Introduction
Senescence is a form of irreversible arrest of cell proliferation. In normal human cells it can occur either due to telomere abrasion that takes place after a certain number of cell divisions (replicative senescence)1 or due to the aberrant activation of oncogenes (oncogene-induced senescence [OIS]).2 Despite its generalized name, the bulk of available literature on OIS considers only two families of oncogenes: RAS2 and RAF.3 In normal rodent cultured cells and in the majority of mouse models, senescence phenotypes caused by constitutively active RAF or RAS proteins are executed by p19ARF-p53 or p16INK4A-pRb pathways (reviewed in ref. 4), however, involvement of these pathways in OIS of human cells appears to be less straightforward.
In humans, naturally occurring OIS is probably best exemplified by nevi that represent benign aggregations of non-proliferative melanocytes that often harbor activating mutations in BRAF (up-to 82% of acquired nevi5) or NRAS genes (up-to 81% of congenital nevi6). Intriguingly, activating mutations in these same genes were detected in several types of malignant melanomas (e.g., from non-chronically sun-damaged skin) at lower frequencies (60 and 20%, respectively7,8). Thus, malignant melanomas that frequently originate from nevi have to develop a mechanism(s) that allow escape from OIS. During the last several years, a series of studies has been devoted to understanding the molecular pathways of such escape, arriving at times to conflicting conclusions. Additionally, it has been reported that senescence-like phenotypes can be caused genetically in metastatic melanoma cells,9–11 suggesting that induction of senescence could be a novel therapeutic approach to treat melanoma. Here, we review the current literature on OIS pathways in normal and cancerous human melanocytic cells.
Activated Forms of RAF and RAS Cause Distinct Types of Senescence in Normal Human Melanocytes
Traditionally, senescent cells are characterized by a combination of phenotypic features, including (1) permanent proliferative arrest,1 (2) changes in cell morphology, including increase in cell size (reviewed in ref. 12), (3) enhanced activity of senescence-associated β-galactosidase (SA-β-Gal) detectable at pH 6.0,13 (4) chromatin reorganization resulting in the emergence of senescence-associated heterochromatin foci (SAHF) detectable by staining with antibodies to tri-methylated lysine 9 of histone H3,14 (4) accumulation of intra-nuclear aggregates of histone H2AX which is phosphorylated at Ser 139 (H2AX-γ foci)15 and (5) elevated levels of several tumor suppressors such as p16INK4A, p53 or p21CIP/WAF (reviewed in ref. 16). Individually, none of these features are sufficient to define senescence and the nature of its bona fide markers is still being debated.
Overexpression of constitutively active mutants of RAS (NRASQ61R/K, HRASG12V) or BRAF (BRAFV600E) in normal human melanocytes (NHM) resulted in a classical senescence-like response which included many of the above features.17 Yet, there were phenotypic differences between melanocytes senescent as a result of overexpression of BRAFV600E or HRASG12V. In the latter case, cells demonstrated increase in size and massive intracellular vacuolization.17 Additionally, senescence phenotypes in HRASG12V-, but not BRAFV600E-, expressing NHM were associated with the expansion of endoplasmic reticulum (ER) and induction of the unfolded protein response (UPR, reviewed in refs. 18 and 9). Moreover, it has been shown that individual genetic inhibition of several key regulators of UPR (ATF6, IRE1, XBP-1 or ATF4)18,19 reduced the proportion of SA-β-Gal-positive cells and partially reverted changes in morphology of HRASG12V-NHM.17 On the other hand, inhibition of the above proteins did not result in resumption of proliferation in these cells but led to cell death, suggesting that ER stress maintains a pro-survival function in melanocytes expressing activated RAS. Accordingly, pharmacological inhibition of PI3K, one of the major downstream effectors of RAS, in HRASG12V-NHM resulted in suppression of ER-stress, decrease of cellular vacuolization and reduction in the activity of SA-β-Gal.17 Interestingly, UPR was induced to a much lesser extent or not at all in normal melanocytes expressing ectopic NRASQ61R,17 or NRASQ61K,20 respectively. In the case of NRASQ61R, this observation was attributed to the reduced ability of this mutant to activate PI3K compared to HRASG12V.17
Tumor Suppressors in Senescence of NHM Induced by Activated NRAS or HRAS
Data obtained in mouse cells often implicate the INK4A/ARF locus in mediating OIS phenotypes (reviewed in ref. 21). This locus encodes for two proteins with an overlapping reading frame: p16INK4a and p19ARF (p14ARF in human).22 Both proteins have been extensively shown to exert tumor-suppressor functions: p16INK4a inhibits CDK4- and CDK6-mediated phosphorylation of pRB resulting in growth arrest23 whereas p19ARF stabilizes p53 via suppression of its inhibitor MDM2, leading to growth arrest or apoptosis.24,25 The INK4A/ARF locus is frequently deleted or mutated in human malignancies including melanoma, where its loss represents a genetic “signature event” (reviewed in ref. 26). However, despite its well-established tumor suppressor functions, the INK4A/ARF locus does not appear to play a prominent role in NRASQ61R/K- or HRASG12V-induced senescence in NHM. Indeed, although levels of both p16INK4a and p14ARF were elevated by activated HRAS or NRAS in NHM, individual or double shRNA-mediated knockdown of p16INK4a or p14ARF did not abolish proliferative arrest, changes in cell morphology or increase in the activity of SA-β-Gal in cells expressing these oncogenes10,17,20,27 (Fig. 1). The only senescence-associated phenotype that depended on p16INK4a was the formation of SAHF20 (Fig. 1).
Figure 1.
Senescence induced in normal human melanocytes by activated RAS. *shRNA-mediated inhibition of both p53 and pRB abolishes senescence. **shRNA-mediated inhibition of p16INK4A suppresses SAHF formation. ***shRNA-mediated inhibition of pRB delays senescence. DDR-DNA damage response, UPR-unfolded protein response, SAHF-senescence associated heterochromatin foci, LY294002-an inhibitor of PI3K.
In contrast to the modest role of p16INK4A depletion in suppression senescence caused by NRASQ61K in NHM, shRNA-mediated silencing of its downstream target pRB significantly delayed emergence of all senescence-associated phenotypes including SA-β-Gal activity, downregulation of proliferation marker Ki67 and formation of SAHF, which was suppressed permanently20 (Fig. 1).
Unlike human melanocytes, suppression of p16INK4a in mouse melanocytes rendered these cells resistant to RAS-induced antiproliferative effects. For instance, loss of p16Ink4A or p19Arf cooperated with activated HRAS in melanomagenesis.30 Similarly, suppression of p16INK4a by β-catenin was identified as a major mechanism by which this protein cooperates with activated NRAS in the formation of melanoma with a short latency in mice.31
Interestingly, normal human fibroblasts (NHF) obtained from melanoma-prone individuals with biallelic mutations in p16INK4A resisted growth arrest caused by this HRASV12G,29,73 unlike NHF with normal genetic background where p16INK4A was depleted or suppressed artificially.2,28,36 Thus, it will be interesting to determine whether melanocytes obtained from melanoma-prone individuals would also be resistant to RAS-induced senescence.
Unlike p16INK4A, mutations of p53 are rare in melanoma.32 Moreover, it has been shown that cells from several metastatic melanoma lines retained not only wild-type p53 but at least some of its downstream pathways.10 On the other hand, a new “epithelial-like” sub-type of melanoma has been described that contains p53 mutations at higher rates33 while another study demonstrated that some of p53-downstream pathways, e.g., apoptosis, are impaired in cells from several melanoma lines.34
Three independent studies found p53 to be dispensable for the senescence induced by activated HRAS or NRAS in NHM10,17,20 and one group reported similar findings in normal mouse melanocytes.35 Similarly, the immediate downstream target of p53, p21CIP/WAF, was also dispensable for NRASQ61R-induced senescence20 (Fig. 1). However, shRNA-mediated silencing of p53 supplemented partial resistance of pRB-depleted NHM to NRASQ61K-induced senescence, making these cells completely refractory to NRASQ61K,20 (Fig. 1). These data suggest that induction of senescence in NHM at least by NRASQ61K requires both p53 and pRB pathways.
RAS-dependent senescence has long been associated with induction of DNA damage (reviewed in ref. 36), which can be detected using the comet assay37 or by monitoring activation of DNA damage response (DDR) proteins, such as ATR, ATM, Chk1, Chk2 or H2AX-γ (reviewed in refs. 38 and 39). Emergence of chromatin foci containing H2AX-γ is also considered as one of the OIS markers.15 Accordingly, shRNA-mediated inhibition of Chk2 or ATM in NHF abolished senescence induced by HRASG12V.40 On the other hand, activation of DDR, such as phosphorylation of ATM and formation of H2AX-γ foci, may occur in senescent cells in the absence of DNA damage as was evidenced by comet assay,41 suggesting that DDR proteins participate in senescence in the absence of DNA damage. Yet, suppression of DDR via caffeine-mediated inhibition of ATM and ATR activities in NHM did not affect any of the NRASQ61K-induced senescence phenotypes20 while still reducing phosphorylation of CHK2 and p53 proteins (direct ATM targets) (Fig. 1). At this point, a more detailed analysis is required to establish the role of individual DDR members in OIS of NHM.
Tumor Suppressors in BRAFV600E-Induced Senescence of NHM
p16INK4A was reported to be dispensable for BRAFV600-induced senescence of NHM in two studies10,17 (Fig. 2). In vivo, loss of p16INK4A did not affect BrafV600-induced senescence nor enhanced melanoma progression in a transgenic mouse carrying an inducible knock-in of BrafV600 gene.43 Additionally, many of the examined human congenital nevi which are composed of senescent melanocytes42,45 were negative for p16INK4A staining,42 although this finding was not confirmed for the benign compound nevi in another report.45 Thus p16INK4A does not appear to play an important role in OIS of NHM.
Figure 2.
Senescence induced in normal human melanocytes by BRAFV600E. *All genes have been identified in ref. 44.
The role of p53 in BRAFV600E-dependent senescence of NHM has been addressed in several studies, however, it still remains controversial. It has been demonstrated that shRNA-mediated depletion of p53 did not affect senescence caused by overexpression of lentivirus-based BRAFV600E cDNA,10,17 or completely overcame senescence caused by overexpression of retrovirus-based BRAFV600E cDNA44 (Fig. 2). The apparent difference in the outcome of these studies, most likely, can be accounted for by the variability in the genetic background among populations of melanocytes independently isolated in each report. Theoretically, p53-deficient NHM may become resistant to BRAFV600E due to the low activity of other tumor suppressors (inherent for a particular population of NHM) whose depletion can functionally cooperate with p53 inhibition. These tumor suppressors may, for example, function up stream or downstream of pRB, whose inactivation cooperated with p53 depletion in overcoming senescence induced by NRASQ61K.20 On the other hand, it has been shown that ectopic expression of BRAFV600E enhanced proliferation of NHM during the first several days before causing growth arrest and senescence.17 Thus, resistance to BRAFV600E in p53-deficient cells may be acquired via epigenetic inhibition of pro-senescence factors during the short hyper-proliferative period following introduction of BRAFV600E.
Recently, a functional screen utilizing a genome-wide shRNA library has identified 17 genes whose individual inhibition can bypass the proliferative arrest induced by BRAFV600E in NHF44 (Fig. 2). Individual testing of these genes revealed that each of them was also required for BRAFV600E-induced senescence in NHM. A detailed analysis of their function in OIS has not been presented for these genes with exception of the one encoding an insulin growth factor binding protein 7 (IGFBP7). Overexpression of IGFBP7 in NHM or supplementing melanocytic media with recombinant IGFBP7 caused senescence phenotypes similar to those induced in these cells by BRAFV600E.44 Interestingly, in the same study, IGFBP7 was found to be not only a downstream target of the BRAFV600E-MEK-ERK cascade but, at the same time, an inhibitor of this pathway, thus establishing a novel negative feed-back regulatory mechanism of MEK activation (Fig. 2). It has also been suggested that a shift from a short hyper-proliferative period to permanent proliferative arrest in melanocytes expressing BRAFV600E occurs due to the gradual accumulation of IGFBP7 amounts, ultimately resulting in inhibition of MEK signaling and activation of senescence programs.
The observation that senescence can be induced via autocrine/paracrine pathways has been further confirmed by the findings that BRAFV600E activates an inflammation-specific transcriptosome in NHF (IMR90) and NHM and that cytokines interleukin 6 and 8 (IL6, IL8) are required for BRAFV600E-induced senescence in NHF.46 Moreover, proliferating NHF, when exposed to the media from BRAFV600E-expressing NHF, underwent senescence in an IL6-dependent manner. Furthermore, IL8 receptor CXCR2 was shown by another group to be required for the induction of replicative and MEK1-induced senescence in IMR90 cells.47 However, the role of the IL6/IL8-dependent network in oncogene-induced senescence of NHM has not been addressed.
Oncogenes as Dominant Suppressors of OIS in NHM
Unlike tumor suppressors, the information about cellular oncogenes controlling BRAFV600E- and/or NRASQ61R/K-induced senescence in NHM is rather scarce. Recently, we reported that the oncogenic transcription factor C-MYC plays a role in OIS of NHM.10 C-MYC is a member of the basic helix-loop-helix/leucine zipper family of transcription factors.48,49 It regulates expression of multiple genes involved in many cellular processes including promotion of proliferation, enhancement of cellular metabolism and induction of apoptosis (reviewed in ref. 50) and is essential for proliferation of all tested normal and tumor human cells.51 C-MYC is frequently upregulated in human malignancies (reviewed in ref. 52) including metastatic melanoma53,54 where its protein levels are significantly higher compared to benign nevi.10 We have demonstrated that levels of endogenous C-MYC gradually decrease in NHM undergoing BRAFV600E- or NRASQ61R/K-induced senescence, whereas ectopic expression of C-MYC partially abolished senescence phenotypes induced by BRAFV600E and, to a lesser extent, by NRASQ61R,10 (Figs. 1 and 2). In the latter cells, C-MYC overexpression failed to suppress the unfolded protein response which is specific to NRASQ61R/K- but not BRAFV600E-induced senescence,17 thus, providing a possible explanation for the relatively poor ability of C-MYC to rescue senescence induced by NRASQ61R.
Recently, a receptor protein tyrosine kinase TYRO3 has been identified in a genome-wide functional screen for positive regulators of microphthalmia-associated transcription factor (MITF, reviewed in ref. 55), a master protein controlling the expression of genes critical for the development and survival of melanocytes.56 TYRO3 and other members of the TAM family of tyrosine receptor kinases (AXL and MER) are involved in regulation of cellular proliferation, transformation and apoptosis (reviewed in ref. 57) and TYRO3 expression was shown to be upregulated in melanoma cell lines and tissues.56 Ectopic expression of TYRO3 led to partial abrogation of senescence caused by BRAFV600E as was evidenced by the decrease in the proportion of SA-β-Gal-positive cells. It should be noted that in another paper, MITF, one of the major downstream targets of TYRO3 in melanocytes, was shown to cooperate with BRAFV600E in transformation of NHM expressing constitutively active CDK4, dominant negative p53 and telomerase.58 It will be, therefore, interesting to determine the role of MITF in TYRO3-dependent suppression of BRAFV600E senescence in NHM.
OIS in Melanoma Cells
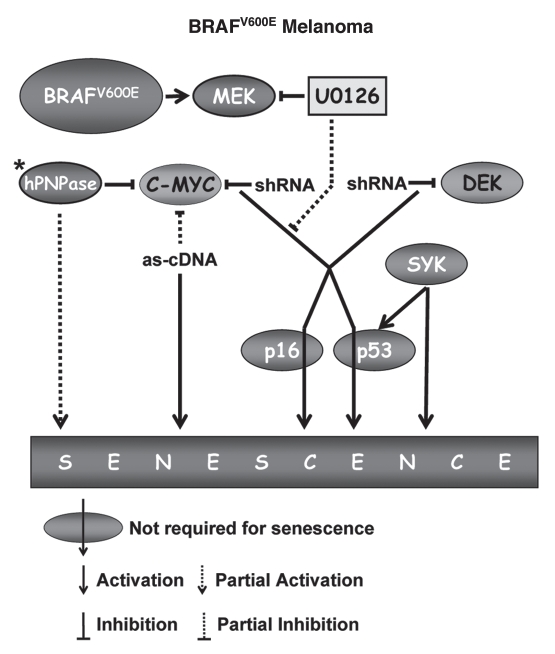
Emergence of senescence-like phenotypes in tumor cells has been reported in response to several stimuli, including genotoxic stress or ectopic overexpression of tumor suppressors (reviewed in ref. 59). This type of response can be considered either as a generic reaction to a certain stress or as a reactivation of senescence pathways that had been driven into dormancy at the earlier stages of tumorigenesis. The latter possibility is very relevant for the progression of melanomas that often originate from nevi and thus have to develop mechanism(s) to overcome OIS. The question then emerges as to whether OIS programs, which are initiated in melanocyte expressing BRAFV600E or NRASQ61R, are completely eradicated or merely suppressed in the course of melanomagenesis. Recently, we have reported that shRNA-mediated depletion of C-MYC in human melanoma cells led to the rapid (4–5 days post-infection) emergence of senescence phenotypes including stable proliferative arrest, formation of SAHF and increase in SA-β-Gal activity10 (Figs. 3 and 4). Intriguingly, the morphological pattern of senescent melanoma cells appeared to correlate with the mutation in BRAF or NRAS genes in such a way that the morphology of MYC-depleted BRAFV600E melanoma cells resembled that of NHM undergoing senescence due to BRAFV600E, whereas MYC-depleted NRASQ61R melanoma cells recapitulated morphology of NRASQ61R-overexpressing senescent melanocytes. Moreover, several senescence phenotypes re-activated by depletion of C-MYC continued to depend on activity of MEK in BRAFV600E melanoma cells and on the activity of PI3K-AKT-mTOR pathway in NRASQ61R melanoma cells (Figs. 3 and 4). Interestingly, senescence phenotypes induced in melanoma cells by C-MYC depletion were p53- or p16INK4A-independent. Taken together, these observations suggest that OIS-specific programs can be suppressed, at least partially, by C-MYC in NHM and continue to exist in MYC-dependent dormancy in melanoma cells.
Figure 3.
Senescence in human melanoma cells expressing NRASQ61R. LY294002-an inhibitor of PI3K, Rapamycin, an inhibitor of mTOR.
Figure 4.
Senescence in human melanoma cells expressing BRAFV600E. *Mutation status of BRAF and NRAS genes in HO-1 melanoma cells has not been determined. U0126, an inhibitor of MEK kinase; as-cDNA, anti-sense cDNA.
C-MYC has also been shown to oppose senescence of human melanoma cells in earlier studies. For instance, partial depletion of C-MYC via ectopic expression of its antisense cDNA resulted in cell crisis after 7 passages in vitro, which was accompanied by downregulation of telomerase reverse transcriptase (TERT) levels, multinucleation, increase in the cell size, vacuolization and elevation of SA-β-Gal activity9 (Fig. 4). A gradual decrease in the amounts of TERT due to a partial inhibition of C-MYC, a well-established transcriptional activator of TERT,60,61 could account for the cell crisis due to the telomere shortening. Thus, in this case, partial depletion of C-MYC appears to induce replicative-rather than OIS-type of senescence.
In another study, C-MYC mRNA was identified as a target for degradation by human polynucleotide phosphorylase (hPNPaseold35),62 an interferon-β-inducible enzyme with exo-ribonuclease activity (reviewed in ref. 63). It has been shown that overexpression of hPNPaseold35 in NHM or melanoma cells induced senescence phenotypes such as growth inhibition, cell flattening and an increase in the number of SA-β-Gal-positive cells (Fig. 4). Furthermore, ectopic expression of C-MYC partially, but significantly, reversed the growth-inhibitory activity of hPNPaseold35.64
Cumulatively, the above data are in agreement with the results obtained in mouse tumors including lymphoma, osteosarcoma and hepatocellular carcinoma where inhibition of C-MYC led to senescence.65 However, in contrast to human melanoma cells, senescence phenotypes induced by c-Myc depletion in mouse tumors were p16- or p53-dependent and MEK-independent.65
With the exception of C-MYC, DEK is the only cellular oncogene that has been shown to control senescence of human melanoma cells.11 DEK is a chromatin remodeling factor (reviewed in ref. 66) that is frequently overexpressed in several human malignancies including melanoma.67 Although the exact mechanism of DEK activity remains unknown, its oncogenic features include the ability to suppress apoptosis and senescence in cervical cancer cells caused by downregulation of E6/E7 viral oncogenes.68 Similarly, shRNA-mediated depletion of DEK in cells from several melanoma lines resulted in progressive inhibition of proliferation that, in approximately two weeks after infection, culminated in the emergence of senescence-like phenotypes including cell flattening, enlargement, vacuolization and increase in SA-β-Gal activity11 (Fig. 4). Given a similarity in the delayed pattern of senescence phenotypes caused by depletion of DEK or inhibition of TERT via partial depletion of C-MYC, it will be interesting to test whether DEK controls TERT levels or activity. Notably, as in suppression of C-MYC, the senescence phenotypes caused by DEK depletion in melanoma cells were independent of p16INK4A or p53.11 On the contrary, p53 has been implicated in the induction of senescence-like phenotypes in melanoma cells by overexpression of non-receptor spleen tyrosine kinase (SYK).69 SYK has long been known to participate in maturation of lymphocytes and signal transduction in many non-lymphoid immune cells (reviewed in ref. 70). Recently, SYK has been identified as a tumor suppressor in breast71 and pancreatic cancers.72 Accordingly, in melanoma cells its expression is epigenetically silenced.69 Reintroduction of SYK cDNA in melanoma cells resulted in proliferative arrest within 4 days accompanied by classical features of senescence including increase in cell size, SA-β-Gal activity and emergence of SAHF69 (Fig. 4). Interestingly, unlike senescence caused by depletion of C-MYC10 or DEK,11 senescence induced by overexpression of SYK led to the activation of p53 by phosphorylation at Ser15 and induction of p21CIP/WAF, although, the functional role of either of these events in SYK-induced senescence has not been established. Thus, human malignant melanoma cells retained the ability to undergo permanent growth arrest reminiscent of replicative or oncogene-induced senescence of normal melanocytes, a feature that can be utilized in the development of novel strategies for treatment of this chemo-refractive malignancy.
In conclusion, despite numerous efforts, our knowledge about molecular mechanisms of OIS in melanocytes does not go far beyond understanding the role of tumor suppressors such as p53, p16INK4A, p21CIP/WAF, etc. Unfortunately, this information does not contribute much to the “big picture.” Indeed, functional involvement of p53 in OIS still remains controversial, whereas other “usual suspects” have been shown to have little or no impact on OIS of melanocytic cells. On the other hand, at least one report demonstrated that simultaneous inhibition of p53 and pRB completely alleviates senescence caused by NRASQ61K.20 Since neither of these proteins are frequently mutated in the course of melanomagenesis, it will be important to identify members of their respective pathways whose altered expression can cooperate in overcoming OIS. It will also be important to determine the nature of the senescence programs that are activated in melanocytes in response to NRASQ61R and/or BRAFV600E. So far, induction of the unfolded protein response (UPR) is the sole pathway shown to be critical for senescence caused by HRASV12G in melanocytes.17 However, UPR does not play a role in BRAFV600E-induced senescence in these cells,20 and the nature of the MEK-downstream pathways, required for BRAFV600E-induced senescence in NHM has not been determined. Finally, the pathways responsible for keeping senescence dormant in melanoma cells are largely unknown. Until now, only two proteins, C-MYC10 and DEK,11 which both can affect transcription of multiple target genes, have been shown to be essential for suppression of OIS in melanoma cells. However, their downstream targets that are required for keeping senescence in check are yet to be specified. Finding answers to these questions will undoubtedly advance our understanding of the mechanisms resulting in the emergence of the first barrier to melanomagenesis and the pathways utilized by melanoma cells to overcome it.
Notes Added to Proofs
When this manuscript was in press, a paper by Scurr et al.74 appeared that contradicted the findings of Wajapeyee et al.44 on the role of IGFBP7 in senescence of normal human melanocytes. In particular, the authors reported that ectopic expression of BRAFV600E induced senescence in human melanocytes that were depleted of IGBP7 via the same shRNAs reported by Wajapeyee et al. Thus, it appears that the only componenets that two studies differ in are the source of melanocytes and the means of delivery of BRAFV600EcDNA [retroviral (Wajapeyee et al.) versus lentiviral (Scurr et al.) vector]. Additionally, Scurr et al. found that BRAFV600E suppresses rather than upregulates44 IGFBP7 levels. Obviously, more independent studies are required for the understanding of IGFBP7 functioning in melanocytic senescence.
Acknowledgements
We are grateful to Dr. Catherine Burkhart (Cleveland Biolabs) and Mrs. Charlene Nitterauer for critical reading of the manuscript. This manuscript was supported by the NIH grant R01-CA120244.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12551
References
- 1.Dimri GP, Testori A, Acosta M, Campisi J. Replicative senescence, aging and growth-regulatory transcription factors. Biol Signals. 1996;5:154–162. doi: 10.1159/000109185. [DOI] [PubMed] [Google Scholar]
- 2.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Woods D, McMahon M, Bishop JM. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 5.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 6.Bauer J, Curtin JA, Pinkel D, Bastian BC. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol. 2007;127:179–182. doi: 10.1038/sj.jid.5700490. [DOI] [PubMed] [Google Scholar]
- 7.Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 8.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 9.Biroccio A, Amodei S, Antonelli A, Benassi B, Zupi G. Inhibition of c-Myc oncoprotein limits the growth of human melanoma cells by inducing cellular crisis. J Biol Chem. 2003;278:35693–35701. doi: 10.1074/jbc.M304597200. [DOI] [PubMed] [Google Scholar]
- 10.Zhuang D, Mannava S, Grachtchouk V, Tang WH, Patil S, Wawrzyniak JA, et al. C-MYC overexpression is required for continuous suppression of oncogene-induced senescence in melanoma cells. Oncogene. 2008;27:6623–6634. doi: 10.1038/onc.2008.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, Kim DS, et al. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res. 2009;69:6405–6413. doi: 10.1158/0008-5472.CAN-09-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacocke M, Campisi J. Cellular senescence: a reflection of normal growth control, differentiation or aging? J Cell Biochem. 1991;45:147–155. doi: 10.1002/jcb.240450205. [DOI] [PubMed] [Google Scholar]
- 13.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- 15.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 16.Campisi J. Senescent cells, tumor suppression and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol. 2006;8:1053–1063. doi: 10.1038/ncb1471. Erratum in: Nat Cell Biol 2006; 8:1309. [DOI] [PubMed] [Google Scholar]
- 18.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 20.Haferkamp S, Tran SL, Becker TM, Scurr LL, Kefford RF, Rizos H. The relative contributions of the p53 and pRb pathways in oncogene-induced melanocyte senescence. Aging. 2009;1:542–556. doi: 10.18632/aging.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–709. doi: 10.1016/s0955-0674(00)00155-1. [DOI] [PubMed] [Google Scholar]
- 22.Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 23.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 24.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 25.Pomerantz J, Schreiber-Agus N, Liégeois NJ, Silverman A, Alland L, Chin L, et al. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 26.Sharpless E, Chin L. The INK4a/ARF locus and melanoma. Oncogene. 2003;22:3092–3098. doi: 10.1038/sj.onc.1206461. [DOI] [PubMed] [Google Scholar]
- 27.Haferkamp S, Scurr LL, Becker TM, Frausto M, Kefford RF, Rizos H. Oncogene-induced senescence does not require the p16(INK4a) or p14ARF melanoma tumor suppressors. J Invest Dermatol. 2009;129:1983–1991. doi: 10.1038/jid.2009.5. [DOI] [PubMed] [Google Scholar]
- 28.Skinner J, Bounacer A, Bond JA, Haughton MF, deMicco C, Wynford-Thomas D. Opposing effects of mutant ras oncoprotein on human fibroblast and epithelial cell proliferation: implications for models of human tumorigenesis. Oncogene. 2004;23:5994–5999. doi: 10.1038/sj.onc.1207798. [DOI] [PubMed] [Google Scholar]
- 29.Huot TJ, Rowe J, Harland M, Drayton S, Brookes S, Gooptu C, et al. Biallelic mutations in p16(INK4a) confer resistance to Ras- and Ets-induced senescence in human diploid fibroblasts. Mol Cell Biol. 2002;22:8135–8143. doi: 10.1128/MCB.22.23.8135-8143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpless NE, Kannan K, Xu J, Bosenberg MW, Chin L. Both products of the mouse Ink4a/Arf locus suppress melanoma formation in vivo. Oncogene. 2003;22:5055–5059. doi: 10.1038/sj.onc.1206809. [DOI] [PubMed] [Google Scholar]
- 31.Delmas V, Beermann F, Martinozzi S, Carreira S, Ackermann J, Kumasaka M, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–2935. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goding CR. Melanocyte development and malignant melanoma. Forum (Genova) 2000;10:176–187. [PubMed] [Google Scholar]
- 33.Shields JM, Thomas NE, Cregger M, Berger AJ, Leslie M, Torrice C, et al. Lack of extracellular signal-regulated kinase mitogen-activated protein kinase signaling shows a new type of melanoma. Cancer Res. 2007;67:1502–1512. doi: 10.1158/0008-5472.CAN-06-3311. [DOI] [PubMed] [Google Scholar]
- 34.Smalley KS, Contractor R, Haass NK, Kulp AN, Atilla-Gokcumen GE, Williams DS, et al. An organometallic protein kinase inhibitor pharmacologically activates p53 and induces apoptosis in human melanoma cells. Cancer Res. 2007;67:209–217. doi: 10.1158/0008-5472.CAN-06-1538. [DOI] [PubMed] [Google Scholar]
- 35.Ha L, Ichikawa T, Anver M, Dickins R, Lowe S, Sharpless NE, et al. ARF functions as a melanoma tumor suppressor by inducing p53-independent senescence. Proc Natl Acad Sci USA. 2007;104:10968–10973. doi: 10.1073/pnas.0611638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 37.Glei M, Hovhannisyan G, Pool-Zobel BL. Use of Comet-FISH in the study of DNA damage and repair: review. Mutat Res. 2009;681:33–43. doi: 10.1016/j.mrrev.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 39.Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. Paving the way for H2AX phosphorylation: chromatin changes in the DNA damage response. Cell Cycle. 2009;8:1494–1500. doi: 10.4161/cc.8.10.8501. [DOI] [PubMed] [Google Scholar]
- 40.Di Micco R, Fumagalli M, Cicalese A, Piccinin S, Gasparini P, Luise C, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 41.Pospelova TV, Demidenko ZN, Bukreeva EI, Pospelov VA, Gudkov AV, Blagosklonny MV. Pseudo-DNA damage response in senescent cells. Cell Cycle. 2009;8:4025–4026. doi: 10.4161/cc.8.24.10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 43.Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 44.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, et al. Cellular senescence in naevi and immortalization in melanoma: a role for p16? Br J Cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 47.Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 48.Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. doi: 10.1146/annurev.cellbio.16.1.653. [DOI] [PubMed] [Google Scholar]
- 49.Nikiforov MA, Popov N, Kotenko I, Henriksson M, Cole MD. The Mad and Myc basic domains are functionally equivalent. J Biol Chem. 2003;278:11094–11099. doi: 10.1074/jbc.M212298200. [DOI] [PubMed] [Google Scholar]
- 50.Cole MD, Nikiforov MA. Transcriptional activation by the Myc oncoprotein. Curr Top Microbiol Immunol. 2006;302:33–50. doi: 10.1007/3-540-32952-8_2. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Mannava S, Grachtchouk V, Zhuang D, Soengas MS, Gudkov AV, et al. c-Myc depletion inhibits proliferation of human tumor cells at various stages of the cell cycle. Oncogene. 2008;27:1905–1915. doi: 10.1038/sj.onc.1210823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 53.Ross DA, Wilson GD. Expression of c-myc oncoprotein represents a new prognostic marker in cutaneous melanoma. Br J Surg. 1998;85:46–51. doi: 10.1046/j.1365-2168.1998.00528.x. [DOI] [PubMed] [Google Scholar]
- 54.Greulich KM, Utikal J, Peter RU, Krähn G. c-MYC and nodular malignant melanoma. A case report. Cancer. 2000;89:97–103. doi: 10.1002/1097-0142(20000701)89:1<97::aid-cncr14>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 55.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhu S, Wurdak H, Wang Y, Galkin A, Tao H, Li J, et al. A genomic screen identifies TYRO3 as a MITF regulator in melanoma. Proc Natl Acad Sci USA. 2009;106:17025–17030. doi: 10.1073/pnas.0909292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chudnovsky Y, Adams AE, Robbins PB, Lin Q, Khavari PA. Use of human tissue to assess the oncogenic activity of melanoma-associated mutations. Nat Genet. 2005;37:745–749. doi: 10.1038/ng1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes Dev. 1998;12:1769–1774. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu KJ, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, et al. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 62.Sarkar D, Leszczyniecka M, Kang DC, Lebedeva IV, Valerie K, Dhar S, et al. Downregulation of Myc as a potential target for growth arrest induced by human polynucleotide phosphorylase (hPNPase old-35) in human melanoma cells. J Biol Chem. 2003;278:24542–24551. doi: 10.1074/jbc.M302421200. [DOI] [PubMed] [Google Scholar]
- 63.Sarkar D, Fisher PB. Human polynucleotide phosphorylase (hPNPase old-35): an RNA degradation enzyme with pleiotrophic biological effects. Cell Cycle. 2006;5:1080–1084. doi: 10.4161/cc.5.10.2741. [DOI] [PubMed] [Google Scholar]
- 64.Sarkar D, Park ES, Emdad L, Randolph A, Valerie K, Fisher PB. Defining the domains of human polynucleotide phosphorylase (hPNPase OLD-35) mediating cellular senescence. Mol Cell Biol. 2005;25:7333–7343. doi: 10.1128/MCB.25.16.7333-7343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu CH, van Riggelen J, Yetil A, Fan AC, Bachireddy P, Felsher DW. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci USA. 2007;104:13028–13033. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waldmann T, Scholten I, Kappes F, Hu HG, Knippers R. The DEK protein—an abundant and ubiquitous constituent of mammalian chromatin. Gene. 2004;343:1–9. doi: 10.1016/j.gene.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 67.Carro MS, Spiga FM, Quarto M, Di Ninni V, Volorio S, Alcalay M, et al. DEK Expression is controlled by E2F and deregulated in diverse tumor types. Cell Cycle. 2006;5:1202–1207. doi: 10.4161/cc.5.11.2801. [DOI] [PubMed] [Google Scholar]
- 68.Wise-Draper TM, Allen HV, Thobe MN, Jones EE, Habash KB, Münger K, et al. The human DEK protooncogene is a senescence inhibitor and an upregulated target of high-risk human papillomavirus E7. J Virol. 2005;79:14309–14317. doi: 10.1128/JVI.79.22.14309-14317.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailet O, Fenouille N, Abbe P, Robert G, Rocchi S, Gonthier N, et al. Spleen tyrosine kinase functions as a tumor suppressor in melanoma cells by inducing senescence-like growth arrest. Cancer Res. 2009;69:2748–2756. doi: 10.1158/0008-5472.CAN-08-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner M, Schweighoffer E, Colucci F, Di Santo JP, Tybulewicz VL. Tyrosine kinase SYK: essential functions for immunoreceptor signalling. Immunol Today. 2000;21:148–154. doi: 10.1016/s0167-5699(99)01574-1. [DOI] [PubMed] [Google Scholar]
- 71.Coopman PJ, Do MT, Barth M, Bowden ET, Hayes AJ, Basyuk E, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–747. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 72.Layton T, Stalens C, Gunderson F, Goodison S, Silletti S. Syk tyrosine kinase acts as a pancreatic adenocarcinoma tumor suppressor by regulating cellular growth and invasion. Am J Pathol. 2009;175:2625–2636. doi: 10.2353/ajpath.2009.090543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones R, Ruas M, Gregory F, Moulin S, Delia D, Manoukian S, et al. A CDKN2A mutation in familial melanoma that abrogates binding of p16INK4a to CDK4 but not CDK6. Cancer Res. 2007;67:9134–9141. doi: 10.1158/0008-5472.CAN-07-1528. [DOI] [PubMed] [Google Scholar]
- 74.Scurr LL, Pupo GM, Becker TM, Lai K, Schrama D, Haferkamp S, Irvine M, Scolyer RA, Mann GJ, Becker JC, Kefford RF, Rizos H. IGFBP7 is not required for B-RAF-induced melanocyte senescence. Cell. 2010;141:717–727. doi: 10.1016/j.cell.2010.04.021. [DOI] [PubMed] [Google Scholar]