Abstract
Lipid droplets (LDs) are intracellular storage sites for triacylglyerols (TAGs) and steryl esters, and play essential roles in energy metabolism and membrane biosynthesis. Adipose triglyceride lipase (ATGL) is the key enzyme for TAG hydrolysis (lipolysis) in adipocytes and LD degradation in nonadipocyte cells. Lipase activity of ATGL in vivo largely depends on its C-terminal sequence as well as coactivation by CGI-58. Here we demonstrate that the C-terminal hydrophobic domain in ATGL is required for LD targeting and CGI-58-independent LD degradation. Overexpression of wild-type ATGL causes a dramatic decrease in LD size and number, whereas a mutant lacking the hydrophobic domain fails to localize to LDs and to affect their morphology. Interestingly, coexpression of CGI-58 is able to promote LD turnover mediated by this ATGL mutant. Recently we have discovered that G0S2 acts as an inhibitor of ATGL activity and ATGL-mediated lipolysis. Here we show that G0S2 binds to ATGL irrelevantly of its activity state or the presence of CGI-58. In G0S2-expressing cells, the combined expression of CGI-58 and ATGL is incapable of stimulating LD turnover. We propose that CGI-58 and G0S2 regulate ATGL via non-competing mechanisms.
Key words: lipolysis, lipase, lipid droplet, triacylglycerol, fatty acid
Composed of a neutral lipid core surrounded by a monolayer of phospholipids with embedded proteins, lipid droplets (LDs) are a dynamic organelle critically involved in lipid synthesis, turnover and trafficking.1–4 Although virtually all cell types are able to generate LDs, the most prominent LDs under physiological conditions are found in white adipocytes. Their large unilocular morphology is well suited for storing fatty acids in the form of triacylglycerols (TAGs). During fasting and exercise, adipose TAG hydrolysis (termed lipolysis) supplies fatty acids and glycerol to muscle, liver and other tissues for energy production. In comparison, LDs in nonadipocyte cells are usually small and multilocular. The TAGs and steryl esters in these LDs can be mobilized to support a variety of local activities including metabolism, membrane synthesis and steroid synthesis. In hyperlipidemic states associated with obesity, the storing capacity of LDs in many nonadipose tissues is often exceeded, resulting in excessive accumulation of unesterified fatty acids that in turn causes cell dysfunction and/or cell death.5 The so-called “lipotoxicity” phenomenon has been well recognized as a causative mechanism for the development of insulin resistance in the liver and the skeletal muscle. Lipid overload in pancreatic cells and cardiac myocytes, on the other hand, can lead to apoptotic cell death that results in the dysregulation of insulin secretion and the development of heart failure, respectively.
Considering the importance of lipid homeostasis at both physiological and pathological levels, it is not surprising that the molecular mechanisms governing LD biogenesis and turnover have attracted immense research effort over the years. LDs in adipocytes as a system have been important historically in providing examples of many concepts of TAG catabolism/lipolysis, including lipase trafficking, function of LD coat proteins as lipolytic barriers, and regulation of lipolysis by hormones through protein phosphorylation. It is now well known that adipose lipolysis is catalyzed sequentially by specific enzymes, releasing one fatty acid at each step with the generation of diacylglycerol (DAG), monoacylglycerol (MAG) and glycerol. The rate-limiting enzyme controlling this process was previously believed to be hormone-sensitive lipase (HSL). The interplay between HSL and the lipid droplet-coat protein perilipin was established as a key mechanism whereby catecholamines/β-adrenergic hormones regulate TAG hydrolysis in adipocytes.6,7 However, the failure of HSL-deficient mice to show an obese phenotype led to intense search for separate TAG lipases and the resultant discovery of adipose triglyceride lipase (ATGL) in 2004.8–10
ATGL is expressed in most tissues examined with highest level detected in white and brown adipose tissue.8,9 Studies using cultured cells and genetically modified animal models demonstrate that ATGL plays a key role in both basal and hormone-stimulated lipolysis in adipocytes.8,11–15 ATGL initiates lipolysis by specifically removing the first fatty acid from TAG to produce DAG substrate, which is then hydrolyzed by HSL to generate an additional fatty acid and MAG substrate. MAGs are converted into fatty acid and glycerol by MAG lipase in the final step of lipolysis.16–19 In contrast to HSL knockout mice exhibiting decreased fat mass,20–23 ATGL null mice showed an expanded adipose tissue along with impaired adipose lipolysis in response to β-adrenergic stimulation.13 Conversely, mice overexpressing ATGL specifically in adipose tissue were leaner with decreased TAG content in adipocytes and were resistant to diet-induced obesity with improved insulin sensitivity.15
While its LD localization is hormone-responsive in adipocytes,12,24 ATGL is constantly located around LDs in cells expressing none or little endogenous perilipin and HSL.25–27 In HeLa cells, ATGL overexpression caused a marked decrease in LD size, whereas siRNA-induced knockdown of endogenous ATGL resulted in an increase in the size of LDs.26 ATGL-null mice showed increased TAG deposition in most nonadipose tissues such as heart, skeletal muscle and liver. In fact, massive lipid accumulation in cardiac myocytes caused heart failure and pre-mature death of ATGL null mice after the age of 12 weeks.13 Exercise-induced lipolysis was also blunted in these mice.14 Additionally, ATGL null mice displayed a 2–3-fold increase in hepatic TAG content along with reduced very low density lipoprotein (VLDL) TAG in the plasma under fasting condition.13 In a separate study, adenoviral hepatic overexpression of ATGL promoted fatty acid oxidation and ameliorated hepatic steatosis in both ob/ob mice and mice with high fat diet-induced obesity.28 Therefore, aside from its function in adipocyte lipolysis, ATGL is critically involved in the TAG catabolism in cardiomyocytes and hepatocytes.
Human and murine ATGL proteins share 86% homology and have a molecular weight of ∼56 kDa. The N-terminal half of ATGL contains a predicted α/β-hydrolase fold29 and an overlapping patatin-like domain. The patatin domain is named after the potato tuber protein patatin,30 a weak acyl lipid hydrolase. The catalytic site of ATGL is located within the patatin-like domain and is characterized by an unconventional catalytic dyad similar to that of human cytosolic phospholipase A2 (cPLA2).29 Mutational analysis revealed that in ATGL the catalytic dyad consists of serine 47 located within a GXSXG motif and aspartate 166 within a DXG motif.26,31 Patatin domains of both ATGL and cPLA2 also contain a glycine-rich sequence that defines the oxyanion hole of the active site. The nitrogen atom in glycine stabilizes the oxyanion formed during substrate cleavage as a result of the nucleophilic attack by the catalytic serine.30 The C-terminal region of ATGL primarily consists of α-helical and loop structures. In humans, a subgroup of neutral lipid storage disease characterized by excessive TAG deposition in nonadipose tissues and mild myopathy (NLSDM) was reported to be caused by mutations in the gene for ATGL.32 Most mutations result in the deletion of the C-terminal region of ATGL. In fibroblasts collected from NLSDM patients, the whole-cell TAG lipase activity was often retained while the LD-associated activity was lost.32,33 Separate mutagenesis studies27,33,34 further demonstrated that though they exhibited substantially increased lipase activity in vitro, ATGL mutants deleted in the C-terminal region were unable to associate with LDs and to catalyze TAG hydrolysis in living cells. Therefore, the LD localization may be essential for ATGL to function as a TAG lipase in vivo.
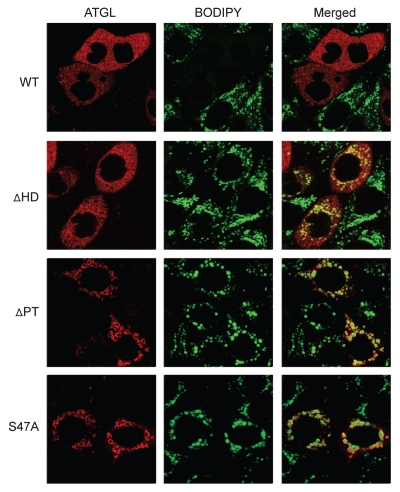
A hydrophobic stretch of 45 amino acids in the C-terminal region has been widely postulated to mediate targeting ATGL to the LDs.19 To directly determine its involvement in the LD degradation, we generated an internal deletion mutant of murine ATGL lacking only the hydrophobic domain. ATGLΔHD possesses a TAG lipase activity comparable to that of the wild type protein.24 For controls, we also constructed two catalytically inactive mutants, ATGLΔPT and S47A. ATGLΔPT is an internal deletion mutant missing the entire patatin-like domain,24 and S47A is a point mutant in which the nucleophilic serine 47 was mutated to alanine. To evaluate their effects on LD turnover, we transfected HeLa cells with each ATGL variant and examined the morphological changes in the intracellular LDs. HeLa cells accumulate multiple small LDs upon treatment with long chain fatty acids, and have been a successful model system for study of ATGL-mediated lipolysis in nonadipocyte cells.26,35 We treated transfected cells with oleic acid overnight to enlarge the LDs followed by a short period of nutrient starvation to promote their degradation. The subcellular localization of ATGL was determined by immunofluorescence staining. The LDs were revealed by staining with BODIPY 493/503, a nonpolar probe selective for neutral lipids such as TAG. As shown in Figure 1, expression of wild type ATGL resulted in a drastic reduction in both the size and the number of LDs in the transfected cells compared with those in the neighboring untransfected cells. As expected, neither S47A nor ATGLΔPT was able to significantly affect the morphology of LDs. Interestingly; ATGLΔHD displayed no ability to degrade LDs in spite of its intact lipase activity. Moreover, ATGLΔPT and S47A were predominantly localized at the surface of LDs, whereas ATGLΔHD resided diffusively throughout the cytoplasm with no apparent presence at the LDs. These results indicate that the hydrophobic domain in the C-terminal region of ATGL indeed is required for the LD targeting and the subsequent degradation of the LDs. The catalytic patatin-like domain, on the other hand, does not appear to play a critical role in the LD localization. This is well in agreement with the finding from Duncan et al. that ATGL lacking almost the entire N-terminal region still retained the LD localization when expressed in COS-7 cells.27
Figure 1.
LD localization of ATGL mutants. HeLa cells transiently expressing murine ATGL wild type, ATGLΔHD, ATGLΔPT or ATGL/S47A were incubated under normal growth conditions with 400 µM of oleic acid complexed to albumin for 18 h. Following 1 h of incubation in serum-free and glucose-free medium, immunofluorescence staining with ATGL antibodies was performed to reveal the transfected cells. Lipid droplets were co-stained with BODIPY 493/503 fluorescence dye.
A major advance in understanding ATGL-mediated lipolysis is the discovery that ATGL activity is highly dependent on association with a co-activator protein called CGI-58 (comparative gene identification-58; also known as α/β hydrolase fold—containing protein 5, ABHD5).31 In humans, mutations of CGI-58 were identified earlier as a cause of Chanarin-Dorfman syndrome (CDS),36 another form of neutral lipid storage disease characterized by ichthyosis (NLSDI) along with TAG deposition in most nonadipose tissues. CGI-58 contains a canonical lipase motif, although the usual catalytic serine in GXSXG motif is replaced by asparagine. As a result, CGI-58 itself does not have a lipase activity.31 Upon interaction with CGI-58, ATGL TAG lipase activity increases from as low as 30–70% to as high as 20-fold in cultured cells,16 depending on experimental conditions. Overexpression of ATGL and CGI-58 synergistically reduced TAG deposition in COS-7 cells.31 Moreover, silencing of CGI-58 was found to drastically reduce PKA-activated fatty acid and glycerol release in both mouse 3T3-L1,31,37 and human hMADs adipocytes.12 In accordance with these in vitro observations, CGI-58-deficiency caused growth retardation along with systemic TAG accumulation and severe hepatic steatosis in newborn mice. Analysis of CGI-58-/- embryonic fibroblasts also revealed a defect in ATGL activation.38
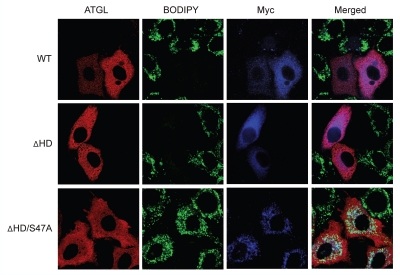
Although compelling evidence has been provided to support the activating role of CGI-58, the precise mechanism how CGI-58 stimulates the lipase activity of ATGL remains elusive. Interactions between the ectopically expressed proteins were detected by ELISA (enzyme-linked immunosorbent assay)31,33 and FRET (fluorescence resonance energy transfer)37 methods. However, co-immunoprecipitation of endogenous ATGL and CGI-58 has not been reported. Therefore, the question arises as to whether a stable association with ATGL is needed for CGI-58 to mediate the stimulating effect. Since in vitro CGI-58 is able to activate ATGL mutants lacking the C-terminal region,33 we tested whether CGI-58 would promote LD degradation by ATGL not residing at the surface of LDs. To this end, we coexpressed Myc epitope-tagged CGI-58 with either wild type ATGL or ATGLΔHD in HeLa cells in which endogenous ATGL was already knocked down using human isoform-specific siRNA oligos. Expectedly, HeLa cells expressing wild type ATGL showed a marked reduction in the size and the number of LDs (Fig. 2, top), regardless of CGI-58 coexpression. Strikingly, though ATGLΔHD alone did not promote LD degradation, coexpression of CGI-58 with ATGLΔHD caused a drastic turnover of the LDs (Fig. 2, middle). Mutation of serine 47 to alanine in ATGLΔHD completely abolished this effect of CGI-58 (Fig. 2 and bottom), demonstrating that the LD degradation was indeed mediated by the catalytic activity of ATGLΔHD with the assistance of CGI-58. Immunofluorescence staining showed that CGI-58 was mainly localized at the surface of LDs, whereas ATGLΔHD/S47A remained diffusive in the cytoplasm. Therefore, CGI-58 was able to stimulate ATGLΔHD's activity towards LDs without recruiting the latter to the LD surface.
Figure 2.
Coexpression of CGI-58 facilitates LD degradation by ATGLΔHD mutant. HeLa cells were treated with human ATGL siRNA and then transfected with each of the murine ATGL variants (wild type, ΔHD or ΔHD/S47A) in the presence of Myc-CGI58. After 18 h of incubation with 400 µM of oleic acid followed by 1 h of nutrient starvation, immunofluorescence staining was performed by using ATGL and Myc antibodies. Lipid droplets were co-stained with BODIPY 493/503 fluorescence dye.
Contributing to the increasingly complex puzzle of lipolysis, our recent studies have demonstrated that a protein encoded by G0/G1 switch gene 2 (G0S2) functions to attenuate ATGL action.24 G0S2 is a small basic protein with 78% identity between mouse and human isoforms. It binds directly to ATGL and is capable of inhibiting its lipase activity. Overexpression of G0S2 decreases basal and stimulated lipolysis in cultured adipocytes and fat explants. Knockdown of endogenous G0S2, on the other hand, enhances lipolysis in mature adipocytes.24 Moreover, G0S2 is highly expressed in adipose tissue as well as liver and heart. The expression of G0S2 increases in response to insulin, glucose and ligands for the PPAR family of transcription factors, and decreases upon treatment with TNFα and β-adrenergic agonist isoproterenol.24,39–42 Therefore, G0S2 likely controls TAG turnover in adipocytes as well as in nonadipocyte cells, and alteration of its expression may be a novel mechanism via which nutritional and hormonal factors regulate the lipid homeostasis.
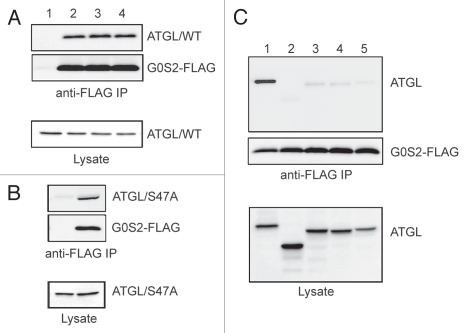
In vitro G0S2 can dose-dependently decrease ATGL activity even in the presence of CGI-58,24 though higher concentrations of G0S2 are needed to achieve the same level of inhibition. To determine whether G0S2 would prevent ATGL-mediated degradation of LDs in the presence of CGI-58, we expressed ATGL along with Myc-CGI-58 in HeLa cells stably transfected with G0S2.24 Consistent with the results from our earlier experiments, ATGL alone in G0S2-expressing cells failed to reduce the size and the number of LDs. Interestingly, the further addition of CGI-58 to ATGL also was unable to promote LD turnover in these cells. Consequently, both ATGL and CGI-58 were found to be co-localized at the surface of LDs. As demonstrated by a separate coimmunoprecipitation experiment, the presence of CGI-58 rendered no effect on the interaction between G0S2 and ATGL. Specifically, we expressed ATGL and G0S2 tagged at the C-terminus with a FLAG epitope (G0S2-FLAG) with or without Myc-CGI-58 in HeLa cells. Anti-FLAG immunoprecipitation pulled down ATGL along with G0S2-FLAG at an equal efficiency in the presence or absence of CGI-58 coexpression. Therefore, G0S2 and CGI-58 do not appear to compete with each other in binding to ATGL.
Our deletion analysis shows that the catalytic patatin-like domain of ATGL is necessary for mediating the complex formation with G0S2.24 To obtain insight into the biochemical mechanism by which G0S2 inhibits ATGL, we investigated whether the lipase activity of ATGL is required for interaction with G0S2. We transiently coexpressed wild type ATGL and G0S2-FLAG in HeLa cells, and evaluated their interaction following pretreatment of cells with Orlistat or (R)-BEL (Fig. 3A). While Orlistat is a cell-permeable general TAG lipase inhibitor, (R)-BEL was shown to efficiently inhibit ATGL in vitro and in mouse primary hepatocytes.10,25 Anti-FLAG immunoprecipitation demonstrated that the specific association of ATGL with G0S2-FLAG was unaffected by either inhibitor (Fig. 3A). In a separate experiment, G0S2 was also found to interact efficiently with S47A (Fig. 3B), the catalytically inactive mutant of ATGL. In an attempt to identify the responsible sequence motif, we generated three stretch-by-stretch deletions of ATGL, with each mutant internally lacking one of the three different regions within the patatin domain. After transfection in HeLa cells, we examined their respective interaction with G0S2-FLAG. As shown in Figure 3C, deletion of any chosen stretch resulted in almost a complete loss of binding to G0S2. Collectively, these observations indicate that though independent of the lipase activity of ATGL, the G0S2 interaction involves nearly the entire region of the patatin-like domain.
Figure 3.
Specific interaction between G0S2 and ATGL. (A) HeLa cells were co-transfected with wild type ATGL along with vector alone (lane 1) or G0S2-FLAG (lanes 2–4). After pretreatment of cells with vehicle only (lanes 1 and 2), 100 µM Orlistat (lane 3) or 5 µM (R)-BEL (lane 4) for 4 h, cells were lysed and G0S2-FLAG was immunoprecipitated with anti-FLAG antibodies. G0S2 and ATGL proteins in immunoprecipitates and lysates were detected by immunoblotting with FLAG and ATGL antibodies, respectively. (B) HeLa cells were co-transfected with ATGL/S47A mutant along with vector alone or G0S2-FLAG. Immunoprecipitation and immunoblotting analysis were performed as described in (A). (C) HeLa cells were co-transfected with G0S2-FLAG and various deletion mutants of ATGL. 1, wild type; 2, ΔPT (lacking residues 10–180); 3, ΔPT-N (lacking residues 10–66); 4, ΔPT-M (lacking residues 67–124); 5, PT-C (lacking residues 125–180). Immunoprecipitation and immunoblotting analysis were performed as described in (A).
In summary, our results show that the hydrophobic domain of ATGL is directly involved in targeting the enzyme to LDs. While the LD localization is a prerequisite for ATGL by itself to mediate LD degradation, CGI-58 is able to facilitate the hydrolysis of TAG substrates within the LDs by cytosolically localized ATGL. Moreover, we show that CGI-58 does not compete with G0S2 in binding to ATGL, and G0S2 possess the capacity to prevent ATGL-mediated LD turnover in the presence of CGI-58. On the basis of our results, we propose that G0S2 and CGI-58 do not directly oppose each other in the control of ATGL action. G0S2 binds to ATGL in a manner that is dependent on the intact 3-dimensional structure of the patatin-like domain, and may function to block the substrate accessibility of ATGL. The activation of ATGL by CGI-58, on the other hand, does not appear to involve a stable interaction between the two proteins.
Recent work by Gruber et al. has demonstrated that a tryptophan-rich stretch in the N-terminal region of CGI-58 is required for binding to the phospholipid monolayer of the LDs.43 Deletion of this lipophilic sequence in CGI-58 caused a complete loss of capacity to activate ATGL without impacting their interaction. These results emphasize the role of lipid binding over direct protein association in CGI-58 activation of ATGL. We speculate that CGI-58 at the surface of LDs may remodel the phospholipid monolayer and thereby allow the access of the TAG core by ATGL. For ATGL localized cytosolically, it may serve as an essential mechanism by which the hydrolytic action is exerted. For LD-localized ATGL with its own hydrophobic domain penetrating the phospholipid monolayer, the TAG core is in close proximity and as a result, the enzyme action may be less reliant on the coactivation by CGI-58. The differential regulation of ATGL could be especially important in the context of adipocyte lipolysis. In the absence of β-adrenergic stimulation, the majority of ATGL reside in the cytosol and CGI-58 may assist in maintaining its basal activity towards TAG substrates in the LDs. Under pathological conditions such as NLSDM caused by ATGL deletions lacking the C-terminal region, the abundant expression of CGI-58 may assist in maintaining ATGL activity and thus the lipolytic rates in adipose tissue. This probably explains, at least in part, that the NLSDM patients generally are not obese.44 Along the same line, it is tempting to hypothesize that the nonadipose tissues that accumulate excess TAG are the ones that express either no or only a low level of CGI-58. For example, the lack of sufficient level of CGI-58 in skeletal muscle cells and cardiomyocytes of these patients may be a permissive factor for the substantial TAG deposition leading to the development of myopathy and muscle weakness.
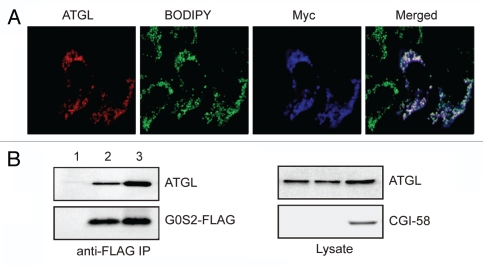
Figure 4.
Coexpression of CGI-58 and ATGL does not promote LD degradation in G0S2-expressing cells. (A) HeLa cells stably expressing G0S2 were transfected with ATGL in the presence of Myc-CGI-58. After 24 h of incubation with 400 µM of oleic acid and 1 h of nutrient starvation, immunofluorescence staining was performed by using anti-ATGL and anti-Myc antibodies. LDs were co-stained with BODIPY 493/503 fluorescence dye. (B) HeLa cells were transfected with ATGL + vector alone (lane 1), ATGL + G0S2-FLAG (lane 2) or ATGL + G0S2-FLAG + Myc-CGI-58 (lane 3). G0S2-FLAG was immunoprecipitated with anti-FLAG antibodies. ATGL, G0S2 and CGI-58 proteins in immunoprecipitates and lysates were detected by immunoblotting with ATGL, FLAG and Myc antibodies, respectively.
Acknowledgements
The work was supported by a NIH grant DK 078742, a Center of Biomedical Research Excellence pilot grant from the University of Kentucky (5P20 RR0202171), and a junior faculty award form American Diabetes Association to J.L.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12181
References
- 1.Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 3.Goodman JM. Demonstrated and inferred metabolism associated with cytosolic lipid droplets. J Lipid Res. 2009;50:2148–2156. doi: 10.1194/jlr.R001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 5.Defronzo RA. The Claude Bernard Lecture 2009; Diabetologia. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Jr, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormonesensitive lipase to the lipid storage droplet. Proc Natl Acad Sci USA. 1992;89:8537–8541. doi: 10.1073/pnas.89.18.8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, et al. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003;161:1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 9.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 11.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- 12.Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, et al. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 14.Huijsman E, van de Par C, Economou C, van der Poel C, Lynch GS, Schoiswohl G, et al. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am J Physiol Endocrinol Metab. 2009;297:505–513. doi: 10.1152/ajpendo.00190.2009. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadian M, Duncan RE, Varady KA, Frasson D, Hellerstein MK, Birkenfeld AL, et al. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes. 2009;58:855–866. doi: 10.2337/db08-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watt MJ, Steinberg GR. Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem J. 2008;414:313–325. doi: 10.1042/BJ20080305. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann R, Lass A, Haemmerle G, Zechner R. Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta. 2009;1791:494–500. doi: 10.1016/j.bbalip.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Raben DM, Baldassare JJ. A new lipase in regulating lipid mobilization: hormone-sensitive lipase is not alone. Trends Endocrinol Metab. 2005;16:35–36. doi: 10.1016/j.tem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci USA. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SP, Laurin N, Himms-Hagen J, Rudnicki MA, Levy E, Robert MF, et al. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obes Res. 2001;9:119–128. doi: 10.1038/oby.2001.15. [DOI] [PubMed] [Google Scholar]
- 22.Mulder H, Sorhede-Winzell M, Contreras JA, Fex M, Strom K, Ploug T, et al. Hormone-sensitive lipase null mice exhibit signs of impaired insulin sensitivity whereas insulin secretion is intact. J Biol Chem. 2003;278:36380–36388. doi: 10.1074/jbc.M213032200. [DOI] [PubMed] [Google Scholar]
- 23.Strom K, Hansson O, Lucas S, Nevsten P, Fernandez C, Klint C, et al. Attainment of brown adipocyte features in white adipocytes of hormone-sensitive lipase null mice. PLoS One. 2008;3:1793. doi: 10.1371/journal.pone.0001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, et al. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung C, Doll JA, Gattu AK, Shugrue C, Cornwell M, Fitchev P, et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48:471–478. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7:106–113. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan RE, Wang Y, Ahmadian M, Lu J, Sarkadi-Nagy E, Sul HS. Characterization of desnutrin functional domains: critical residues for triacylglycerol hydrolysis in cultured cells. J Lipid Res. 51:309–317. doi: 10.1194/jlr.M000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids and ameliorates steatosis. J Biol Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dessen A, Tang J, Schmidt H, Stahl M, Clark JD, Seehra J, et al. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 1999;97:349–360. doi: 10.1016/s0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- 30.Rydel TJ, Williams JM, Krieger E, Moshiri F, Stallings WC, Brown SM, et al. The crystal structure, mutagenesis and activity studies reveal that patatin is a lipid acyl hydrolase with a Ser-Asp catalytic dyad. Biochemistry. 2003;42:6696–6708. doi: 10.1021/bi027156r. [DOI] [PubMed] [Google Scholar]
- 31.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 33.Schweiger M, Schoiswohl G, Lass A, Radner FP, Haemmerle G, Malli R, et al. The C-terminal region of human adipose triglyceride lipase affects enzyme activity and lipid droplet binding. J Biol Chem. 2008;283:17211–17220. doi: 10.1074/jbc.M710566200. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K, Inoguchi T, Maeda Y, Nakashima N, Kuwano A, Eto E, et al. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J Clin Endocrinol Metab. 2008;93:2877–2884. doi: 10.1210/jc.2007-2247. [DOI] [PubMed] [Google Scholar]
- 35.Soni KG, Mardones GA, Sougrat R, Smirnova E, Jackson CL, Bonifacino JS. Coatomer-dependent protein delivery to lipid droplets. J Cell Sci. 2009;122:1834–1841. doi: 10.1242/jcs.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J Biol Chem. 285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zandbergen F, Mandard S, Escher P, Tan NS, Patsouris D, Jatkoe T, et al. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochem J. 2005;392:313–324. doi: 10.1042/BJ20050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teunissen BE, Smeets PJ, Willemsen PH, De Windt LJ, Van der Vusse GJ, Van Bilsen M. Activation of PPARdelta inhibits cardiac fibroblast proliferation and the transdifferentiation into myofibroblasts. Cardiovasc Res. 2007;75:519–529. doi: 10.1016/j.cardiores.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 41.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 42.Parikh H, Carlsson E, Chutkow WA, Johansson LE, Storgaard H, Poulsen P, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4:158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruber A, Cornaciu I, Lass A, Schweiger M, Poeschl M, Eder C, et al. The N-terminal region of comparative gene identification-58 (CGI-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J Biol Chem. 285:12289–12298. doi: 10.1074/jbc.M109.064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297:289–296. doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]