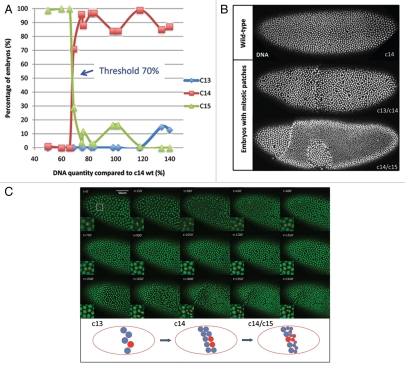
Cell cycle control plays a central role in the development and homeostasis of multicellular organisms. Mis-regulation of cell cycles often leads to abnormal tissue sizes and functions in development and can cause various diseases.1 Even though many pathways controlling the cell cycle machinery have been characterized, the signals regulating the cell cycle during development remain poorly understood. The stereotyped nuclear division pattern in the early Drosophila embryonic development has been a good model system to study how development and cell cycle are co-regulated.2 Drosophila embryos develop as a syncytium and undergo thirteen rounds of synchronous mitosis without cytokinesis generating about six thousand nuclei in a common cytoplasm. Subsequently, embryos pause in the interphase of cycle 14 for approximately 1 hour when cellularization, a specialized form of cytokinesis, encloses nuclei into cell membranes. After cellularization, embryos undergo rapid morphogenetic changes (gastrulation) and individual cells resume mitosis asynchronously forming different mitotic domains. The transition from the early synchronous division to cell cycle pause, onset of morphogenetic change and asynchronous cell cycle is similar to the transition observed in other metazoa and is known as “mid-blastula transition” (MBT). The mechanism controlling the onset of the MBT seems to monitor the nucleocytoplasmic (N/C) ratio,3,4 i.e., the ratio of DNA content to unknown cytoplasmic factor(s). During the early syncytial cycles, this ratio is low enough to allow the next nuclear division, such that the DNA content of the embryo continues to double with each cycle. It has been proposed that when this ratio passes a pre-set threshold, all the phenomena of MBT, including cell cycle arrest, take place. However, how nuclei sense or measure the N/C ratio and how they achieve the synchronous divisions preceding the MBT remain unknown. To address these questions, we have used a series of compound fly stocks to generate embryos with different amount of DNA, thus different values of N/C ratio during early divisions.5 We found that the threshold for the N/C ratio to trigger MBT is about 70% of wild-type DNA (Fig. 1A): above this threshold, embryos behave like wild-type embryo, pausing at interphase of cycle 14; below the threshold, embryos undergo one extra mitosis and pause in the interphase of cycle 15. We were intrigued to observe that embryos with N/C values close to the threshold frequently form mitotic patches, with one part pausing in one cycle and the rest undergoing an extra mitosis and pausing in the next cycle. Embryos from cross C(3R);F(3L) × C(3)EN, which gave highest percentage of patchy embryos, were collected, stained with Hoechst dye to visualize DNA and examined for geometric characteristics of the mitotic patches. Cycle 15 patches occupy on average 74.5% of the whole c14/c15 embryo area with standard deviation of ±12.7% (n = 12) while the position of the patches is variable (Fig. 1B). To explain the formation of the mitotic patches, we consider three possible models. First, in what we refer to as the “independence model,” individual nuclei make independent decisions for whether entering next division cycle. If the N/C ratio is measured and the decision to pause is made immediately at the MBT, the nuclear distributions in patchy embryos with threshold DNA contents should show a salt and pepper pattern. However the large mitotic patches actually observed are not compatible with such a model. Using a statistical mechanics model to analyze the distribution of random cluster sizes on a lattice,6 we show that the observed pattern of nuclear density is incompatible with the independent decisions made at cycle 14 (p value < 10^–240). An alternative explanation for the large patch size is that the N/C ratio is measured at an earlier stage and “remembered” in all the progeny of a given nucleus, resulting in a large patch of coherent lineage-related nuclei. To test this possibility, we introduced the Histone-GFP transgene into the compound stocks used to produce embryos with threshold DNA content and used live-imaging to determine if two sister nuclei descending from the same mother nucleus immediately prior to the MBT cell cycle decision always divide in the same way as would be predicted in the lineage model. We monitored the dynamics of patch formation in five embryos and focused our analysis on the nuclei at the boundary between the patches, since these nuclei can provide the required information to link lineage and different division behaviors. As shown in Figure 1C and Supplementary Movie 1, the mother nucleus labeled with a red dot divides into two daughter nuclei. Subsequently, one of the daughter nuclei divides again while the other arrests in interphase. This is the case for 4 out of 11 boundary nuclei we tracked. This observation argues that the decision to divide is made immediately before the MBT and that the coherent behavior of adjacent nuclei cannot be attributed to their common lineage. Instead, the coherent behavior suggests that the decision to arrest the cell cycle may be made collectively by neighboring nuclei through the exchange of some unknown molecule(s). Alternatively, it may reflect subtle local inhomogeneities in the cytoplasmic component of the N/C ratio that only impact the cell cycle decision when DNA content is close to threshold values.
Figure 1.
Patchy cell cycle pause during Drosophila mid-blastula transition. (A) Cell cycle behaviors of embryos with different amount of DNA compared with wild-type. Below the threshold of 70%, embryos undergo one extra division. Above the threshold, embryos pause at cycle 14 as wild-type. (B) Embryos with threshold DNA form mitotic patches during MBT. Embryos are fixed and stained with Hoechst. (C) Snapshot of a movie of mitotic patch formation. The white square region is enlarged for better visualization. Red dots label the lineage of a nucleus. The cartoon below depicts the lineage history of a nucleus colored in red at the patch boundary. Scale bar = 50 µm.
We favor the communication model because it provides the embryo with a mechanism ensuring coherent behavior across all nuclei during MBT even if individual nuclei make errors in the measurement of the N/C ratio or if the level of the cytoplasmic component is subject to local fluctuations. Communication between nuclei would dampen this randomness and may also synchronize the behavior of individual nuclei during the earlier stage of embryonic development, when nuclei undergo synchronous wave-like cleavage divisions. Similar community effects have also been suggested to generate homogeneity during Xenopus embryonic development and organismal level organization for cell cycle regulation may be a general feature of many organisms.7 It would be interesting to know the molecular nature of the exchanged information and if such inter-nuclear or inter-cellular signaling pathways are conserved. We suggest that molecular and genetic approaches might reveal the nature of the exchange molecules.
Acknowledgments
We thank all Wieschaus lab members or helpful discussion and Stefano Di Talia for manuscript revision. This work was supported by the Howard Hughes Medical Institute and by National Institute of Child Health and Human Development Grant 5R37HD15587.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12557
Supplementary Material
References
- 1.Hanahan D. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Foe V. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Newport J. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- 4.Edgar BA. Cell. 1986;44:365–372. doi: 10.1016/0092-8674(86)90771-3. [DOI] [PubMed] [Google Scholar]
- 5.Lu XM. Development. 2009;136:2101–2110. doi: 10.1242/dev.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leath PL. Physical Review B. 1976;14:5046–5055. [Google Scholar]
- 7.Gurdon JB. Cell. 1993;75:831–834. doi: 10.1016/0092-8674(93)90526-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.