Abstract
Mitochondrial DNA (mtDNA) mutations were reported in primary head and neck squamous cell carcinoma (HNSCC) patients. However, very little information is available on the mtDNA mutation pattern in the histologically negative surgical margins and tumors of HNSCC patients who experienced tumor recurrence. The present study aimed at understanding the nature and timing of mtDNA mutation in histologically negative margins, and tumors in HNSCC patients who developed local recurrence during the follow ups. The entire 16.5-kb mitochondrial genome was sequenced in matched normal lymphocytes, histologically normal margins, and tumors of 50 recurrent HNSCC patients. The mtDNA mutations were then compared with clinical parameters. Forty-eight percent (24 of 50) patients harbored at least one somatic mtDNA mutation in the tumor, and a total of 37 somatic mtDNA mutations were detected. The mtDNA mutations were mostly heteroplasmic in nature and nucleotide transitions (A↔G; T↔C). Forty-six percent of the mutations (17 of 37) were detected in the tumors and were also detectable in the corresponding histologically normal margin of the patients. The mtDNA mutations involved both coding and noncoding regions of the mtDNA. Majority (9 of 17, 53%) of the noncoding mutations involved tRNAs. Seventy-five percent (15 of 20) of the coding mtDNA mutations were nonsynonymous in nature and mainly affected cytochrome c oxidase (Complex IV), frequently altered in different human mitochondrial diseases including cancer. Analysis of mtDNA mutation could be an invaluable tool for molecular assessment of histologically negative margins and as well for monitoring HNSCC patients with locoregional recurrences.
Introduction
Mitochondria are unique organelles that posses their own DNA (mtDNA), inherited maternally, and replicated and transcribed semiautonomously (1, 2). Human mtDNA is a 16.5-kb double-stranded closed circular molecule that encodes 12S and 16S rRNAs, 22 tRNAs, and 13 proteins (total of 37 genes) required for the oxidative phosphorylation system (1, 2). Most human cells contain hundreds of copies of mtDNA, and nearly all of these mtDNA copies are identical (i.e., homoplasmic) at birth (1, 2). Introduction of a new mtDNA mutation in a cell may result in a state of mixed population of mtDNA known as heteroplasmy (2). During replicative segregation, the balance can be biased toward homoplasmic mutant or wild-type (2). MtDNA is particularly susceptible to damage by environmental carcinogens because it contains no introns, protective histones, or nonhistone proteins, and is exposed to endogenous reactive oxygen species generated as a byproduct of the oxidative phosphorylation system (1). Possibly as a consequence, the mtDNA is more susceptible to mutation in cancer cells than nuclear DNA (1).
Head and neck squamous cell carcinoma (HNSCC) is the fifth leading cause of cancer-related death with an annual incidence of 500,000 cases worldwide (3). Approximately 40,000 new cases of HNSCC are diagnosed annually in the United States (3). The 5-year posttherapeutic survival rate is among the lowest of the major cancers, despite significant improvement in therapeutic modalities with locoregional relapse being the major cause of death (3). Due to their poor survival outcome, development of suitable methods for early disease detection, monitoring, and evaluation of surgical margins are of paramount importance (3). Somatic mtDNA mutations have been reported mostly in primary HNSCC patients (4, 5). However, very little information is available on the mtDNA mutation pattern in the histologically negative surgical margins of HNSCC patients who experienced local recurrence. In the present study, we detected numerous tumor-clonal somatic mtDNA mutations in histologically negative margins of these HNSCC patients mainly affecting cytochrome c oxidase (Complex IV).
Materials and Methods
Patient’s history and tissue samples
We obtained matched normal lymphocytes and tumor tissues from 50 HNSCC patients who underwent surgical resection with curative intent after signed informed consent on a Johns Hopkins Institutional Review Board–approved protocol. Subjects in this study went on to experience local tumor recurrence. Sixty-four margins specimens (two to five margins from each patient) available from 24 of these 50 patients were also collected as per the above protocol. All the tumors were squamous cell carcinoma. Clinical and histologic staging of all the specimens were determined by a pathologist (W.H.W). All the patients were heavy smokers. Detailed information and the frequency of mtDNA mutations in tumors and margins of the recurrent HNSCC patients are shown in Table 1A. Mutation analysis was done in a blinded fashion without the prior knowledge of the samples.
Table 1.
Pattern of mtDNA mutation
| A: Pattern of mtDNA mutation in the HNSCC patients with local recurrence | |||||
|---|---|---|---|---|---|
| Patient ID | Mutation in tumor | Mutation in margin | Age*/stage | Recurrence† | Survival‡ |
| HN1 | 1 | 1 | 59/IV | 1A | 7 |
| HN2 | 1 | 1 | 66/II | 1A | 17 |
| HN3 | 1 | 0 | 58/II | 1A | 8 |
| HN4 | 3 | 1 | 48/II | 1A | 18 |
| HN5 | 2 | 1 | 77/II | 1A | 37 |
| HN6 | 1 | 0 | 64/II | 1A | 36 |
| HN7 | 3 | 1 | 63/II | 1C | 7 |
| HN8 | 3 | 1 | 75/III | 1A | 5 |
| HN9 | 3 | 1 | 83/IV | 1A | 15 |
| HN10 | 1 | 1 | 46/III | 1A | 19 |
| HN11 | 1 | 0 | 59/II | 1A | 9 |
| HN12 | 1 | 1 | 63/III | 1A | 18 |
| HN13 | 1 | 1 | 51/II | 1A | 47 |
| HN14 | 2 | 0 | 57/IV | 1A | 3 |
| HN15 | 2 | 1 | 61/II | 1A | 11 |
| HN16 | 1 | 1 | 80/I | 1A | 56 |
| HN17 | 1 | 1 | 62/IV | 1A | 15 |
| HN18 | 1 | 0 | 69/I | IB | 64 |
| HN19 | 2 | 1 | 66/IV | IC | 17 |
| HN20 | 1 | 0 | 81/I | IC | 34 |
| HN21 | 1 | 1 | 38/III | IC | 3 |
| HN22 | 2 | 0 | 67/IV | 1A | 23 |
| HN23 | 1 | 1 | 68/I | 1A | 33 |
| HN24 | 1 | 1 | 65/IV | 1A | 27 |
| B: Pattern of mtDNA mutation in the HNSCC patients without any recurrence | |||||
|---|---|---|---|---|---|
| Patient ID§ | Mutation in tumor | Mutation in margin | Age/stage | Recurrence | Survival |
| NR1 | 0 | 0 | 59/II | None | 66 |
| NR2 | 0 | 0 | 34/II | None | 74 |
Age of first diagnosis.
1A, local only; 1B, neck only; 1C, local and neck only.
Survival in months.
NR, nonrecurrent.
Mitochondrial whole genome amplification and sequencing
Genomic DNA was extracted according to our standard protocol from the microdissected tumor tissues and undissected margin specimens (6). We amplified whole mitochondrial genomic DNA from 10 ng of genomic DNA template using the REPLI-g mtDNA amplification kit according to the manufacturer’s protocol as described earlier (7). The amplified DNA was then purified using the DNA MiniAmp Cleaning kit (Qiagen). We examined the purity of the amplified mtDNA and nuclear DNA contamination by PCR analysis using a primer specific for mitochondria-encoded COI/COII and nuclear-encoded β-actin. Four to five hundred nanograms of purified mtDNA were used for sequencing on the Mitochip 2.0 platform (Affymetrix; ref. 7).
Mitochip 2.0 sequencing array analysis
We performed fragmentation, labeling, and Chip hybridization of the mtDNA as per Affymetrix protocol with appropriate controls as described earlier (7). Data analysis was done using the Affymetrix GSEQ software, and the Revised Cambridge Reference Sequence (rCRS) was used as the reference sequence. The MitoAnalyzer software was also used to verify the mtDNA mutations at different nucleotide positions as described earlier (7).
Determination of mtDNA mutation in tumor and margin specimens
Somatic mtDNA sequence variants were identified as bp changes in mtDNA of tumor when compared with mtDNA sequence of matched normal lymphocytes (6–8). Clonal mtDNA sequence variants were identified as identical bp changes in matched tumor and margin samples compared with the normal lymphocytes. Germ line sequence variants were identified as bp changes present in the normal lymphocytes as well as tumor tissues and/or margin samples compared with rCRS (9). In each case, mtDNA sequences were interrogated at the available Human Mitochondrial Genome Databases for defining each sequence variant (10–12).
Calculation of the mtDNA mutation load in different respiratory complexes of the mitochondrial genome
The total number of somatic mtDNA mutations detected in the tumors and margins in individual complex were considered for calculating the mutation load (Table 3). The mtDNA mutation load on each complex was calculated as follows: total number of nucleotides altered per complex/total number of nucleotides per complex × 100 (Table 3).
Table 3.
Calculation of mutation load in mitochondria-encoded genes of the respiratory Complex I, III, IV, and V
| Genome size (bp)* | Total number of amino acids | Total number of altered nucleotides† | Mutation load | |
|---|---|---|---|---|
| Gene name (Complex I) | ||||
| ND1 | 954 | 318 | ||
| ND2 | 1041 | 347 | ||
| ND3 | 345 | 115 | ||
| ND4 | 1377 | 459 | ||
| ND4L | 297 | 99 | ||
| ND5 | 1812 | 604 | ||
| ND6 | 525 | 175 | ||
| Total genome size, 6351 bp | 4 | 4/6,351 × 100 = 0.06% | ||
| Gene name (Complex III) | ||||
| CYTB | 1,140 | 380 | 1 | 1/1,140 × 100 = 0.08% |
| Gene name (Complex IV) | ||||
| COXI | 1,542 | 514 | ||
| COXII | 675 | 225 | ||
| COXIII | 783 | 261 | ||
| Total genome size, 3,000 bp | 8 | 8/3,000 × 100 = 0.26% | ||
| Gene name (Complex V) | ||||
| ATP6 | 681 | 227 | ||
| ATP8 | 207 | 69 | ||
| Total genome size, 888 bp | 0 | 0/888 × 100 = 00% | ||
Human Mitochondrial Genome Database (http://www.genpat.uu.se/mtDB/).
Only nonsynonymous coding mtDNA mutations were considered.
Statistical analysis
χ2 or Fisher’s exact tests were used as appropriate. Logistic regression was used to assess the effects of age and stage on the probability of mtDNA mutations. Sample t test or nonparametric Wilcoxon rank-sum test was also used to compare numbers of tumor and margin mtDNA mutations across the different groups. All P values are two sided, and all confidence intervals are at the 95% level. Computations for all the analysis were done using the Statistical Analysis System.
Results
Pattern of somatic mtDNA mutation in the tumors of the HNSCC patients with local recurrence
To understand the frequency and nature of mtDNA mutation in HNSCC patients who developed recurrence during the follow-ups, we analyzed microdissected tumor tissues and matched normal lymphocytes from 50 HNSCC patients on a reliable high-throughput mtDNA sequencing platform (6–8). Average call rate on the Mitochip version 2.0 was 92.5%. The patients belonged to the major Euro-pean haplogroup H and V (Table 2). Forty-eight percent (24 of 50) of the HNSCC tumors harbored at least 1 somatic mtDNA mutation (Tables 1A and 2). The mtDNA mutations were mostly heteroplasmic in nature and nucleotide transitions (A↔G; T↔C). A total of 37 somatic mtDNA mutations were detected spanning both the coding and noncoding region of the mitochondrial genome (Tables 1A and 2). Seventeen were from the noncoding regions of the mtDNA, whereas a majority of them (9 of 17, 53%) was in the tRNAs. Of the rest of the eight mtDNA mutations, one was from the D-Loop; four were from the 12SrRNA; and three were from 16SrRNA. The remainder of the 20 mutations was found in the coding regions; one was from ND2; four were from ND5 (Complex I); three were from CYTB (Complex III); eight were from COXI; three were from COX-II; and one was from COXIII (Complex IV). Of the 20 coding mtDNA mutations, 13 (65%) were nonsynonymous in nature (Table 2).
Table 2.
Pattern of somatic mtDNA mutations in the tumors of the HNSCC patients
| Patient | Haplotype | Nucleotide position* | rCRS | Normal† | Tumor | Margin | Amino acid change | mtDNA region |
|---|---|---|---|---|---|---|---|---|
| HN1 | H | 6027 | g | g | a | a | G-S | COI |
| HN2 | H | 15249 | a | a | g | g | Y-C | CYTB |
| HN3 | H | 5756 | g | g | a | g | — | OLR |
| HN4 | H | 7538 | t | t | c | t | — | tRNAAsp |
| 13814 | t | t | c | t | V-A | ND5 | ||
| 14803 | c | c | t | t | I-I | CYTB | ||
| HN5 | V | 2188 | a | a | g | a | — | 16S rRNA |
| 2517 | t | t | c | c | — | 16S rRNA | ||
| HN6 | H | 1606 | g | g | a | a | — | tRNAVal |
| HN7 | V | 705 | c | c | t | t | — | 12S rRNA |
| 5700 | t | t | c | t | — | tRNAAsp | ||
| 9320 | c | c | t | c | H-H | COIII | ||
| HN8 | H | 7242 | t | t | c | t | Y-R | COI |
| 13322 | t | t | c | c | I-T | ND5 | ||
| 15984 | t | t | c | t | — | tRNAPro | ||
| HN9 | H | 7284 | t | t | c | c | S-P | COI |
| 12447 | a | a | g | a | K-K | ND5 | ||
| 15640 | c | c | t | c | I-I | CYTB | ||
| HN10 | V | 1444 | a | a | g | a | — | 12S rRNA |
| HN11 | H | 10457 | t | t | c | t | — | tRNA Arg |
| HN12 | V | 8170 | a | a | g | g | Q-E | COII |
| HN13 | H | 1643 | a | a | g | a | — | tRNAVal |
| HN14 | H | 7767 | t | t | c | c | M-T | COII |
| 7865 | t | t | c | t | S-P | COII | ||
| HN15 | H | 5537 | a | a | g | a | — | tRNATrp |
| 13808 | t | t | c | c | L-P | ND5 | ||
| HN16 | H | 6027 | g | a | a | a | G-S | COI |
| HN17 | V | 912 | t | t | a | a | — | 12SrRNA |
| HN18 | H | 1323 | g | g | a | g | — | 12SrRNA |
| HN19 | H | 2069 | t | t | c | t | — | 16SrRNA |
| 5570 | t | t | c | c | — | tRNATryp | ||
| HN20 | H | 5799 | a | a | g | a | — | tRNCys |
| HN21 | H | 4689 | a | a | g | g | I-V | ND2 |
| HN22 | H | 6542 | c | c | t | c | R-R | COI |
| 7029 | c | c | t | c | H-T | COI | ||
| HN23 | H | 6380 | a | a | g | g | L-L | COI |
| HN24 | H | 6794 | a | a | g | g | M-M | COI |
All mtDNA sequences were interrogated at Human Mitochondrial genome database (http://www.genpat.uu.se/mtDB/).
Matched normal lymphocytes.
Pattern of somatic mtDNA mutation in the histologically negative margins of the HNSCC patients
We further assessed histologically negative surgical margin obtained from the 24 patients having mtDNA mutation in the tumors (Tables 1 and 2). We procured 64 histologically negative margin samples from these 24 patients and sequenced them on the same Mitochip version 2.0 sequencing platform. Seventy-one percent (17 of 24) of the patients have shown at least one mtDNA mutation in the margin. All the mtDNA mutations were also clonally detected in the corresponding tumor tissues of the recurrent HNSCC patients (Table 2).
Association between clinical characteristics and mtDNA mutation in the HNSCC patients
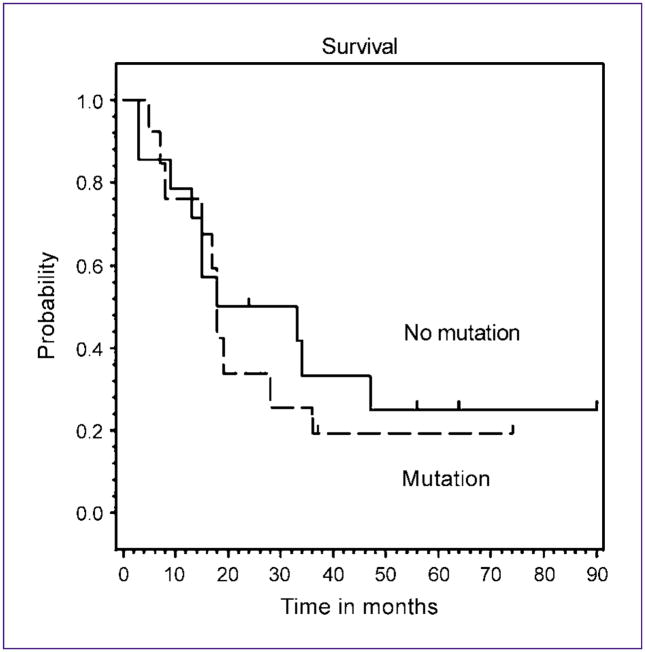
We compared the mtDNA mutation pattern with different clinical parameters such as stage and survival in this study. All HNSCC cases went on to develop local recurrence after resection with or without postoperative radiation. There was a significant difference in the distribution of mtDNA mutations among the stage I to II versus III to IV patients (Student’s t test, P = 0.03). No correlation was found with the survival (Fig. 1). The median overall survival was 22 months in both groups. Hazard ratio for mtDNA mutations was 1.01 (95% confidence interval, 0.96–1.08, P = 0.52).
Fig. 1.
Impact of mtDNA mutation on survival in the recurrent HNSCC patients. Survival was not significantly different among patients with and without mtDNA mutations.
Coding mtDNA mutations affect mitochondrial respiratory Complex IV and may be pathogenic
We also determined the effect of somatic mtDNA mutation on different respiratory complexes encoded by the mitochondrial genome (Tables 2 and 3). Only the coding nonsynonymous mtDNA mutations observed in the patients were considered, and the mutational load for each complex was calculated as described in Materials and Methods. As shown in Fig. 2, the mutational load was significantly higher (P < 0.001) in Complex IV compared with the other complexes.
Fig. 2.
The effect of mtDNA mutation on different respiratory complexes. Mutation load was significantly higher (*, P < 0.001) in Complex IV compared with Complex I and III.
Germ line mtDNA sequence variants in the recurrent HNSCC patients
Other than the somatic mtDNA mutation, we also examined the pattern of germ line mtDNA sequence variation in matched normal lymphocytes and tumors as well as corresponding margin samples by comparing each patient with the reference rCRS. A total of five coding germ line mtDNA sequence variants were detected in these patients with only one of which was previously reported as per the available human mitochondrial genomic databases (Table 4). Eighty-percent (four of five) germ line sequence variants were nonsynonymous in nature. One of the germ lime mtDNA sequence variants resulted in the termination codon of a Complex I gene ND4 (Table 4).
Table 4.
Germ line mtDNA sequence variants in the recurrent HNSCC patients
| Nucleotide position | rCRS | Normal* | Tumor | Margin | Amino acid change | mtDNA region |
|---|---|---|---|---|---|---|
| 4767 | a | g | g | g | M-V | ND2 |
| 9665 | a | g | g | g | E-E | COIII |
| 11717 | g | a | a | a | G-Ter† | ND4 |
| 15419 | a | g | g | g | T-P | CYTB |
| 15563 | t | c | c | c | Y-H | CYTB |
Matched normal lymphocytes.
Ter, mutation resulted in termination codon.
Discussion
In the present study, we examined the pattern of mtDNA mutations in histologically negative margins and tumors obtained from 50 recurrent HNSCC patients by sequencing the entire mitochondrial genome on the Affymetrix Mito-chip 2.0 sequencing platform (6–8). To our knowledge, this is the first report of the scrutiny of mtDNA mutation pattern in normal surgical margins of HNSCC patients. This high-throughput mtDNA resequencing platform overcomes conventional DNA sequencing method as reliable mutation detection is possible (6, 7). Stringent criteria were followed and appropriate databases were used to classify every single mtDNA sequence variant, considering the recent findings of erroneous reporting of mtDNA mutation in different cancers (13–17). Based on the standard data interpretation criterion, we detected both somatic and germ line mtDNA sequence variants in these patients and also confirm that the resultant sequence variations are not known single nucleotide polymorphisms in different population as per the available mtDNA databases (10–12).
Locoregional relapse is the major cause of death in HNSCC, which clearly warrants for suitable methods for disease monitoring or to predict relapse. In this context, evaluation of surgical margins using mtDNA mutation could be useful as mtDNA mutation analysis has potential implication for cancer biomarker development (18). One may argue in favor of the clonal detection of tumor cells using nuclear genome as done earlier using p53 mutation (19). But due to the large copy number of mutated mtDNA compared with the nDNA in cancer cells, detection is more advantageous and suitable for cancer diagnostic application (18). Moreover, profiling of a panel of mtDNA mutation affecting different respiratory complexes along with nDNA changes could also be useful for screening population at risk such as heavy smokers. Recently, we identified clonal mtDNA mutation in histologically normal respiratory epithelium of some follow-up lung cancer patients (7).
In the present study, all the patients were heavy smokers, and the mtDNA analysis was done in a blinded fashion. Strikingly, the normal margin samples without any microscopic evidence of hyperplastic or dysplastic changes exhibited tumor clonal mtDNA mutation. It is very unlikely that the high-resolution Mitochip v2.0 system has a low sensitivity for mutation detection; otherwise, we would not have detected matched mtDNA mutations in a few cells procured from several margins of the same patient. Due to lack of biospecimens, we could only assess six negative margins and tumor samples obtained from two nonrecurrent HNSCC patients (Table 1B). No mtDNA mutations were detected in the tumor or margin samples of these nonrecurrent patients. Thus, it seems that mtDNA mutation screening could be an invaluable tool for critical evaluation of negative margins.
We also detected numerous somatic mtDNA mutations in the tumors of the recurrent HNSCC patients significantly associated with the disease progression. A substantial number of noncoding mutations were in the tRNAs, a frequent target in different tumors (4). A considerable number of these mutations were clonally associated with the corresponding normal margins. This result suggests for their functional role in HNSCC. Recently, we have shown the effect of forced overexpression of mitochondria-encoded Complex I/III gene mutation in cervical and bladder cancer progression (4, 20, 21). Those mutations were originally found in the patients. Thus, it seems that mtDNA mutation associated with HNSCC progression is detectable using this high-throughput sequencing platform. Identification of a panel of frequently occurring mutations at different stages could be useful for monitoring HNSCC progression.
Most of the mtDNA mutations observed in this study were at the coding regions of the mtDNA, and only one mutation was detected in the regulatory D-Loop region where transcription of the all the 13 mtDNA-encoded genes starts. We hypothesize that for progression, cells prefer functional D-Loop region with growth-promoting coding mtDNA mutations. The coding mtDNA mutation load seemed to be higher in Cytochrome c oxidase (Complex IV). Cytochrome c oxidase subunits I to III comprise the catalytic core of the enzyme and are all synthesized from mtDNA (22). The remaining subunits (IV–VIII) are synthesized from cellular nuclear DNA (22). Cytochrome c oxidase dysfunction due to mutation in different subunits has been reported in prostate cancer and other mitochondrial diseases (22). Due to the lack of representative tumor sections, we could not evaluate Complex IV expression status at the protein level in the respective patients showing mutation in the Complex IV.
The presence of germ line mtDNA sequence variants was suggested as an indicator of a high susceptibility genetic background, which might facilitate concomitant somatic mutation in mtDNA and nDNA (9). Detection of germ line variants and simultaneous somatic mtDNA mutations in tumors and margins of the overlapping group of HNSCC patients does support this notion. Clearly, the presence of clonal germ line mtDNA mutations in negative margins is likely to increase their susceptibility of acquiring additional independent somatic mtDNA/nDNA alteration necessary for tumorigenic transformation. One of the germ line sequence variants in Complex I (Table 4) resulted in a termination codon, which may disrupt the proper assembly and functioning of the complex and contribute to tumor progression. Identification of the patients with such notable mtDNA changes could also be useful for diagnostics and disease monitoring.
Taken together, our results show the presence of tumor-clonal somatic mtDNA mutations in histologically normal margins of recurrent HNSCC patients, implicating for potential application of mtDNA mutation in cancer diagnostics (18). Functional analysis of the specific mtDNA mutations observed will guide us to further understand their role in HNSCC progression.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Verma M, Kagan J, Sidransky D, Srivastava S. Proteomic analysis of cancer-cell mitochondria. Nat Rev Cancer. 2003;3:789–95. doi: 10.1038/nrc1192. [DOI] [PubMed] [Google Scholar]
- 2.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang SS, Califano J. Current status of biomarkers in head and neck cancer. J Surg Oncol. 2008;97:640–3. doi: 10.1002/jso.21023. [DOI] [PubMed] [Google Scholar]
- 4.Zhou S, Kachhap S, Sun W, et al. Frequency and phenotypic implications of mitochondrial DNA mutations in human squamous cell cancers of the head and neck. Proc Natl Acad Sci U S A. 2007;104:7540–5. doi: 10.1073/pnas.0610818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mithani SK, Taube JM, Zhou S, et al. Mitochondrial mutations are a late event in the progression of head and neck squamous cell cancer. Clin Can Res. 2007;13:4331–5. doi: 10.1158/1078-0432.CCR-06-2613. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S, Kassauei K, Cutler DJ, et al. An oligonucleotide microarray for high-throughput sequencing of the mitochondrial genome. J Mol Diagn. 2006;4:476–82. doi: 10.2353/jmoldx.2006.060008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasgupta S, Yung RC, Westra WH, Rini DA, Brandes J, Sidransky D. Following mitochondrial footprints through a long mucosal path to lung cancer. PLoS One. 2009;4:e6533. doi: 10.1371/journal.pone.0006533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maragh S, Jakupciak JP, Wagner PD, et al. Multiple strand displacement amplification of mitochondrial DNA. BMC Medical Genetics. 2008;9:7. doi: 10.1186/1471-2350-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gochhait S, Bhatt A, Sharma S, Singh YP, Gupta P, Bamezai RN. Concomitant presence of mutations in mitochondrial genome and p53 in cancer development—a study in north Indian sporadic breast and esophageal cancer patients. Int J Cancer. 2008;123:2580–6. doi: 10.1002/ijc.23817. [DOI] [PubMed] [Google Scholar]
- 10.Available from: http://www.genpat.uu.se/mtDB/
- 11.Available from: http://www.cstl.nist.gov/biotech/strbase/mitoanalyzer.html
- 12.Available from: http://www.phylotree.org
- 13.Sales A, Yao Y-G, Macaulay V, Vega A, Carracedo A, Bandelt H-J. A critical reassessment of the role of mitochondria in tumorigenesis. PLoS Medicine. 2005;2:1158–66. doi: 10.1371/journal.pmed.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandelt H-J, Sales A, Bravi CM. What is a novel mtDNA mutation—and does novelty really matter? J Hum Genet. 2006;51:1073–82. doi: 10.1007/s10038-006-0066-5. [DOI] [PubMed] [Google Scholar]
- 15.Bandelt H-J, Sales A, Taylor RW, Yao Y-G. Exaggerated status of novel and pathogenic mtDNA sequence variants due to inadequate database searches. Hum Mutat. 2008;30:191–6. doi: 10.1002/humu.20846. [DOI] [PubMed] [Google Scholar]
- 16.Bandelt H-J, Yao Y-G, Sales A. The search of novel mtDNA mutations in hypertrophic cardiomyopathy: MITOMAPing as a risk factor. Int J Cadiol. 2008;126:439–42. doi: 10.1016/j.ijcard.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 17.Bandelt H-J, Sales A. Contamination and sample mix-up can best explain some patterns od mtDNA instabilities in buccal cells and oral squamous cell carcinoma. BMC Cancer. 2009;9:113. doi: 10.1186/1471-2407-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Wu J, Dressman DC, et al. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature. 2010 doi: 10.1038/nature08802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–35. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 20.Dasgupta S, Hoque MO, Upadhyay S, Sidransky D. Mitochondrial cytochrome B gene mutation promotes tumor growth in bladder cancer. Cancer Res. 2008;68:700–6. doi: 10.1158/0008-5472.CAN-07-5532. [DOI] [PubMed] [Google Scholar]
- 21.Dasgupta S, Hoque MO, Upadhyay S, Sidransky D, Sidransky D. Forced Cytochrome B gene mutation expression induces mitochondrial proliferation and prevents apoptosis in human uroepithelial SV-HUC-1 cells. Int J Cancer. 2009;125:2829–35. doi: 10.1002/ijc.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann PC, Gillespie JW, Charboneau L, et al. Mitochondrial proteome: altered cytochrome c oxidase subunit levels in prostate cancer. Proteomics. 2003;3:1801–10. doi: 10.1002/pmic.200300461. [DOI] [PubMed] [Google Scholar]