase 3 is required for the apoptosis-inducing effect of Nodal/ALK7.
Using RT-PCR, we detected both Nodal and ALK7 transcripts in MDA-MB-231 and MCF-7 cells. Expression of Nodal mRNA in mammary tissues has been reported in mice [28], however, to our knowledge, this is the first time the expression of ALK7 transcripts have been observed in breast cancer cells. We have previously cloned four ALK7 transcripts derived from alternative splicing of the ALK7 gene[15]. Transcripts 1, 2, 3, and 4 encode, respectively, for a full-length receptor, a truncated receptor that is missing part of the ligand binding domain, and two soluble proteins that lack the transmembrane and GS domains [15]. Similar to our previous findings in trophoblast [17] and epithelial ovarian cancer cells [18, 20], the breast cancer cell lines we examined also expressed all four ALK7 transcripts. Expression of type IIB activin receptor, the known partner of ALK7, and Smads in breast cell lines has been previously reported [29, 30]. Expression of Nodal and its signaling molecules in breast cancer cells suggests that Nodal may play an autocrine/paracrine role in regulating breast cancer cell functions.
The present study demonstrates that Nodal exerts an antiproliferative effect on breast cancer cells. Overexpression of Nodal decreased live cell numbers and inhibited DNA synthesis as measured by BrdU incorporation assays. The finding that Nodal inhibits cell proliferation is in agreement with findings from our previous studies in human trophoblast cells [17] and ovarian cancer cells [18]. The effect of Nodal on cell growth was mimicked by ALK7-ca but blocked by dominant negative ALK7, suggesting that Nodal acts through ALK7 and then activates Smad2/3 to inhibit cell proliferation. Since it has been shown that Nodal mainly signals through ALK4 during embryogenesis [11, 31, 32], the involvement of ALK4 in the action of Nodal on breast cancer cells can not be excluded. Similarly, ALK7 is capable of mediating not only Nodal, but also Activin-B and Activin-AB, signaling [26, 33]. However, while activin-A has been shown to exert growth-inhibitory effects on MCF-7 cells [34], but has no effect on MDA-MB-231 cells [35], the role of activin-B and activin-AB in these cell lines is not known. Whether ALK4 and Activin B/AB are involved in Nodal/ALK7-regulated breast cancer cell proliferation/apoptosis remains to be investigated.
In both MDA-MB-231 and MCF-7 cells, we observed that Nodal and ALK7-ca up-regulates cell cycle inhibitors such as p27 and p21. Our recent studies in human trophoblast cells also demonstrate that activation of the Nodal-ALK7 pathway leads to an inhibition of cell cycle progression from G1 to S phase and this is caused in part by activation of p27 and inhibition of cdk2 [17]. Similarly, ALK7 was found to up-regulate p15 and p21 in PC12 cells [16]. These findings suggest that the cell cycle inhibitors may be involved in ALK7-induced cell growth arrest. Interestingly, we found that BRCA2 was transiently up-regulated by Nodal and ALK7-ca. Since BRCA2 has been found to suppress cell proliferation [36, 37], it is possible that it may in part mediate the antiproliferative effect of Nodal/ALK7 in breast cancer cells.
The results from cell counting and MTT assays show that Nodal and ALK7-ca have more potent effects on MDA-MB-231 cells than in MCF-7 cells. The different responsiveness between the two cell lines is not due to different transfection efficiency because transfection efficiency in MCF-7 cells was higher than that in MDA-MB-231 cells. In both TUNEL and DNA fragmentation assays, we found that Nodal and ALK7-ca potently induced apoptosis in MDA-MB-231 cells but only had minor effects in MCF-7 cells. On the other hand, BrdU assays revealed that Nodal and ALK7-ca have similar effects in inhibiting proliferation in both cell lines. Therefore, we further examined the mechanisms underlying the action of Nodal and ALK7 and the steps in the apoptosis pathway that are responsible for the decrease in response to the pro-apoptotic effect of Nodal and ALK7-ca. Proteins from the Bcl-2 family are key regulators of caspase activation and apoptosis. The functional blockade of some members of this family, notably Bcl-2 and Bcl-XL, can lead to apoptosis in breast cancer cells [38, 39]. We measured Bcl-2 and Bcl-XL protein levels after Nodal and ALK7-ca transfection and found that Nodal and ALK7-ca decreased Bcl-2 and Bcl-XL levels in both cell lines. Furthermore, we found caspase-3 was activated significantly by both Nodal and ALK7-ca in MDA-MB-231 cells, however, consistent with previous reports [27] that MCF-7 cells lack caspase 3, no caspase 3 activation was detected in MCF-7. These results suggest that in MDA-MB-231 cells, activation of the Nodal-ALK7 pathway down-regulates Bcl-2 and Bcl-XL expression, which in turn activates caspase-3, to induce apoptosis. This notion is further supported by the finding that a caspase 3 inhibitor blocked Nodal-induced apoptosis. However, it is unclear why expression of caspase 3 failed to restore the response to Nodal and ALK7. Since we did not detect active caspase 3 in these cells following Nodal and ALK7-ca transfection (data not shown), it is possible that other mediator(s) of Nodal, which are required for caspase 3 activation, are also defective in MCF-7 cells.
In addition to the caspase 3-dependent pathway, Nodal/ALK7 may also induce apoptosis in a caspase 3-independent manner. Although the basal level of apoptosis, as detected by TUNEL assay, was low, Nodal and ALK7-ca increased the number of TUNEL positive cells. The number of dead cells as measured by trypan blue exclusion was also increased by overexpression of Nodal or activation of ALK7. Previous studies have suggested that Bax can induce apoptosis through caspase 3-independent mechanisms [24]. Since we have recently shown that Bax partially mediates the apoptosis-inducing effect of Nodal/ALK7 [20], it is possible that Nodal/ALK7 upregulates Bax in breast cancer cells and this leads to the induction of apoptosis through a caspase 3-independent pathway.
Based on our observation that activation of the Nodal-ALK7 pathway results in the inhibition of cell proliferation and induction of apoptosis in breast cancer cells, it appears that Nodal has tumor-suppressing activities in breast cancer cells. This is somewhat at odds with the findings that Cripto, a co-receptor of Nodal, has oncogenic effects in mouse mammary epithelial cells and human breast cancer cells. Overexpression of Cripto-1 has been shown to induce tumor formation, cell migration and invasion, as well as tumor angiogenesis [40–43]. While it is well-documented that Cripto is required for Nodal signaling through ALK4 during embryogenesis [11, 32], Nodal signaling through ALK7 is not dependent on Cripto [11]. Cripto also has functions independent of Nodal signaling. For example, Cripto inhibited activin signaling through ALK4 and antagonized the growth-inhibitory effect of activin-B in human breast cancer cells [43]. In addition, Cripto activated MAPK and PI3K pathways in a Nodal-independent manner [28, 44, 45]. Whether or not Cripto plays a role in Nodal-inhibited cell growth and survival in breast cancer and other cells remains to be determined.
While our studies in breast cancer and ovarian cancer cells support a role for Nodal/ALK7 signaling in inhibiting proliferation and inducing apoptosis, a recent study has shown that Nodal signaling increased melanoma aggressiveness, indicating that Nodal has tumorigenic activities [46]. Thus, the role of Nodal in cancer may be very complex depending on the type and/or stage of the cancer. It is well established that TGF-β plays dual roles in cancer development. At early stages of tumorigenesis, TGF-β acts as a suppressor of tumor growth. However, as the tumor progresses, changes in TGF-β expression and cellular responses allow TGF-β to stimulate tumor progression, invasion and metastasis [47–50]. More studies are required to investigate the role of Nodal in tumorigenesis.
Taken together, these results demonstrated that the Nodal-ALK7 signaling pathway exerts antiproliferative and proapoptotic effects in breast cancer cells and that caspase 3 is important for Nodal-ALK7-induced apoptosis.
These findings suggest that Nodal may play a role in breast carcinogenesis and further studies are required to understand the involvement and signaling events of Nodal in breast cancer.
Acknowledgments
This study was supported by grants from Canadian Institute of Health Research (MOP-74622 and MOP-81370) and an Ontario Premier's Research Excellent Award to CP. GX is a recipient of an OWHC Scholars Award and CP is a recipient of a Mid-Career Award from OWHC/CIHR). GY received a fellowship from Toronto Ovarian Cancer Research Network. We thank Dr. B. Fang for providing MCF-7/caspase 3 cells.
References
- 1.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 2.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 3.Graham H, Peng C. Activin receptor-like kinases: structure, function and clinical implications. Endocr Metab Immune Disord Drug Targets. 2006;6:45–58. doi: 10.2174/187153006776056585. [DOI] [PubMed] [Google Scholar]
- 4.Abe Y, Minegishi T, Leung PC. Activin receptor signaling. Growth Factors. 2004;22:105–110. doi: 10.1080/08977190410001704688. [DOI] [PubMed] [Google Scholar]
- 5.Chang CF, Westbrook R, Ma J, Cao D. Transforming growth factor-beta signaling in breast cancer. Front Biosci. 2007;12:4393–4401. doi: 10.2741/2396. [DOI] [PubMed] [Google Scholar]
- 6.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7:1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 7.Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002;16:2339–2344. doi: 10.1101/gad.1016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Sakuma R, Ohnishi Yi Y, Meno C, Fujii H, Juan H, Takeuchi J, Ogura T, Li E, Miyazono K, Hamada H. Inhibition of Nodal signalling by Lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells. 2002;7:401–412. doi: 10.1046/j.1365-2443.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 11.Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibanez CF, Brivanlou AH. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchida K, Sawchenko PE, Nishikawa S, Vale WW. Molecular cloning of a novel type I receptor serine/threonine kinase for the TGF beta superfamily from rat brain. Mol Cell Neurosci. 1996;7:467–478. doi: 10.1006/mcne.1996.0034. [DOI] [PubMed] [Google Scholar]
- 13.Ryden M, Imamura T, Jornvall H, Belluardo N, Neveu I, Trupp M, Okadome T, ten Dijke P, Ibanez CF. A novel type I receptor serine-threonine kinase predominantly expressed in the adult central nervous system. J Biol Chem. 1996;271:30603–30609. doi: 10.1074/jbc.271.48.30603. [DOI] [PubMed] [Google Scholar]
- 14.Bondestam J, Huotari MA, Moren A, Ustinov J, Kaivo-Oja N, Kallio J, Horelli-Kuitunen N, Aaltonen J, Fujii M, Moustakas A, Ten Dijke P, Otonkoski T, Ritvos O. cDNA cloning, expression studies chromosome mapping of human type I serine/threonine kinase receptor ALK7 (ACVR1C) Cytogenet Cell Genet. 2001;95:157–162. doi: 10.1159/000059339. [DOI] [PubMed] [Google Scholar]
- 15.Roberts HJ, Hu S, Qiu Q, Leung PC, Caniggia I, Gruslin A, Tsang B, Peng C. Identification of novel isoforms of activin receptor-like kinase 7 (ALK7) generated by alternative splicing and expression of ALK7 and its ligand, Nodal, in human placenta. Biol Reprod. 2003;68:1719–1726. doi: 10.1095/biolreprod.102.013045. [DOI] [PubMed] [Google Scholar]
- 16.Jornvall H, Blokzijl A, ten Dijke P, Ibanez CF. The orphan receptor serine/threonine kinase ALK7 signals arrest of proliferation and morphological differentiation in a neuronal cell line. J Biol Chem. 2001;276:5140–5146. doi: 10.1074/jbc.M005200200. [DOI] [PubMed] [Google Scholar]
- 17.Munir S, Xu G, Wu Y, Yang B, Lala PK, Peng C. Nodal and ALK7 inhibit proliferation and induce apoptosis in human trophoblast cells. J Biol Chem. 2004;279:31277–31286. doi: 10.1074/jbc.M400641200. [DOI] [PubMed] [Google Scholar]
- 18.Xu G, Zhong Y, Munir S, Yang BB, Tsang BK, Peng C. Nodal induces apoptosis and inhibits proliferation in human epithelial ovarian cancer cells via activin receptor-like kinase 7. J Clin Endocrinol Metab. 2004;89:5523–5534. doi: 10.1210/jc.2004-0893. [DOI] [PubMed] [Google Scholar]
- 19.Kim BC, van Gelder H, Kim TA, Lee HJ, Baik KG, Chun HH, Lee DA, Choi KS, Kim SJ. Activin receptor-like kinase-7 induces apoptosis through activation of MAPKs in a Smad3-dependent mechanism in hepatoma cells. J Biol Chem. 2004;279:28458–28465. doi: 10.1074/jbc.M313277200. [DOI] [PubMed] [Google Scholar]
- 20.Xu G, Zhou H, Wang Q, Auersperg N, Peng C. Activin receptor-like kinase 7 induces apoptosis through up-regulation of Bax and down-regulation of Xiap in normal and malignant ovarian epithelial cell lines. Mol Cancer Res. 2006;4:235–246. doi: 10.1158/1541-7786.MCR-05-0174. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N, Kumar M, Xu G, Ju W, Yoon T, Xu E, Huang X, Gaisano H, Peng C, Wang Q. Activin receptor-like kinase 7 induces apoptosis of pancreatic beta cells and beta cell lines. Diabetologia. 2006;49:506–518. doi: 10.1007/s00125-005-0095-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Jiang JY, Zhu C, Peng C, Tsang BK. Role and regulation of nodal/activin receptor-like kinase 7 signaling pathway in the control of ovarian follicular atresia. Mol Endocrinol. 2006;20:2469–2482. doi: 10.1210/me.2005-0446. [DOI] [PubMed] [Google Scholar]
- 23.Xu G, Bernaudo S, Fu G, Lee DY, Yang BB, Peng C. Cyclin G2 is degraded through the ubiquitin-proteasome pathway and mediates the antiproliferative effect of activin receptor-like kinase 7. Mol Biol Cell. 2008;19:4968–4979. doi: 10.1091/mbc.E08-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagawa S, Gu J, Honda T, McDonnell TJ, Swisher SG, Roth JA, Fang B. Deficiency of caspase-3 in MCF7 cells blocks Bax-mediated nuclear fragmentation but not cell death. Clin Cancer Res. 2001;7:1474–1480. [PubMed] [Google Scholar]
- 25.Bast RC, Jr, Boyer CM, Jacobs I, Xu FJ, Wu S, Wiener J, Kohler M, Berchuck A. Cell growth regulation in epithelial ovarian cancer. Cancer. 1993;71:1597–1601. doi: 10.1002/cncr.2820710426. [DOI] [PubMed] [Google Scholar]
- 26.Bernard DJ, Lee KB, Santos MM. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reprod Biol Endocrinol. 2006;4:52. doi: 10.1186/1477-7827-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 28.Bianco C, Adkins HB, Wechselberger C, Seno M, Normanno N, De Luca A, Sun Y, Khan N, Kenney N, Ebert A, Williams KP, Sanicola M, Salomon DS. Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells. Mol Cell Biol. 2002;22:2586–2597. doi: 10.1128/MCB.22.8.2586-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rios R, Fernandez-Nocelos S, Carneiro I, Arce VM, Devesa J. Differential response to exogenous and endogenous myostatin in myoblasts suggests that myostatin acts as an autocrine factor in vivo. Endocrinology. 2004;145:2795–2803. doi: 10.1210/en.2003-1166. [DOI] [PubMed] [Google Scholar]
- 30.Pouliot F, Labrie C. Expression profile of agonistic Smads in human breast cancer cells: absence of regulation by estrogens. Int J Cancer. 1999;81:98–103. doi: 10.1002/(sici)1097-0215(19990331)81:1<98::aid-ijc17>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Mironova E, Whitaker LL, Edwards L, Yost HJ, Ramsdell AF. ALK4 functions as a receptor for multiple TGF beta-related ligands to regulate left-right axis determination and mesoderm induction in Xenopus. Dev Biol. 2004;268:280–294. doi: 10.1016/j.ydbio.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 32.Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
- 33.Tsuchida K, Nakatani M, Yamakawa N, Hashimoto O, Hasegawa Y, Sugino H. Activin isoforms signal through type I receptor serine/threonine kinase ALK7. Mol Cell Endocrinol. 2004;220:59–65. doi: 10.1016/j.mce.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Liu QY, Niranjan B, Gomes P, Gomm JJ, Davies D, Coombes RC, Buluwela L. Inhibitory effects of activin on the growth and morpholgenesis of primary and transformed mammary epithelial cells. Cancer Res. 1996;56:1155–1163. [PubMed] [Google Scholar]
- 35.de Winter JP, Roelen BA, ten Dijke P, van der Burg B, van den Eijnden-van Raaij AJ. DPC4 (SMAD4) mediates transforming growth factor-beta1 (TGF-beta1) induced growth inhibition and transcriptional response in breast tumour cells. Oncogene. 1997;14:1891–1899. doi: 10.1038/sj.onc.1201017. [DOI] [PubMed] [Google Scholar]
- 36.Tian XX, Rai D, Li J, Zou C, Bai Y, Wazer D, Band V, Gao Q. BRCA2 suppresses cell proliferation via stabilizing MAGE-D1. Cancer Res. 2005;65:4747–4753. doi: 10.1158/0008-5472.CAN-05-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang SC, Shao R, Pao AY, Zhang S, Hung MC, Su LK. Inhibition of cancer cell growth by BRCA2. Cancer Res. 2002;62:1311–1314. [PubMed] [Google Scholar]
- 38.Real PJ, Cao Y, Wang R, Nikolovska-Coleska Z, Sanz-Ortiz J, Wang S, Fernandez-Luna JL. Breast cancer cells can evade apoptosis-mediated selective killing by a novel small molecule inhibitor of Bcl-2. Cancer Res. 2004;64:7947–7953. doi: 10.1158/0008-5472.CAN-04-0945. [DOI] [PubMed] [Google Scholar]
- 39.Tortora G, Caputo R, Damiano V, Caputo R, Troiani T, Veneziani BM, De Placido S, Bianco AR, Zangemeister-Wittke U, Ciardiello F. Combined targeted inhibition of bcl-2, bcl-XL, epidermal growth factor receptor, and protein kinase A type I causes potent antitumor, apoptotic, and antiangiogenic activity. Clin Cancer Res. 2003;9:866–871. [PubMed] [Google Scholar]
- 40.Bianco C, Strizzi L, Normanno N, Khan N, Salomon DS. Cripto-1: an oncofetal gene with many faces. Curr Top Dev Biol. 2005;67:85–133. doi: 10.1016/S0070-2153(05)67003-2. [DOI] [PubMed] [Google Scholar]
- 41.Wechselberger C, Ebert AD, Bianco C, Khan NI, Sun Y, Wallace-Jones B, Montesano R, Salomon DS. Cripto-1 enhances migration and branching morphogenesis of mouse mammary epithelial cells. Exp Cell Res. 2001;266:95–105. doi: 10.1006/excr.2001.5195. [DOI] [PubMed] [Google Scholar]
- 42.Normanno N, De Luca A, Bianco C, Maiello MR, Carriero MV, Rehman A, Wechselberger C, Arra C, Strizzi L, Sanicola M, Salomon DS. Cripto-1 overexpression leads to enhanced invasiveness and resistance to anoikis in human MCF-7 breast cancer cells. J Cell Physiol. 2004;198:31–39. doi: 10.1002/jcp.10375. [DOI] [PubMed] [Google Scholar]
- 43.Adkins HB, Bianco C, Schiffer SG, Rayhorn P, Zafari M, Cheung AE, Orozco O, Olson D, De Luca A, Chen LL, Miatkowski K, Benjamin C, Normanno N, Williams KP, Jarpe M, LePage D, Salomon D, Sanicola M. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J Clin Invest. 2003;112:575–587. doi: 10.1172/JCI17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianco C, Strizzi L, Rehman A, Normanno N, Wechselberger C, Sun Y, Khan N, Hirota M, Adkins H, Williams K, Margolis RU, Sanicola M, Salomon DS. A Nodal-and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003;63:1192–1197. [PubMed] [Google Scholar]
- 45.Xing PX, Hu XF, Pietersz GA, Hosick HL, McKenzie IF. Cripto: a novel target for antibody-based cancer immunotherapy. Cancer Res. 2004;64:4018–4023. doi: 10.1158/0008-5472.CAN-03-3888. [DOI] [PubMed] [Google Scholar]
- 46.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J, Hendrix MJ. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 47.Akhurst RJ. TGF-beta antagonists: why suppress a tumor suppressor? J Clin Invest. 2002;109:1533–1536. doi: 10.1172/JCI15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhurst RJ, Derynck R. TGF-beta signaling in cancer–a double-edged sword. Trends Cell Biol. 2001;11:S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 49.Buck MB, Knabbe C. TGF-beta signaling in breast cancer. Ann N Y Acad Sci. 2006;1089:119–126. doi: 10.1196/annals.1386.024. [DOI] [PubMed] [Google Scholar]
- 50.Galliher AJ, Neil JR, Schiemann WP. Role of transforming growth factor-beta in cancer progression. Future Oncol. 2006;2:743–763. doi: 10.2217/14796694.2.6.743. [DOI] [PubMed] [Google Scholar]