Abstract
Mitogen-activated protein kinase (MAPK) pathways are major signal transduction systems by which eukaryotic cells convert environmental cues to intracellular events such as proliferation and differentiation. We have identified a Trypanosoma cruzi homologue of the MAPK family that we have called TcMAPK2. Sequence analyses demonstrates TcMAPK2 has high homology with lower eukaryotic ERK2 but has significant differences from mammalian ERK2. Enzymatic assays of both recombinant TcMAPK2 and native protein obtained by immunoprecipitation using anti-TcMAPK2 demonstrated that both preparations of TcMAPK2 were catalytically active. Immunofluorescence analysis of the subcellular localization of TcMAPK2 determined it is mainly cytoplasmic in epimastigotes, along the flagella in trypomastigotes and on the plasma membrane of intracellular amastigotes. Phosphorylated TcMAPK2 was highest in trypomastigotes and lowest in amastigotes. Recombinant TcMAPK2 was able to phosphorylate the recombinant protein of a cAMP specific phosphodiesterase. Overexpression of TcMAPK2 in epimastigotes inhibited growth and development leading to death. TcMAPK2 has an important role in the stress response of the parasite and may be important in regulating proliferation and differentiation.
Key words: Trypanosoma cruzi, mitogen-activated protein kinase, phosphorylation
Introduction
Trypanosoma cruzi is the causative agent of Chagas disease. At least 15 million people are infected with this pathogenic protozoan (www.who.int/tdr/diseases/chagas/direction.htm). T. cruzi has a complex life cycle involving four morphogenetic stages.1 The epimastigote and metacyclic trypomastigote are insect-specific stages, whereas the blood form trypomastigote and amastigotes are mammalian host-specific, extracellular and intracellular stages, respectively. In endemic areas, the main mode of transmission is through an insect vector, the triatomine bug.2 A triatomine becomes infected with T. cruzi by feeding on the blood of an infected person or animal. The bugs bite and ingest blood, and then they defecate on the person. Triatomine pass T. cruzi parasites (metacyclic trypomastigotes) in feces left near the site of the bite wound. Scratching the site of the bite causes the parasites to enter the host through the wound or through intact mucous membranes, such as the conjunctiva. Once inside the host the trypomastigotes invade cells, where they differentiate into intracellular amastigotes. The amastigotes multiply by binary fission and differentiate into trypomastigotes, which are then released into the bloodstream. This cycle is repeated in each newly infected cell. Replication resumes only when the parasites enter another cell or are ingested by another vector.2
The molecular mechanisms regulating the differentiation of T. cruzi remain unclear. Signaling via cAMP, cAMP-dependent protein kinase (protein kinase A or PKA) and other interacting signal transduction pathways are crucial components of differentiation in most eukaryotes.3 In axenic culture, log phase epimastigotes grow and eventually enter stationary phase from which metacyclogenesis occurs, suggesting that cell density and/or nutrition depletion may trigger this differentiation process. Trypomastigotes differentiate to intracellular amastigotes once they invade the host cells, but can be induced in vitro to become extracellular amastigotes by pH shifts.4 These phenomena suggest that environmental cues are important in proliferation and differentiation.
Mitogen-activated protein kinases (MAPK) are well-known mediators of signal transduction of higher eukaryotes regulating important processes like proliferation, differentiation, stress response and apoptosis. They display a high level of evolutionary conservation and are essential for many cell functions in response to extracellular stimuli.5–7 In mammalian cells, four mammalian MAP kinase cascades are currently recognized, including Extracellular signal-regulated kinase (ERK); c-Jun NH2-terminal kinase/stress-activated protein kinase (JNK/SAPK); p38 and big mitogen-activated protein kinase-1/ERK5 (BMK-1/ERK5) pathways.5–7 These MAP kinases are activated by phosphorylation that occurs at a specific threonine and tyrosine residue localized within the activation loop motif TxY (T = Threonine, x = any amino acids, Y = Tyrosine) of kinase subdomain VIII. A prototypical three-component cascade is involved in the activation. MAP kinases are activated by a range of diverse stimuli.
Studies of MAP kinases in pathogenic protozoa have revealed the importance of this kinase family in the development of many organisms. Identification of novel MAP kinases, TgMAPK1 and TgMAPK2, in Toxoplasma gondii has been reported.8 TgMAPK1 and TgMAPK2 are evolutionarily very distant from other MAP kinase family. In malaria parasites, P. berghei and P. falciparum, MAP kinase-2 is required for male gamete formation.9–11 An atypical activator of MAPK was reported in P. falciparum12–14 and the typical three-component cascade signaling does not exist in malaria parasites, suggesting that mechanisms for the activation of MAP kinase in malaria are different than their mammalian host. Giardia lamblia, an intestinal protozoan parasite, possesses homologues of ERK1 and ERK2, which are involved in encystations.15 However, there have been no studies of MAP kinases in T. cruzi. In the current paper we report the functional characterization of the T. cruzi homologue of ERK2 (TcMAPK2) and provide evidence of differential subcellular localization as well as activation of TcMAPK2 in T. cruzi.
Results
Sequence characterization and analysis of TcMAPK2.
We cloned TcMAPK2 by direct PCR amplification from CL Brener epimastigote cDNA (ORF 1,362 bp, 453 amino acids and 51,014 kDa molecular weight). The entire open reading frame was amplified by PCR using cDNA and primers with appropriate restriction sites and subcloned into a pTrcHisA expression vector. There are two copies of TcMAPK2 in the T. cruzi genome (Tc00.1047053510295.50 and Tc00.1047053506007.40). This kinase contains the typical twelve sub-domains of a mitogen-activated protein kinase. This includes consensus sequences in subdomain I, GXGXXGXV (GQGAYGIV 20–27); II, AXK (ALK 40–42); III, RXXRE (RTFRE 56–60); VI, HRDXKPXN (HRDMKPSN, 133–140); VII, DFG (DFG, 153–155); and VIII, TXYXXXRXYRXPE (TDYIMTRWYRPPE, 174–186). Typical MAPK features are seen in TcMAPK2 including subdomains I to III, which are for binding and orienting ATP, and a conserved TXY motif for dual phosphorylation (Thr174–Tyr176), which is involved in the activation of the enzyme. BLAST analysis demonstrates that TcMAPK2 has an 81% identity with a Trypanosoma brucei MAKP (Gene ID: 3662091 Tb10.70.2070), 66% identity with a Leishmania major MAPK (Gene ID: 5655322 LmjF36.072), and 60% identity with a Giardia intestinalis ERK2 (GenBank accession: AAN73430.1). TcMAPK2 has a low homology with mammalian ERK2, however, it has 51% identity with a human MAPK 15 (DBSOURCE: accession CH471162.2). These sequence alignments are demonstrated in Figure 1.
Figure 1.
Comparison of the TcMAPK2 with other MAPKs. Amino acid sequence alignment of TcMAPK2 from different species. TcMAPK2 (TcERK2) in the T. cruzi genome (Tc00.1047053510295.50 and Tc00.1047053506007.40); GIERK2: Giardia intestinalis ERK2 (GenBank accession: AAN73430.1). Lm-MAPK2: Leishmania major (MAPK Gene ID: 5655322 LmjF36.072); HuMAPK15: a human MAPK 15 (DBSOURCE: accession CH471162.2). TcMAPK2 includes consensus in subdomain I, GXGXXGXV (GQGAYGIV 20–27); II, AXK (ALK 40–42); III, RXXRE (RTFRE 56–60); VI, HRDXKPXN (HRDMKPSN, 133–140); VII, DFG (DFG, 153–155); VIII, TXYXXXRXYRXPE (TDYIMTRWYRPPE, 174–186). Typical features of MAPK exist in this kinase. Subdomains I–III are for binding and orienting ATP. The conserved TXY motif for dual phosphorylation (Thr174–Tyr176) for the activation of the enzyme is also present. The shaded residues are those that are found in the consensus sequence.
TcMAPK2 was catalytically active.
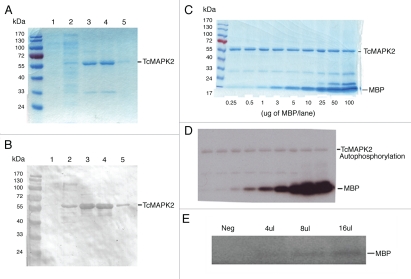
In order to perform functional characterization of TcMAPK2, recombinant enzyme was expressed and purified using a His Trap FF crude column (pre-charged with Ni2+). SDS-polyacrylamide gel electrophoresis under reducing conditions and Coomassie blue R250 staining as well as immunoblot analysis using a HIS6 mAb were performed to analyze the purity of the recombinant protein. As demonstrated in Figure 2A and B, we were able to obtain purified recombinant TcMAPK2 (recTcMAPK2). The catalytic activity of the recombinant TcMAPK2 was determined using MBP as the substrate. An in vitro kinase assay revealed that TcMAPK2 was catalytically active (Fig. 2C and D) and phosphorylated MBP efficiently. We next evaluated if anti-TcMAPK2 could pull down native TcMAPK2 as an active kinase from parasite lysate. Anti-TcMAPK2 immune complexes from epimastigote lysate were able to phosphorylate MBP indicating that anti-TcMAPK2 can pull down native active kinase (Fig. 2E).
Figure 2.
Expression and purification of TcMAPK2 and analyses of recombinant and nativeTcMAPK2. (A) Coomassie blue R250 staining lane 1: 20 µl of non-induced bacteria; lane 2: 20 µl of 1 mM IPTG IP induced bacteria; lane 3: first eluate; lane 4: second eluate; lane 5: third eluate from the Trap FF crude column. (B) Immunoblot using HIS6 mAb. Immunoblot examined identical samples in a duplicate SDS gel using antibody to HIS6. Note that the expression and purification technique for TcMAPK2 was successful (see Materials and Methods for details). (C) After in vitro phosphorylation reaction, the samples were subjected to the resolution of SDS gel and Coomassie blue R250 staining. The purified recombinant TcMAPK2 and the substrates were visualized in the gel. MBP concentrations were indicated at the bottom of each lane. (D) The same gel was dried and used for autoradiography for five minutes. Phosphorylation of MBP and autophosphorylation of TcMAPK2 are detected by autoradiography. (E) Anti-TcMAPK2 precipitated immune complexes from epimastigotes phosphorylate MBP. The bead with immune complexes was resuspended in 50 µl of buffer A and then used for kinase assay using MBP as a substrate. “Neg” indicates the negative control (using 16 µl bead suspension from parasite lysate with pre-immune serum). 4 µl, 8 µl and 16 µl indicate the amount of bead suspension with Anti-TcMAPK2 immune complexes. Note that Anti-TcMAPK2 immune complexes can phosphorylate MBP in the kinase assay, indicating that native TcMAPK2 is active.
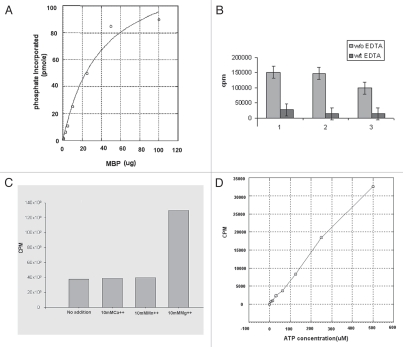
Enzymatic parameters were calculated using KaleidaGraph 4 software. At the standard reaction conditions using 5 µg of the recombinant enzyme and varying concentration of MBP, the specific activity of recTcMAPK2 is 6.4 ± 0.5 nmole PO4/incorp./µmole TcMAPK2/second and the apparent Km is in the nanomolar range (1.2 ± 0.2 nM) (Fig. 3A). Enzymatic activity was significantly inhibited by EDTA (Fig. 3B). Purified recTc-MAPK2 is MgCl2 dependent. Mg2+ significantly increased the enzymatic activity while Mn2+ and Ca2+ had no effect (Fig. 3C). An increase in ATP concentration increased the incorporation of phosphate into MBP (Fig. 3D). Purified recTcMAPK2 was not sensitive to FR180204, a human ERK2 inhibitor16 (data not shown).
Figure 3.
Biochemical analysis of TcMAPK2. (A) Computer software analysis of enzymatic activity of TcMAPK2. Enzymatic parameters were calculated using KaleidaGraph 4 software. At the standard reaction conditions using 5 µg of the recombinant enzyme and varying concentration of MBP, the specific activity of recombinant TcMAPK2 is 6.4 ± 0.5 nmole PO4/incorp./µmole TcMAPK2/second and the apparent Km is in the nanomolar range (1.2 ± 0.2 nM). (B) EDTA abolished the enzymatic activity. Light color columns are reactions without EDTA while dark color columns with 250 mM EDTA. Three separate replicates were performed. (C) TcMAPK2 is Mg2+ dependent. 10 mM of either Ca2+ or Mn2+ shows no effect on the enzymatic activity as compared to standard kinase assay without adding divalent metal compounds while 10 mM Mg2+ significantly increases more than three folds of the enzymatic activity. The plot is a representative of three separate experiments. (D) TcMAPK2 is ATP dependent. Increase the concentration of ATP increases the incorporation of phosphate into the MBP. This linear curve is a representative of three separate experiments.
Altered localization, protein level and phosphorylation of TcMAPK2.
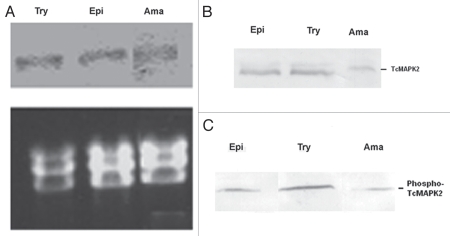
To examine whether there are alterations in TcMAPK2 mRNA in the three life stages (epimastigote, trypomastigote and amastigote) of T. cruzi, we performed a northern blot analysis. All forms display a single hybridization signal at about 4 kb, which is consistent with the predicted size of TcMAPK2. There was no change at the level of mRNA (Fig. 4A). Immunoblotting of these life stages of T. cruzi was performed using anti-TcMAPK2 and densitometry software (AlphaEaseFC software, Alpha Innotech). The results indicated that the TcMAPK2 protein levels were similar both in trypomastigotes and epimastigotes, but amastigotes had a lower TcMAPK2 protein level (Fig. 4B). Immunoblot analysis of the three life stages of T. cruzi using anti-phospho-TcMAPK2 revealed that trypomastigotes have highest level of phospho-TcMAPK2, followed by epimastigotes and then extracellular amastigotes (Fig. 4C). Based on the data from densitometry, we found that the ratio of phospho-TcMAPK2 vs. total TcMAPK2 is 0.94 for trypomastigote and 0.45 for both epimastigote and amastigotes. These data indicate that trypomastigotes have proportionally more phosphorylated TcMAPK2 (which would be the active enzyme) than either epimastigote or amastigotes. This is consistent with another observation that immune-complexes obtained using anti-TcMAPK2 from a lysate of 6 × 106 trypomastigotes were sufficient to phosphorylate MBP while at the same experimental conditions immune-complexes from 6 × 106 extracellular amastigotes were not sufficient to phosphorylate the same amount of MBP (data not shown).
Figure 4.
Developmental studies of TcMAPK2 in three forms of T. cruzi. (A) Northern blot analysis of T. cruzi. Try (trypomastigotes), Epi (epimastigotes) and Ama (extracellular amastigotes). Top panel shows TcMAPK2 probe hybridization signals (∼4 kb) of total RNAs from three life forms of T. cruzi. Bottom panel shows Ethidium bromide-stained gels demonstrated the equal loading of T. cruzi ribosomal RNA and confirmed the integrity of the samples. There was no change in the level of mRNA among three forms of T. cruzi. (B) Immunoblot analysis of T. cruzi using anti-TcMAPK2. The results indicated that the TcMAPK2 protein level was at the same level in trypomastigotes and epimastigotes but TcMAPK2 protein level in amastigotes is lower than that in other forms. (C) Immunoblot analysis of T. cruzi using anti-phospho-TcMAPK2. Trypomastigotes have highest level of phospho-TcMAPK2, followed by epimastigote and then extracellular amastigotes. Based on the data from analysis of densitometry, the ratio of phospho-TcMAPK2 vs. TcMAPK2 is two folds higher in trypomastigotes as compared to that of epimastigotes and amastigotes.
IFA using anti-TcMAPK2 revealed that cytoplasmic staining is evident in epimastigotes. In addition, there is some staining in the flagella (Fig. 5, top part). Staining in trypomastigotes is clearly evident along the flagellar (Fig. 5, second part). Staining of intracellular amastigotes or newly release amastigotes demonstrated that TcMAPK2 mainly localized to the plasma membrane (Fig. 5, third and bottom parts). Pre-immure sera did not show any staining with any stage of T. cruzi (not shown).
Figure 5.
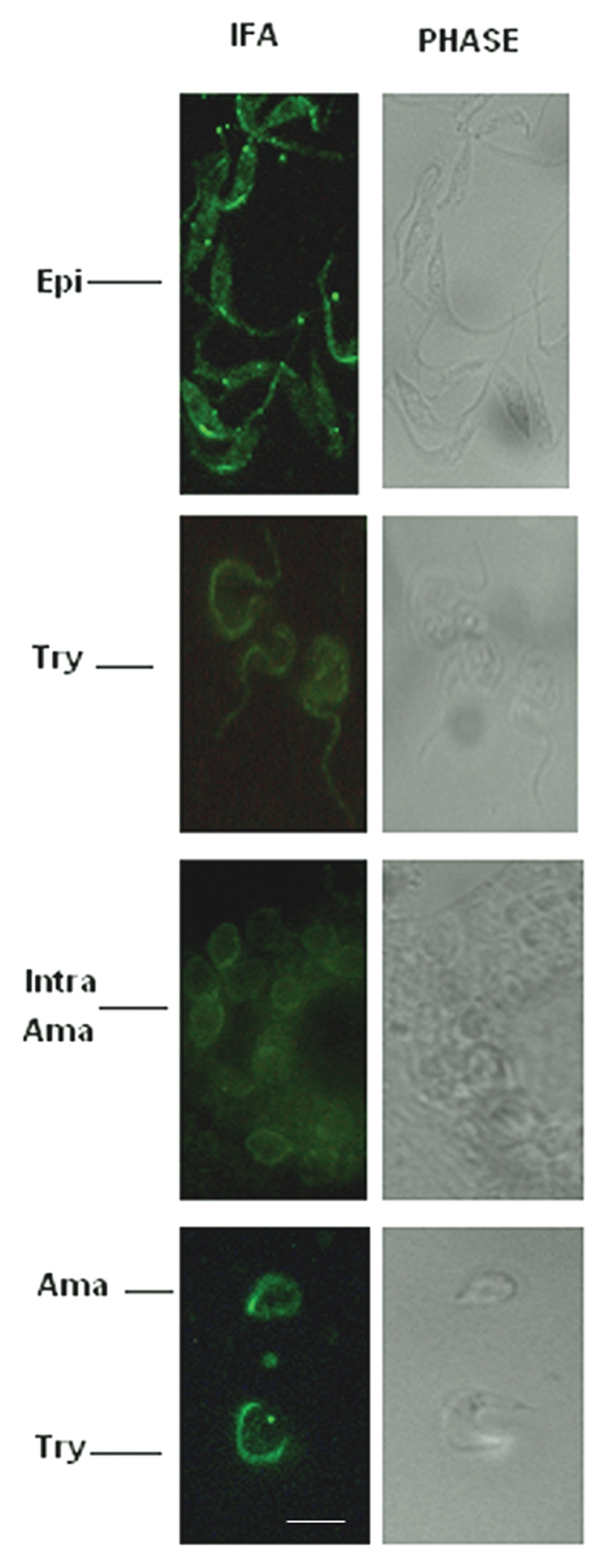
IFA of T. cruzi with anti-TcMAPK2. Epi indicates epimastigotes; Try indicates trypomastigotes, Intra Ama indicates intracellular amastigotes and Ama indicates amastigotes (newly released). IFA revealed cytoplasmic staining in epimastigotes. In addition, there are some intensive spots staining in the flagellar side (top part). Staining in trypomastigotes was evident along the flagellar side (second part). Staining of intracellular amastigotes or newly released amastigotes shows that TcMAPK2 mainly localized in the plasma membrane (third and bottom parts). Pre-immure sera did not show any staining (not shown). Scale bars = 10 µm.
TcMAPK2 interacts and phosphorylates a T. cruzi cAMP specific phosphodiesterase.
Previously, we demonstrated that TcPKAc interacted with and phosphorylated TcMAPK2 and T. cruzi cAMP specific phosphodiesterase (TcPDEC2) (GenBank accession no. DQ008164). In addition, by yeast two hybrid analysis TcMAPK2 and TcPDEC2 interacted.17 In the current study, we demonstrated in vitro that purified recTcMAPK2 phosphorylates recTcPDEC2 (Fig. 6). In TcPDEC2, there are two typical consensus sequences that can be phosphorylated by TcMAPK2 (PVTP at 555–558 and VTTP at 612–615).
Figure 6.
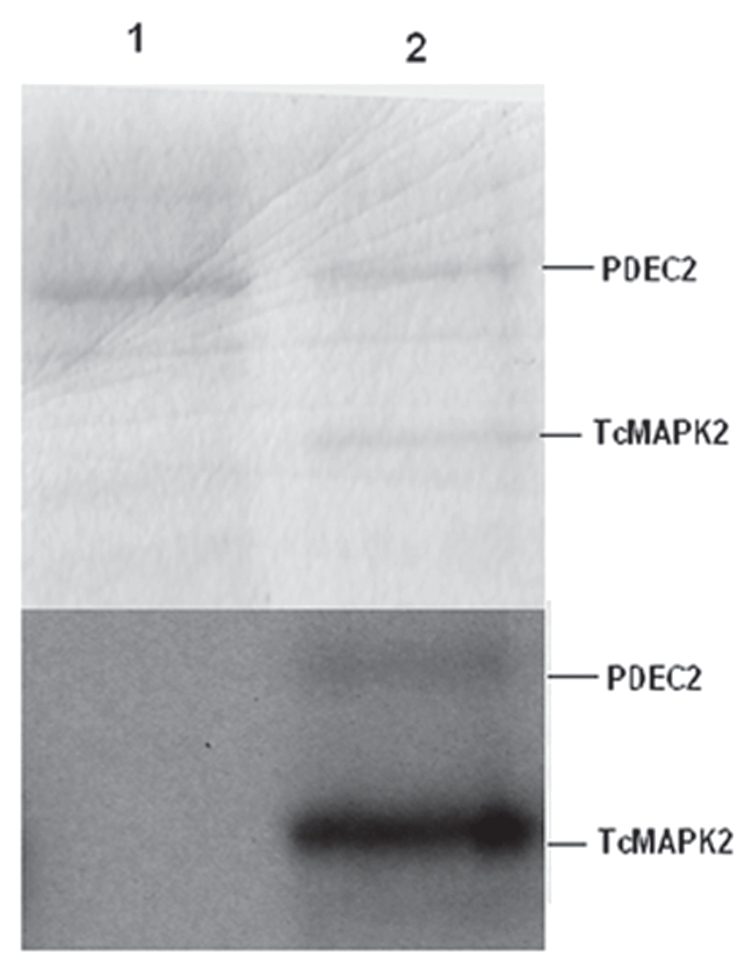
TcMAPK2 phosphorylates TcPDEC2. Top part, Coomassie blue R250 staining; Bottom part, the same gel was used for autoradiography. Lane1, purified recombinant TcPDEC2 alone (without purified recombinant TcMAPK2). Lane2 purified recombinant TcMAPK2 and purified recombinant TcPEDC2 together for in vitro kinase assay reaction (see Materials and Methods for details). Note that TcMAPK2 phosphorylated TcPEDC2. Also, autophosphorylation occurred in TcMAPK2.
Overexpression of TcMAPK2 is detrimental to epimastigotes.
pTREX-TcMAPK2 was introduced into epimastigotes (CL Brener) by electroporation. Transfected parasites were selected by G418. After four weeks, parasites without vectors died. While parasites carrying pTREX grew. Parasites carrying pTREX-TcMAPK2 were also resistant to G418 and grew; however, these parasites with overexpression of TcMAPK2 had much slower growth and soon stopped proliferating and died. This experiment was repeated three times with identical results. This result indicates that increased TcMAPK2 expression suppresses the growth of epimastigotes.
Discussion
The homolog of ERK2 is a highly conserved ubiquitously expressed kinase in many organisms which plays a pivotal role in regulating complex cellular processes in response to environmental cues. In our previous study, we found that the orthologue of lower eukaryotic ERK2, TcMAPK2, interacted with a TcPKAc and TcPDE2 by yeast-two hybrid screen, indicating that it is highly likely that TcMAPK2 is an important component for cAMP homeostasis. Sequence analysis indicated that TcMAPK2 contained all of the MAPK subdomains that are conserved in other eukaryotic MAPKs. TcMAPK2 has highest homology with a Trypanosoma brucei MAKP (81% identity), followed by a Leishmania major MAPK (66% identity) and a Giardia intestinalis ERK2 (60% identity). However, TcMAPK2 contains many insertions inside the gene as compared to mammalian ERK2 and has a long carboxy-terminal extension. Therefore, TcMAPK2 has a molecular weight of 51 kDa which is bigger than the mammalian ERK2.
By multiple species gene BLAST, TcMAPK2 was found to be most similar to human ERK8 or human MAPK15 (51% identity) (see Fig. 1). Long carboxy-terminal extensions have also been found in mammalian ERK5, ERK7 and ERK8.18–21 The ERK7 carboxy-terminal extension has been shown to regulate kinase localization and functions as a negative regulator of growth.21 It has been reported in Trypanosoma brucei that the carboxy-terminal extension of a MAPK, TbECK1 is involved in the regulation of the kinase activity in procyclic trypanosomes.22 The biological functions of the extension in TcMAPK2 remain to be investigated.
MAPKs with a TDY (T = Threonine, D = Aspartic acid, Y = Tyrosine) signature often regulate stress responses. For example, MAPKs with a TDY signature were found to regulate plant stress responses and host defenses.24 In the T. cruzi genome there are thirteen T. cruzi MAPKs and six of them possess a TDY signature. This is similar to Leishmania major MAPKs (there are eight TDY MAPKs among the 15 identified L. major MAPKs).23 The TxY motif in TcMAPK2 for dual phosphorylation is TDY and this suggests that this enzyme may play an important role in the stress response in T. cruzi.
Analysis of the kinetic parameters of recombinant TcMAPK2 indicated that it was sufficient for phosphorylating MBP. It is possible that full activation of TcMAPK2 requires activating phosphorylation at the TXY motif by an upstream kinase, a MEK homolog, which could be partially achieved by the recombinant TcMAPK2 through autophosphorylation (Fig. 2B). Immunoprecipitated native TcMAPK2 was also able to phosphorylate MBP. These data demonstrate that TcMAPK2 is catalytically active. Consistent with other MAPKs, EDTA suppressed its enzymatic activity (Fig. 3B). This kinase is Mg2+ and ATP dependent. Mg2+ significantly increased its activity while Ca2+ and Mn2+ have no effect (Fig. 3C). Also, an increase in the concentration of ATP in the enzymatic reactions enhances the incorporation of phosphate into the substrates (Fig. 3D). The newly developed mammalian ERK2 inhibitor, FR180204, failed to inhibit TcMAPK2 in vitro. This suggests that this parasitic kinase can be used to screen multiple inhibitors and discover a specific inhibitor since its inhibition profile is different from that of the mammalian ERK2.
Northern blot analysis indicated that there was no change in mRNA levels in the three life stages of T. cruzi (Fig. 4A). However, the phospho-TcMAPK2 (active form) existed in the highest level in the non-dividing trypomastigotes (Fig. 4C). In general, kinetoplastid parasites regulate their proteins at the translational level but not at the transcriptional level, which is consistent with what was observed with TcMAPK2. Trypomastigotes are extracellular forms which directly encounter the hostile host immune responses and osmotic stresses. The increase in the level and activation of TcMAPK2 in trypomastigotes is consistent with a role for this enzyme pathway in the parasite's response to environment stresses. Both total TcMAPK2 and phospho-TcMAPK2 have the lowest level in amastigotes, indicating a downregulation may be necessary for the transition from trypomastigotes (non replicating) to amastigotes (replicating). In epimastigotes, the level of total TcMAPK2 is similar to that of trypomastigotes. However, the ratio of phospho-TcMAPK2 vs. total TcMAPK2 is much lower than that of trypomastigote. Interestingly, overexpression of TcMAPK2 in epimastigotes resulted in a slower growth and detrimental effects. This suggests that TcMAPK2 activity may signal the parasite to slow down its growth and adapt to the new environments.
IFA revealed that cytoplasmic staining is evident in epimastigotes. In addition, there are some intensive spots staining in the flagellar. Staining in trypomastigotes is evident along the flagellar side. Members of MAP kinases in Leishmania parasites have been found to play important roles in flagellar length as well as parasite survival in the infected host25–30 and TcMAPK2 may have the similar functions in T. cruzi. IFA in intracellular amastigotes or newly release amastigotes shows that TcMAPK2 mainly localized in the plasma membrane (Fig. 5). This enzyme may be important for the adaptation of the parasite to the host cytoplasmic environments.
Previously, we demonstrated that TcPKAc interacted with and phosphorylated TcMAPK2 and TcPDEC2 and that TcMAPK2 and TcPDEC2 interacted by yeast two hybrid analysis.17 This suggested that a network for the regulation of cAMP homeostasis exists in T. cruzi and that this network was similar to other eukaryotes. This further suggests that two major pathways, i.e., PKA and MAPK, may have cross talk to allow the regulation of critical functions during differentiation or stress responses. In this paper, we report that purified recTcMAPK2 phosphorylates recTc PDEC2 (Fig. 6). This is the first identification of a substrate of MAPK in T. cruzi. In addition, this provides a useful technique for the purification of an active recTcMAPK2. This recTcMAPK2 can be used to confirm additional MAPK substrates in vitro.
Based on all of the data, we speculate that TcMAPK2 activation is necessary for trypomastigote survival and invasion. However, downregulation of TcMAPK2 is necessary for amastigote replication. TcMAPK2 may also be involved in flagella functions and stress responses from hostile host environments. This enzyme is significantly different from the mammalian counterparts and could represent a target for drug development. Further investigations will shed light on its functions in this pathogen.
Materials and Methods
cell culture
T. cruzi epimastigotes (HO 3/15, Brazil, Tulahuen and CL Brener) were grown at 26°C in liver infusion tryptose broth supplemented with 10% FCS (Life Technologies, Gaithersburg, MD). Trypomastigotes were obtained by growth in L6E9 myoblast cultures. The trypomastigotes were harvested 5–8 days post-inoculation, depending on the strain. Prior to harvesting the trypomastigotes, the cell cultures were washed with medium (Dulbecco's modified Eagle medium without serum) in order to remove small numbers of extracellular amastigotes that are frequently present. Extracellular amastigotes were obtained by the methods described before.30,31
PCR amplification.
TcMAPK2 was found to interact with a T. cruzi protein kinase. A catalytic subunit (TcPKAc) and a T. cruzi cAMP specific phosphodiesterase (TcPDEC2) in our initial yeast-two hybrid screen experiments.17 A TcMAPK2 homologue of Giardia lamblia was reported to mediate differentiation of the organism. In addition, this candidate contains all conserve functional domains with a longer insertion in the C-terminal as compared to the mammalian homologue. Based on these findings, we decided to characterize this kinase. The entire open reading frame was amplified by PCR using cDNA and primers with appropriate restriction sites: forward primer (Xho1)-CCG CTC GAG ATG TCA TCT GAA ATA GAG C, reverse (EcoR1)-CCG GAA TTC CTA CTT TTG CAT GGT TCG T and subcloned into a pTrcHisA expression vector (Invitrogen), which expresses six histidines in the N-terminal of recombinant protein.
Northern blot.
To examine whether there are alterations in TcMAPK2 mRNA in the three stages of T. cruzi, we performed a northern blot analysis as described previously.31 Briefly, total RNA (20 µg) isolated from T. cruzi epimastigotes, trypomastigotes and amastigotes was electrophoresed on 1% agarose/formaldehyde gels and capillary blotted to nylon membranes using standard procedures. The membranes were probed with the TcMAPK2 open reading frame (ORF) labeled with digoxigenin. Hybridization was performed at 42°C overnight followed by extensive washing with Saline Sodium Citrate Solution (SSC)/0.5% SDS at 42 and 55°C. A mAb (Roche) against digoxigenin was used to detect the hybridized probe in the nylon membranes. Ethidium bromide-stained gels demonstrated the equal loading of T. cruzi ribosomal RNA and confirmed the integrity of the samples.
Expression and purification of recombinant protein.
The expression construct was introduced into E. coli BL21 (DE3) (Invitrogen) by transformation and plated in LB ampicillin agar plates. One colony was inoculated into 50 ml of LB broth with 100 µg ampicillin/ml. The bacteria were allowed to grow overnight to a density of 0.7–1 Abs600 with constant agitation 250 RPM at 30°C and were then induced to express the recombinant protein by adding 1 mM isopropylthiogalactoside (IPTG). The induced bacteria were maintained with the same agitation at 30°C for 3 hrs. Protein purification was performed using the His Trap FF crude Kit (GE Healthcare).
Briefly, the bacterial pellet was resuspended in 20 ml of sonication buffer (1x phosphate buffer with 20 mM imidazole with one protease inhibitor cocktail tablet per 10 ml) and sonicated on ice. Afterwards, the lysate was centrifuged at 27,000 g, 4°C for 30 min. The supernatant was then passed into a 1-ml His Trap FF crude column (pre-charged with Ni2+), the column was washed with 40 ml (1x phosphate buffer with 20 mM imidazole) of binding buffer and protein was eluted using 5 ml elution buffer (1x phosphate buffer containing 40 mM imidazole) by collecting the eluate in 1 ml fractions. Aliquots of bacteria before and after induction and eluates were analyzed by SDS-polyacrylamide gel electrophoresis under reducing conditions and stained with Coomassie blue R250. Immunoblot was then performed using a HIS6 mAb (Novagen) to verify the expression of recombinant proteins. We were able to purify large amount of pure recombinant protein. The fractions of eluates containing pure recombinant protein were further dialyzed with buffer (20 mM Tris-HCL pH 7.5, 1 mM DTT, 10% glycerol and 1 complete protease inhibitor cocktail tablet per 100 ml) at 4°C for 24 hrs and protein concentration was determined using Bio-Rad assay. The recombinant protein was stored at −80°C.
Generation of TcMAPK2 and Phospho-TcMAPK2 antibodies.
To generate a polyclonal antibody against TcMAPK2, recombinant protein was used to immunize mice with Freund's adjuvant (Sigma, St. Louis, MO) at a 1:1 dilution with boosting at 4 and 12 weeks. The specificity of this antiserum was confirmed by immunoblot. To generate a polyclonal antibody against Phospho-TcMAPK2 at the double phosphorylation sites, phospho-peptide ARPALT* DY* IMTR [* = modified by phosphorylation] was generated and used to immunized rabbits using a standard commercial immunization protocol (Proteintech Group, Inc., Chicago, IL). These antibodies were tested by immunoblot against mammalian cell lysate (L6E9) and did not find any cross reactivity. All antibodies were produced under protocols approved by our Institutional Animal Use and Care Committee (IACUC).
Immunoblotting and immunoprecipitation.
Immunoblotting for T. cruzi lysates were described previously.31,32 Briefly, samples of lysates from the three life stages of T. cruzi (50 µg total protein per lane for anti-TcMAPK2 and 20 µg per lane for anti-phospho-TcMAPK2) were resolved on a 10% SDS-polyacrylamide gel and transferred onto nitrocellulose membrane, blocked with 5% non-fat milk and detected with appropriate primary antibodies by using anti-TcMAPK2, antiphospho-TcMAPK2 (both by 1:1,000 dilution) and secondary antibody conjugated with alkaline phosphatase (1:5,000 dilution for anti-TcMAPK2 and 1:10,000 for anti-phospho-TcMAPK2), and then visualized by using BCIP/NBT as substrate (Roche, Bavaria, Germany) using standard techniques. To dtermine whether the anti-TcMAPK2 can pull down native active kinase in parasite lysate, immunoprecipitation and kinase assay were performed. Parasites suspension in 2 ml, 6 × 106 of trypomastigotes or 2 × 109 epimastigotes, in buffer A (20 mM Tris-HCL pH 7.5, 1 protease inhibitor cocktail tablet per 5 ml) were sonicated and centrifuged at 1,500 g to precipitate insoluble materials. A TcMAPK2 antibody was added the supernatants at a 1:100 dilution and incubated at 4°C overnight with a rocking motion. Pre-immune mouse serum was used as a negative control. The antibody-antigen complexes were then isolated by incubating the reaction mixture with protein A-agarose, followed by centrifugation at 10,000x g and washing three times with buffer A prior to assays. The protein A-agarose containing the precipitated native TcMAPK2 was then resuspended in buffer A and used to perform the kinase assay using Myelin basic protein (MBP) (Sigma, St. Louis, MO) as a substrate.
Immunofluorescence analysis (IFA).
Briefly, the three forms of T. cruzi (CL Brener) as well as T. cruzi infected L6E9 monolayer were fixed with 4% paraformaldehyde, adhered to poly-L-lysine coverslips, permeabilized for 5 min with Dulbecco's phosphate-buffered saline (PBS), 0.3% Triton X-100, blocked for 1 h with PBS, 3% bovine serum albumin, 1% fish gelatin, 5% goat serum, 50 mM NH4Cl, and then incubated with primary antibody (anti-TcMAPK2, 1:10) for 1 h at 37°C, washed three times in PBS and then incubated with secondary antibody (goat anti-mouse IgG Fluoresceiin linked, 1:5,000) for 45 min at 37°C. Coverslips were then washed three times in PBS, incubated with 40, 6-diamidino-2-phenylindole-2 HCl (DAPI) solution (5 ug/ml, Molecular Probes) to allow visualization of nuclei and kinetoplasts. Subsequently, 200 µl of DABCO solution 2.5% of DABCO [1, 4-diazabicyclo (2.2.2) octane (Sigma, St. Louis, MO) and 20% of glycerol in PBS] was placed on the slides. The slides were examined using a Fluorescence microscope (Nikon, Model: HB-10101AF, using a 100X oil lens) with Fluoresceiin (Nikon filter cube: UV2B) and blue filter (Nikon filter cube: B2A) sets. Pre-immune serum or a secondary antibody alone was used as negative control.
Kinase assay.
To examine the kinase activities of purified recombinant TcMAPK2, kinase assays were performed. Briefly, 5 µg of recombinant TcMAPK2 was used in a 40-µl reaction containing 100 mM Tris-HCl, pH 7.5, 100 µM ATP, 1 mM MgCl2 and 4 µci [γ32P]ATP (3,000 ci/mMol, GE-Health Care, Fairfield, CT). Different concentrations of MBP were used as substrates. The reaction mixture was incubated at 30°C for 30 min and 10 µl of 5x Laemmli sample buffer was added into each reaction and boiled for 5 min and resolved in SDS-PAGE (12%). The gel was stained with Coomassie blue R250 and phosphorylation of MBP was detected by autoradiography.
To determine the enzyme kinetics, separate reactions were set up in which [γ-32P] ATP was added into cold ATP and specificity was then determined. The reaction conditions were as noted above. The reaction was stopped with EDTA and spotted onto P81 paper discs which were then washed with 0.75% phosphoric acid and acetone. Bound radioactivity was detected in a liquid scintillation counter. Enzymatic parameters were calculated using KaleidaGraph 4 software (Synergy Software).
In vitro phosphorylation.
Previously, we demonstrated that TcPKAc interacted with and phosphorylated TcMAPK2 and a TcPDEC2, (GenBank accession no. DQ008164). There are two putative consensuses sites that can be phosphorylated by TcMAPK2 (PVTP in 555–558; VTTP in 612–615). To verify that this TcPDEC2 could be phosphorylated by TcMAPK2 an in vitro phosphorylation assay was performed. The reaction mixture contained 25 mM Tris (pH 7.4), 10 mM MgCI2, 1 µCi [γ-32P] ATP (3,000 Ci/mmol) 1 ug of recombinant TcPDEC2 and 0.5 ug of recombinant TcMAPK2. A negative control was performed using the same reagents without the TcMAPK2 recombinant protein. The reactions were incubated in 30°C for 30 min and then 4 ul of 6x SDS sample buffer (60% Glycerol, 300 mM Tris (pH 6.8), 12 mM EDTA, 12% SDS, 864 mM 2-mercaptoethanol and 0.05% bromophenol blue) was added to each tube, which was heated at 100°C for 5 min and then analyzed by SDS-polyacrylamide gel electrophoresis under reducing conditions. Coomassie blue R250 staining was used to visualize the bands and loadings, followed by gel drying and autoradiography.
Overexpression of TcMAPK2.
The entire TcMAPK2 gene was subcloned into pTREX vector.33 pTREX and pTREX-TcMAPK2 were introduced into epimastigotes (CL Brener) by electroporation as previously described17 and the effect of overexpression of TcMAPK2 evaluated.
Acknowledgements
This work was supported by National Institutes of Health Grants AI 058893 (H.H.), AI076248.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12372
References
- 1.Tanowitz HB, Machado FS, Jelicks LA, Shirani J, de Carvalho AC, Spray DC, et al. Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease) Prog Cardiovasc Dis. 2009;51:524–539. doi: 10.1016/j.pcad.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reisenman CE, Lawrence G, Guerenstein PG, Gregory T, Dotson E, Hildebrand JG. Infection of kissing bugs with Trypanosoma cruzi, Tucson, Arizona USA. Emerg Infect Dis. 2010;16:400–405. doi: 10.3201/eid1603.090648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naula C, Seebeck T. Cyclic AMP Signaling in Trypanosomatides. Parasitol Today. 2000;16:35–38. doi: 10.1016/s0169-4758(99)01582-3. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson S, Vandekerckhove F, Frevert U, Nussenzweig V. The induction of Trypanosoma cruzi trypomastigote to amastigote transformation by low pH. Parasitol. 1995;110:547–554. doi: 10.1017/s0031182000065264. [DOI] [PubMed] [Google Scholar]
- 5.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 6.Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. The Inter J of Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Avruch J. MAPP kinase pathways: The first twenty years. BBA. 2007;1773:1150–1160. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacey MR, Brumlik MJ, Yenni RE, Burow ME, Curiel TJ. Toxoplasma gondii expresses two mitogen-activated protein kinase genes that represent distinct protozoan subfamilies. J Mol Evol. 2007;64:4–14. doi: 10.1007/s00239-005-0197-x. [DOI] [PubMed] [Google Scholar]
- 9.Dorin-Semblat D, Quashie N, Halbert J, Sicard A, Doerig C, Peat E, et al. Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Mol Microbiol. 2007;65:1170–1180. doi: 10.1111/j.1365-2958.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 10.Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol. 2005;58:1253–1263. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- 11.Rangarajan R, Bei AK, Jethwaney D, Maldonado P, Dorin D, Sultan AA, et al. A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei. EMBO Rep. 2005;6:464–469. doi: 10.1038/sj.embor.7400404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorin D, Semblat JP, Poullet P, Alano P, Goldring JP, Whittle C, et al. PfPK7, an atypical MEK-related protein kinase, reflects the absence of classical three-component MAPK pathways in the human malaria parasite Plasmodium falciparum. Mol Microbiol. 2005;55:184–196. doi: 10.1111/j.1365-2958.2004.04393.x. [DOI] [PubMed] [Google Scholar]
- 13.Lye YM, Chan M, Sim TS. Pfnek3: an atypical activator of a MAP kinase in Plasmodium falciparum. FEBS Lett. 2006;580:6083–6092. doi: 10.1016/j.febslet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Low H, Lye YM, Sim TS. Pfnek3 functions as an atypical MAPKK in Plasmodium falciparum. Biochem Biophys Res Commun. 2007;361:439–444. doi: 10.1016/j.bbrc.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Ellis JG, 4th, Davila M, Chakrabarti R. Potential involvement of extracellular signal-regulated kinase 1 and 2 in encystations of a primitive eukaryote, Giardia lamblia. J Biol Chem. 2003;278:1936–1945. doi: 10.1074/jbc.M209274200. [DOI] [PubMed] [Google Scholar]
- 16.Ohori M, Kinoshita T, Okubo M, Sato K, Yamazaki A, Arakawa H, et al. Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem Biophys Res Commun. 2005;336:357–363. doi: 10.1016/j.bbrc.2005.08.082. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Weiss LM, Braunstein VL, Huang H. The role of Protein kinase A in Trypanosoma cruzi. Infect Immun. 2008;76:4757–4763. doi: 10.1128/IAI.00527-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou GC, Bao ZQ, Dixon JE. Components of a new human protein-kinase signal-transduction pathway. J Biol Chem. 1995;270:12665–12669. doi: 10.1074/jbc.270.21.12665. [DOI] [PubMed] [Google Scholar]
- 19.Lee JD, Ulevitch RJ, Han J. Primary structure of BMK1: a new mammalian MAP kinase. Biochem Biophys Res Commun. 1995;213:715–724. doi: 10.1006/bbrc.1995.2189. [DOI] [PubMed] [Google Scholar]
- 20.Abe MK, Kuo WL, Hershenson MB, Rosner MR. Extracellular signal-regulated kinase 7 (ERK7), a novel ERK with a C-terminal domain that regulates its activity, its cellular localization and cell growth. Mol Cell Biol. 1999;19:1301–1312. doi: 10.1128/mcb.19.2.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abe MK, Saelzler MP, Espinosa R, III, Kahle KT, Hershenson MB, Le Beau MM, et al. ERK8, a new member of the mitogen-activated protein kinase family. J Biol Chem. 2002;277:16733–16743. doi: 10.1074/jbc.M112483200. [DOI] [PubMed] [Google Scholar]
- 22.Ellis J, Sarkar M, Hendriks E, Matthews K. A novel ERK-like, CRK-like protein kinase that modulates growth in Trypanosoma brucei via an autoregulatory C-terminal extension. Mol Microbiol. 2004;53:1487–1499. doi: 10.1111/j.1365-2958.2004.04218.x. [DOI] [PubMed] [Google Scholar]
- 23.Wiese M. Leishmania MAP kinases—familiar proteins in an unusual context. Int J Parasitol. 2007;37:1053–1062. doi: 10.1016/j.ijpara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Reyna NS, Yang Y. Molecular analysis of the rice MAP kinase gene family in relation to Magnaporthe grisea infection. Mol Plant Microbe Interact. 2006;19:530–540. doi: 10.1094/MPMI-19-0530. [DOI] [PubMed] [Google Scholar]
- 25.Wiese M. A mitogen-activated protein (MAP) kinase homologue of Leishmania mexicana is essential for parasite survival in the infected host. EMBO J. 1998;17:2619–2628. doi: 10.1093/emboj/17.9.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiese M, Kuhn D, Grünfelder CG. Protein kinase involved in flagellar-length control. Eukaryot Cell. 2003;2:769–777. doi: 10.1128/EC.2.4.769-777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Melzer MI, Kruse M, Juelch CS, Wiese M. LmxPK4, a mitogen-activated protein kinase (MAP) kinase homologue essential for promastigotes and amastigotes of Leishmania mexicana. Kinetoplastid Biol Dis. 2005;4:6. doi: 10.1186/1475-9292-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn D, Wiese M. LmxPK4, a mitogen-activated protein kinase kinase homologue of Leishmania mexicana with a potential role in parasite differentiation. Mol Microbiol. 2005;56:1169–1182. doi: 10.1111/j.1365-2958.2005.04614.x. [DOI] [PubMed] [Google Scholar]
- 29.Bengs F, Scholz A, Kuhn D, Wiese M. LmxMPK9, a mitogen-activated protein kinase homologue affects flagellar length in Leishmania mexicana. Mol Microbiol. 2005;55:1606–1615. doi: 10.1111/j.1365-2958.2005.04498.x. [DOI] [PubMed] [Google Scholar]
- 30.Erdmann M, Scholz A, Melzer IM, Schmetz C, Wiese M. Interacting protein kinase involved in the regulation of flagellar length. Mol Biol Cell. 2006;17:2035–2045. doi: 10.1091/mbc.E05-10-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, Werner C, Weiss LM, Wittner M, Orr GA. Molecular cloning and expression of the catalytic subunit of protein kinase A from Trypanosoma cruzi. Int J Parasitol. 2002;32:1107–1115. doi: 10.1016/s0020-7519(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 32.Huang H, Weiss LM, Nagajyothi F, Tanowitz HB, Wittner M, Orr GA, et al. Molecular cloning and characterization of the protein kinase A regulatory subunit of Trypanosoma cruzi. Mol Biochem Parasitol. 2006;149:242–245. doi: 10.1016/j.molbiopara.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez MP, Levin MJ. Functional analysis of the intergenic regions of TcP2beta gene loci allowed the construction of an improved Trypanosoma cruzi expression vector. Gene. 1999;239:217–225. doi: 10.1016/s0378-1119(99)00386-8. [DOI] [PubMed] [Google Scholar]