Abstract
Advanced age is considered a risk factor for pancreatic cancer, but this relationship at the molecular and genetic level remains unclear. We present a clinical case series focusing on an association between pancreatic adenocarcinoma and Werner syndrome (WS) that is an autosomal recessive genetic disorder characterized by accelerated aging and cancer predisposition, and is caused by loss-of-function mutations in the WS RecQ helicase gene (WRN). Although pancreatic adenocarcinoma mostly occurs in a sporadic fashion, a minority of cases occurs in the context of susceptible individuals with hereditary syndromes. While WS has not been previously recognized as a risk factor for developing malignant tumors of the exocrine pancreas, the clinicopathologic features of three reported patients suggest a contributory role of WRN deficiency in pancreatic carcinogenesis. Molecular genetic analyses support the role of WRN as a tumor suppressor gene, although recent evidence reveals that WRN can alternatively promote oncogenicity depending on the molecular context. Based upon the clinico-pathologic features of these patients and the role of WRN in experimental models, we propose that its loss-of-function predisposes the development of pancreatic adenocarcinoma through epigenetic silencing or loss-of-heterozygosity of WRN. To test this hypothesis, we are investigating the mechanistic role of WRN in pancreatic cancer models including a pancreatic adenocarcinoma cell line generated from a human with WS. These studies are expected to provide new insight into the relationship between aging and pancreatic tumorigenesis, and facilitate development of novel strategies for patient-tailored interventions in this deadly malignancy.
Key words: Werner syndrome, WRN, RecQ helicase, exonuclease, pancreatic adenocarcinoma, tumor suppressor gene, adult-onset progeria, DNA repair syndrome
Introduction
Pancreatic cancer is a deadly malignancy with a rising incidence and a dismal prognosis.1 Until preventive interventions and early detection of pancreatic cancer are feasible, the development of efficacious therapy is critical to improve the survival of afflicted patients. The vast majority of patients die of metastatic disease that is refractory to the conventionally used cytotoxic chemotherapeutic agents such as gemcitabine, 5-fluorouracil, and capecitabine. Rational design of molecular target-based and patient-tailored therapy can be facilitated by translational approaches that utilize the genetic alterations contributing to pancreatic carcinogenesis.2,3 Conceivably, genes that influence the aging process may contribute to development of pancreatic neoplasia and potentially serve as novel therapeutic targets, since the majority of patients with pancreatic cancer are diagnosed in the geriatric population.
Pancreatic carcinogenesis involves senescence of pancreatic epithelia associated with deregulation of developmental pathways, oncogenes and tumor suppressors.4 Pancreatic intra-epithelial neoplasia (PanIN) initially exhibit genomic instability and senescence-associated telomere shortening.5,6 Progression of PanIN to invasive adenocarcinoma is accompanied by aberrant activation of Hedgehog signaling, oncogenic K-RAS mutations and inactivation of the tumor suppressors p16INK4A, SMAD4 and p53.7–9 Important insights into the molecular mechanisms that govern tumorigenesis have also been gained through genetic analyses of pancreatic cancer in the context of hereditary syndromes.
About 5–10% of pancreatic adenocarcinoma develops in patients with genetic syndromes that cause a hereditary predisposition to a variety of neoplasia.10,11 Werner syndrome (WS) is a disorder of accelerated aging with onset during adolescence that is inherited in an autosomal recessive fashion. It is caused by loss-of-function mutations of the WRN gene that encodes a DNA helicase of the RecQ family possessing an exonuclease domain.12–14 This syndrome manifests by the development of the signs and symptoms of medical problems typically associated with aging during the second decade of life accompanied by a predisposition to a variety of cancers.15–20 Cultured fibroblasts from WS patients exhibit genomic instability causing a variety of chromosomal abnormalities such as sister chromatid exchanges and variegated mosaicism.21,22 To date, WS has not been considered as a predisposing hereditary syndrome for exocrine pancreatic cancer. However, emerging cases describing pancreatic adenocarcinoma in patients with WS suggest a potential role of WRN in the initiation and progression of pancreatic neoplasia.23–25
In this clinical case series, we will compare the clinical, pathological and molecular features of the three reported cases of pancreatic adenocarcinoma in patients with WS. This will be followed by a review of the biochemical function, molecular genetics, and role of WRN as a tumor suppressor gene (TSG), and a discussion of the potential role of WRN silencing in the pathogenesis of pancreatic adenocarcinoma. The predicted use of WRN as a diagnostic and prognostic biomarker as well as a preventive and therapeutic target in pancreatic adenocarcinoma will be addressed.
Clinical Case Series
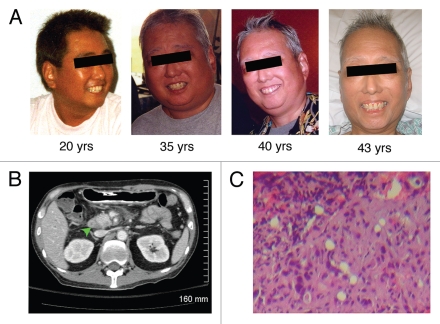
The clinical, pathological and molecular features of pancreatic cancer in three patients with WS are summarized in Table 1. All of these patients are of Japanese ethnicity consistent with the epidemiology of WS showing that greater than 75% of cases occur in this ethnic group.26 The high prevalence of WS in the Japanese population is attributed to multiple founder mutations of the WRN gene in mountainous regions of Japan where geography has historically limited gene flow, thereby allowing amplification of mutant alleles. These patients developed the classical signs of WS, including premature graying of hair, diffuse muscular and dermal atrophy, voice changes and classic “bird-like facies” (Fig. 1). Their medical history also includes cardiovascular disease, type 2 diabetes mellitus, bilateral cataracts and hypertension.
Table 1.
Clinical, pathologic and molecular features of WS patients with pancreatic tumors
| Age/Gender | Risk factors | Clinical presentation | Histopathology | Mutation of WRN | References |
| 43-y.o. male | none identified | Failure to thrive and obstructive jaundice | Moderately-differentiated ductal adenocarcinoma | 009, 039 | 25 |
| 53-y.o. male | not reported | Obstructive jaundice | Carcinoma | Not reported | 23 |
| 61-y.o. female | not reported | Obstructive jaundice | Papillary carcinoma | Not reported | 24 |
Note: Age refers to the age when pancreatic cancer was diagnosed. Clinical presentation preceding diagnosis of pancreatic tumor. Mutation of WRN refers to WS causative mutation number as listed in the Werner International Registry's mutational database (http://www.pathology.washington.edu/research/werner/database). y.o., years-old.
Figure 1.
The 43 year-old patient with WS developed accelerated aging with onset during the second decade of life and subsequently pancreatic adenocarcinoma. (A) on physical examination, the patient appeared older than his stated age and developed gray hair and “bird-like” facies due to atrophy of subcutaneous fat. (B) computed-tomographic image of the abdomen. The arrowhead points to the hypodense lesion/tumor in the head of pancreas. (C) histologic examination of the primary tumor in the pancreas revealed moderately-differentiated ductal adenocarcinoma.
The clinical presentations of pancreatic adenocarcinoma in these patients are typical, with obstructive jaundice caused by a tumor in the pancreatic head (Table 1). However, the average age of these WS patients (52.3 ± 9) is younger than the average age at which sporadic pancreatic adenocarcinoma is typically diagnosed during the seventh decade of life.27,28 A man with classic clinical findings of WS developed exocrine pancreatic cancer at age 53.23 Another woman with genetically confirmed WS developed pancreatic papillary ductal carcinoma at age 61, and she was successfully treated by pancreaticoduodenectomy.24 We have recently reported a case of metastatic pancreatic adencarcinoma in a 43-year-old man who was a compound heterozygote with two different loss-of-function mutations of WRN (Fig. 2).25 In our reported case, the pancreatic tumor was further characterized by molecular analyses. Of particular note in the 43-year-old patient's tumor was the absence of the oncogenic K-RAS mutations, especially as mutations that constitutively activate K-RAS signaling have been identified in more than 95% of sporadic pancreatic adenocarcinoma.4 Taken together, the clinicopathologic findings and molecular features of pancreatic adenocarcinoma in these patients with WS raise the possibility of a causal relationship between development of pancreatic adenocarcinoma and their underlying WS.
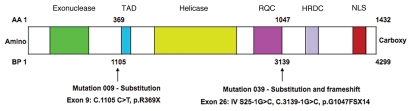
Figure 2.
A schematic diagram of WRN with the loss-of-function mutations identified in the 43-year-old patient who developed pancreatic adenocarcinoma. The WRN gene encodes a helicase of the RecQ family containing an exonuclease domain, and also possesses a transactivation domain (TAD), RecQ conserved domain (RQC), helicase and RNase D C-terminus domain (HRDC) and nuclear localization sequence (NLS). This patient possessed mutations 009 and 039 as defined by the Werner International Registry. Mutation 009 caused a stop codon at exon 9 through a cytosine-to-thymine transition (C1105T), leading to the substitution of arginine by a stop codon (R369stop), and resulting in the truncation of the helicase and exonuclease domains. Mutation 039 caused a guanine-to-cytosine transversion (G3139-1C) at the junction of intron 25 and exon 26, leading to the substitution of phenylalanine for glycine (G1047F), and resulting in a frameshift and insertion of a stop codon 14 amino acids distal to the nucleotide change that abolished the NLS domain. AA, amino acid; BP, DNA base pair.
DNA repair disorders such as hereditary breast and ovarian cancer syndrome, ataxia telangiectasia and Lynch syndrome cause a hereditary predisposition to develop pancreatic cancer.10 We propose that WS is a novel hereditary pancreatic cancer predisposition syndrome by causing a DNA repair defect and chromosomal instability in pancreatic epithelia (Table 2, Fig. 3). Although the possibility that pancreatic cancer and WS coincidentally occurred in these patients cannot be excluded, their early age of onset suggests that WRN mutations predisposed their cancer. Importantly, the absence of any identifiable risk factors in the 43-year-old whose pancreatic tumor harbored no oncogenic mutations in K-RAS argues for a role of WRN mutations in his cancer. Based on these observations, we hypothesize a causal relationship between loss-of-function of WRN and exocrine pancreatic cancer (Figs. 3 and 4). We are currently studying the functional significance of WRN in pancreatic neoplasia in order to test this hypothesis.
Table 2.
Genetic syndromes with mutations in repair of DN A damage as predisposing risk factors of progeria and/or cancer
| Disorders | Mutated protein | Progeria | Cancer | References |
| Werner syndrome | WRN RecQ helicase/exonuclease | Premature aging | Soft tissue sarcoma; gastric, colorectal, thyroid and pancreatic carcinoma | 22–25, 37, 46 |
| Fanconi anemia | BRCA2 | NA | Breast, leukemia, genitourinary, head and neck | 47 |
| Hereditary non-polyposis colon cancer | MSH and MLH | NA | Colon, gastric, genitourinary, ovarian, skin, hepatobiliary, pancreatic | 48 |
| Xeroderma Pigmentosum | *XPA, XPB, XPC, XPD , XPE , XPF and XPG | Premature aging | Skin, central nervous system | 50, 51 |
| Xeroderma Pigmentosum-DeSantis Cacchione's syndrome | XP-DSC | Premature aging | Internal organs | 52 |
| Cockayne's syndrome | CSA and CSB | Premature aging | NA | 53 |
| Bloom's syndrome | BLM | Premature cancer at early age | Breast, gastric, cervical, leukemia, skin, colorectal, hepatocellular | 54 |
| Rothmund-Thomson's syndrome | RECQ4 | Premature aging | Osteosarcoma, skin | 55 |
| Hutchinson-Gilford Progeria | Lamin A | Premature aging | NA | 56 |
| Trichothiodystrophy | XPD , XPB, TTDA | Premature aging | NA | 57 |
XP is mostly caused by mutations in XPA. NA, not associated. Note: In some of the above syndromes caused by defects in DN A damage repair, progeria is not associated with cancer predisposition. Tentatively, those afflicted individuals die at young age, such that the multi-step development of cancer that requires lengthy period of time is precluded. On the other hand, those syndromes predisposing cancer are not associated with progeria, suggesting complex genetic involvement in premature aging and cancer.
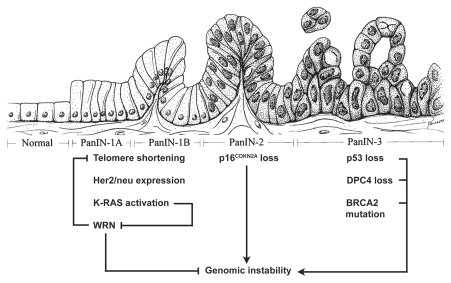
Figure 3.
A working model for the role of WRN in the multiple steps of pancreatic carcinogenesis. We propose that suppression of WRN expression by K-RAS activation contributes to telomere shortening and genomic instability that is observed in PanINs. WRN may also be silenced in PanINs through loss-of-heterozygosity, methylation of its CpG-promoter region, and the loss-of-function mutations seen in WS. DP C4, deleted in pancreatic cancer 4; BRCA2, the DN A repair enzyme causing hereditary breast and ovarian cancer syndrome. The illustrated diagram is modified and reprinted from Am J Pathol 2000, 156: 1821–1825 with permission from the American Society for Investigative Pathology. →, activates; ⊣, inhibits.
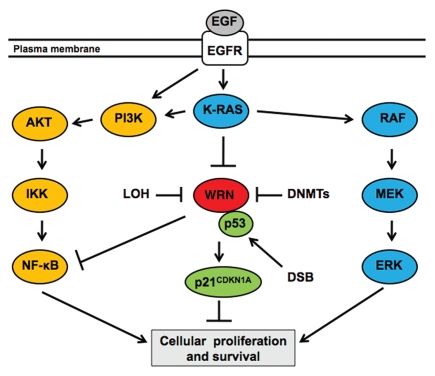
Figure 4.
A working model for the role of WRN in the signaling pathways involved in pancreatic cancer cells. The epidermal growth factor receptor (EGFR) pathway is a key mediator of cellular proliferation and survival in exocrine pancreas and pancreatic adenocarcinoma. We propose that downregulation of WRN expression by oncogenic K-RAS signaling, DNA methyltransferases (DNMTs) or a loss-of-heterozygosity (LOH), contributes to cellular proliferation and survival in pancreatic neoplasia by preventing p53-mediated transcription of p21CDKN1A and promoting the NF-κB signaling cascade. EGFR, receptor dimer; AKT, a serine/threonine-specific protein kinase; DSB, double-stranded DN A break; ER K, extracellular-signal related kinases; IKK, IkB kinase; MEK, mitogen-activated protein kinase; RAF, a protein serine/threonine protein kinase. →, activates; ⊣, inhibits.
Biochemical Functions and Molecular Genetics of WRN RecQ Helicase/Exonuclease
The WRN gene is located on chromosome 8p12, and it encodes a DNA helicase of the E. coli RecQ family. WRN is the only member of the RecQ helicase family that possesses an exonuclease domain.20 In an ATP-hydrolysis-dependent fashion, WRN unwinds the DNA double helix in the 3′ to 5′ direction, thereby promoting DNA replication during the S-phase of the cell cycle.12–14 During DNA synthesis, WRN binds to the large subunit of human replication protein A (RPA), resulting in the stimulation of its helicase activity.30 WRN favors interaction with particular DNA substrates such as forked DNA, Holliday junctions, D-loops, triplexes, G4 tetraplexes and partial duplexes, suggesting highly specific roles in DNA replication and transcription.22
Subsequent to unwinding DNA substrates, WRN proofreads DNA with its exonuclease domain that similarly excises DNA base pairs in a 3′ to 5′ direction. This exonuclease function enables repair of DNA damage by facilitating resolution of hindered DNA replication forks during synthesis of complicated sequences, or by base pair excision and recombination when DNA polymerase encounters an obstruction.22,31 Further supporting its dual roles in DNA replication and repair, the WRN protein is known to interact with DNA repair enzymes such as Polβ, Ku, DNA-PKcs, PARP-1 and APE1.22 In addition to directly proofreading DNA base pairs, WRN also plays roles in maintaining genomic integrity through a cell cycle “gatekeeper” function by inducing p53-dependent transcription of the cyclin-dependent kinase inhibitor p21CDKN1A.32 By both directly regulating the fidelity of the DNA template through base pair excision and recombination, and by modulating cell cycle gatekeepers, WRN is considered to be a “genome caretaker” that maintains the structure and integrity of genomic and somatic DNA in proliferating cells.
The structure of the WRN protein is large and complex, and ongoing efforts are underway to fully understand the functional significance of its structural domains. The WRN gene possesses 35 exons that encode a 1,432 amino acid protein with a molecular weight of 165 kDa. The carboxy (C)-terminus of the WRN protein contains its enzymatic functions, possessing both its helicase and exonuclease activity. Consistent with its enzymatic functions, a number of WRN mutations that truncate transcription of C-terminal exons are known to cause WS. The C-terminus has also been found to possess a transactivation domain located between its helicase and exonuclease regions, and its precise transcriptional targets are under investigation. While the C-terminus contains the enzymatic functions of WRN, its amino (N)-terminus contains its conserved RecQ helicase domain, nuclear localization signal, and p53 binding component. Although the majority of causative WS mutations occur in the C-terminus, mutations that abolish the nuclear localization domain in the N-terminus have also been reported to cause WS.22 While the biochemical functions of the WRN protein's structural domains have been characterized, further study is needed to establish whether their function is dependent upon specific cellular contexts. Of particular interest will be the precise transcriptional targets of WRN and whether it is a novel epigenetic modulator that allows transcriptional activation of TSGs by directly unwinding DNA substrates.
The biochemical roles of WRN as a helicase/exonuclease are supported by the clinical phenotype of patients with WS. In these individuals, proliferating tissues undergo premature senescence and neoplastic transformation, whereas non-proliferating tissues are spared from age-related pathologies. These clinical observations are supported by analysis of fibroblasts in cell culture from WS patients that exhibit decreased proliferative capacity, hindered progression through the S-phase of the cell cycle,33 and enhanced sensitivity to the clastogenic effects of DNA damaging agents such as ionizing radiation and DNA cross-linking agents. By contrast, the non-proliferating tissues are spared from senescent changes as suggested by the failure to develop senile dementia or cerebral atrophy in WS. For example, histologic analysis of the neural tissue from our recently reported patient with WS and pancreatic adenocarcinoma at autopsy lacked any signs of neuronal atrophy or neurodegenerative changes.25
These biochemical roles of WRN as a genome caretaker along with the tumor predisposition seen in WS support the rigorous study of its role in epithelial malignancies such as pancreatic adenocarcinoma.
Role of WRN in Tumorigenesis
Several lines of evidence suggest that WRN possesses a TSG function in the initiation, progression and maintenance of a variety of neoplasia. With respect to tumor initiation, the genomic instability in WS is consistent with the observation that patients develop a variety of malignancies including colorectal, thyroid, pancreatic and skin cancers, and they have a very high incidence of mesenchymal maligancies such as soft tissue sarcomas.17 Another role that WRN silencing may play in the initiation of neoplasia is by regulating telomere length, especially as shortened telomeres are characteristic of both pre-cancerous lesions and in WS.34,35 The physical interaction of WRN with telomeres allows the maintenance of telomere length,36 thereby preventing loss of telomere caps that normally protect chromosomes from genetic instability at their 3′ ends. Furthermore, forced exogenous expression of telomerase abolishes the WS phenotype in WRN-deficient mice, suggesting that the interaction of WRN with telomeres provides a key mechanism by which WRN prevents senescence and genomic instability. While the initiation of tumorigenesis is complex, evidence suggests that WRN acts in a pleiotropic fashion to suppress tumor initiation in humans and the myriad of cellular events that accompanies aging.
Recent evidence has suggested that WRN may act as a TSG in the progression and maintenance of established tumors in cancer cell lines in vivo.37 This finding is supported by the observation of frequent loss-of-heterozygosity (LOH) at the WRN loci located at chromosome 8p11.2-p12,29,38 as well as by the epigenetic silencing of WRN in multiple tumor types.37 In a panel of tumor specimens with epigenetically silenced WRN through methylation of CpG-islands of its promoter, forced ectopic overexpression of wild-type WRN reduced colony formation in vitro and growth of mouse tumor xenografts.37 However, in cancers with intact WRN signaling, WRN has been found to promote cell cycle progression and oncogenicity.36 These conflicting findings suggest that the role of WRN in neoplastic cells is context-dependent and that analysis of WRN expression may be necessary to develop patient-tailored therapy especially when using epigenetic modulators that affect WRN expression.
The ability of WRN to influence expression of genes involved in cell cycle progression such as inflammatory signaling cascades have recently been implicated as a potential mechanism by which it acts as a TSG.40 While expression of WRN potentially blocks cell cycle progression by enhancing p53-dependent transcription of p21CDKN1A,32 short interfering RNA (siRNA)-induced knockdown of WRN expression alternatively promotes cell cycle progression by inducing transcription of E2F family members.40 Consistent with a TSG role in neoplasia, expression of WRN can be suppressed by activation of RAS and the RB/E2F pathway.41 Furthermore, silencing of WRN with siRNA also induces the expression of other pro-oncogenic cascades such as MYC, IL-6 and NFκB signaling.40
The mechanism by which WRN suppresses oncogenesis may also involve cellular responses to free radical reactive oxygen species (ROS). Suppressing the formation of the free radical scavenger-reduced glutathione using 2-deoxy-glucose (2-DG) causes the up-regulation of WRN expression.42 This event is consistent with the observation that fibroblasts derived from patients with WS are resistant to hydrogen peroxide induced cell cycle arrest.43 Moreover, pre-clinical studies have shown the anti-tumor efficacy of 2-DG in combination with 5-fluorouracil, and ionizing radiation in pancreatic cancer.44 Further analysis of WRN signaling in the context of free radical biology will be necessary to clearly delineate its role in ROS-induced senescence,45 tumorigenesis and response to therapeutic small molecules and radiation.
Other genetic syndromes with mutations in enzymes involving DNA damage and repair have been implicated in premature aging and/or various human cancers (Table 2).46–57 Collectively, the loss of their enzymatic functions impairs the normal activities of these enzymes, resulting in genomic instability and cancer predisposition.58 Although population studies of heterozygous WRN loss-of-function mutations have not clearly indicated a risk of tumorigenesis, it has been observed that families of WS patients have a high rate of cancer.56 Further study of individuals carrying heterozygous loss-of-function WRN mutations will determine whether they can benefit from early cancer screening, as with other DNA repair disorders.
Potential Role of WRN in Pathogenesis and Treatment of Pancreatic Adenocarcinoma
Several lines of evidence suggest a contributory role of WRN silencing in malignant transformation of pancreatic ductal epithelia. First, chromosome 8p12 exhibits deletions and LOH in pancreatic neoplasia,59 implicating the loss of functional WRN in pancreatic adenocarcinoma. Second, activation of RAS and the RB/E2F pathway suppresses the expression of WRN,41 suggesting WRN is a downstream effector of RAS signaling. Third, genetic instability and telomere shortening occurs in greater than 90% of PanINs,6 and WRN may be involved in telomere maintenance and neoplastic transformation of pancreatic epithelia. Fourth, cigarette smoking is an environmental risk factor for developing tumors of the exocrine pancreas,60,61 and provided that cigarette smoke downregulates WRN in respiratory epithelia,61 a similar molecular phenomenon could occur in the exocrine pancreas. Taken together, these lines of evidence along with this clinical case series have provided rationale for our rigorous examination of the functional role of WRN in the pathogenesis of pancreatic neoplasia.
Currently, we are examining the expression level of WRN in human primary pancreatic cancer tissue, with the aim of determining whether WRN could serve as a clinical biomarker and a therapeutic target. Analysis of epigenetic silencing of WRN through mRNA expression and CpG-promoter methylation may yield prognostic information by identifying pancreatic tumors that are sensitive to DNA damaging agents.63 Cells deficient in WRN signaling have been proposed to be sensitive to DNA alkylating agents and γ-irradiation,29,64 and determination of relative WRN expression could provide valuable information to select patients whose tumors would respond to such therapies.63 Given a potential tumor suppressor role in pancreatic adenocarcinoma, WRN also represents a “druggable” therapeutic target as its expression is regulated by the DNA methyltransferase (DNMT) inhibitor 5-azacytidine,37 and potentially with histone deacetylase (HDAC) inhibitors.65 Our ongoing studies will clarify the role of WRN as a biomarker that may be useful in the development patient-tailored interventions for pancreatic adenocarcinoma.
In summary, numerous lines of evidence strongly suggest a contributory role of WRN in the initiation, progression and maintenance of pancreatic adenocarcinoma. Using pancreatic cancer models, we aim to delineate the molecular mechanisms by which WRN mediates its role. A pancreatic cancer cell line established from the hepatic metastasis of the 43-year-old patient with WS (Fig. 1, Table 1) will serve as a novel translational tool and provide an experimental platform to interrogate the milleu of inter-related mechanisms involving cancer and aging.25
Conclusion and Prospective
There are a few known risk factors associated with development of pancreatic adenocarcinoma, including aging, cigarette smoking and certain hereditary syndromes.66–84 As shown in the clinical case series in this report, pancreatic adenocarcinoma in patients with WS is rare, but increasingly being recognized (Table 1). We propose that WS is a heritable disease that predisposes affected individuals to develop pancreatic adenocarcinoma (Table 3). Moreover, we hypothesize that epigenetic silencing of WRN or its loss-of-function causes genomic instability that leads to neoplastic transformation in pancreatic ductal epithelia (Fig. 3), as well as the promotion of cellular proliferation in pancreatic neoplasia (Fig. 4). Analyzing the role of WRN silencing in PanINs is expected to help determine whether WRN is a feasible biomarker and/or target for intervention prior to their evolution to invasive adenocarcinoma for the goal of early detection and treatment.
Table 3.
Genetic syndromes with inherited predisposition to pancreatic cancer
| Familial syndrome | Gene product (locus) | References |
| Werner syndrome | WRN RecQ helicase/exonuclease 8p11.2-p12 | 23–25 |
| Peutz-Jeghers syndrome | STK11/LKB1 (19p13.3) | 10, 66 |
| Familial pancreatitis | PR SS/TRY1 (7q35) | 67, 68 |
| Familial atypical multiple mole melanoma | CDKN2A/p16/MTS1 (9p21) | 69 |
| Li-Fraumeni syndrome | p53 (17p13) | 70, 71 |
| Hereditary non-polyposis colorectal cancer (Lynch syndrome) | HMSH2 (2p22-21), hMLH1 (3p21.3) | 72 |
| Ataxia-telangiectasia | ATM (11q22-23) | 73, 74 |
| Hereditary breast-ovarian cancer syndrome | BRCA2 (13q12) | 75–78 |
| Cystic fibrosis carrier | CFTR1 (7q31) | 79, 80 |
| Familial adenomatous polyposis | APC (5q21) | 81, 82 |
| Family X of pancreatic insufficiency and diabetes mellitus | not identified (4q32-34) | 83, 84 |
Multiple DN A repair disorders cause a hereditary predisposition to the development of exocrine pancreatic cancer including Werner syndrome, Lynch syndrome, ataxia-telangiectasia and hereditary breast-ovarian cancer syndrome.
Pancreatic cancer remains one of the greatest oncologic challenges and novel therapeutic targets and an understanding of its molecular basis is desperately needed to advance care in this deadly malignancy. Currently, our research is actively focusing on the role of WRN in the pathogenesis of pancreatic neoplasia and how these findings can be translated into screening and therapeutic approaches for pancreatic adenocarcinoma. Results of these studies will have important clinical implications. First, relatively young patients with WS and early stage pancreatic adenocarcinoma are eligible for potentially curative surgical intervention. Second, if WRN acts as a TSG in pancreatic adenocarcinoma, it is a feasible pharmacologic target for epigenetic therapy with DNMT and HDAC inhibitors, 2-DG or other small molecules. Clarification of its precise molecular roles in pancreatic adenocarcinoma, as well as analysis of WRN expression in patient tumors, will form the basis to guide such approaches.
Acknowledgements
We wish to express our gratitude to the family of our reported patient for granting us permission to contribute this case to the medical literature and to establish a cell line. We thank Dr. Peter Bryant-Greenwood (University of Hawaii) for valuable discussion during the preparation of this manuscript. This work was supported by research grant 123-6260-4 from the Cancer Research Center of Hawaii of the University of Hawaii (S.G.C.), the Pilot Grant in Translational Research from the Department of Internal Medicine of the University of Iowa Carver College of Medicine (N.S.Y.), the Cancer Center Designated Gift Fund for pancreatic cancer research (N.S.Y.) and the Cancer Center Support Grant (P30 CA 086862) by the National Cancer Institute to the Holden Comprehensive Cancer Center at the University of Iowa (N.S.Y.).
Abbreviations
- WS
Werner syndrome
- WRN
Werner syndrome RecQ helicase gene or protein
- RecQ
E. coli recombination gene Q
- TSG
tumor suppressor gene
- HDAC
histone deacetylases
- DNMT
DNA methyltransferases
- LOH
loss-of-heterozygosity
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- PanIN
pancreatic intra-epithelial neoplasia
- CpG
cytosine in phosphodiester bond to guanine
- siRNA
small interfering RNA
- DSB
double-stranded DNA break
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/12763
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:15–20. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Strimpakos A, Saif MW, Syrigos KN. Pancreatic cancer: from molecular pathogenesis to targeted therapy. Cancer Met Rev. 2008;27:495–522. doi: 10.1007/s10555-008-9134-y. [DOI] [PubMed] [Google Scholar]
- 3.Yee NS. Zebrafish as a biological system for identifying and evaluating therapeutic targets and compounds. In: Han H, Grippo PJ, editors. Drug discovery in pancreatic cancer. New York: Springer; 2010. pp. 95–112. [Google Scholar]
- 4.Koorstra JBM, Hustinx SR, Offerhaus GJA, Maitra A. Pancreatic carcinogenesis. Pancreatology. 2008;8:110–125. doi: 10.1159/000123838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hruban RH, Wilentz RE, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol. 2000;156:1821–1825. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaino M. Alterations in the tumor suppressor genes p53, RB, p16/MTS1 and p15/MTS2 in human pancreatic cancer and hepatoma cell lines. J Gastroenterol. 1997;32:40–46. doi: 10.1007/BF01213295. [DOI] [PubMed] [Google Scholar]
- 8.Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56:1134–1152. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun SG, Zhou W, Yee NS. Combined targeting of histone deacetylases and hedgehog signaling enhances cytotoxicity in pancreatic cancer. Cancer Biol Ther. 2009;8:1328–1339. doi: 10.4161/cbt.8.14.8633. [DOI] [PubMed] [Google Scholar]
- 10.Yee NS, Furth EE, Pack M. Clinicopathologic and molecular features of pancreatic adenocarcinoma associated with Peutz-Jeghers syndrome. Cancer Biol Ther. 2003;2:38–47. doi: 10.4161/cbt.191. [DOI] [PubMed] [Google Scholar]
- 11.Lochan R, Daly AK, Reeves HL, Charnley RM. Genetic susceptibility in pancreatic ductal adenocarcinoma. Br J Surg. 2008;95:22–32. doi: 10.1002/bjs.6049. [DOI] [PubMed] [Google Scholar]
- 12.Gray MD, Shen JC, Kamath-Loeb AS, Blank A, Sopher BL, Martin GM, et al. The Werner syndrome protein is a DNA helicase. Nat Genet. 1997;17:100–103. doi: 10.1038/ng0997-100. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki N, Shimamoto A, Imamura O, Kuromitsu J, Kitao S, Goto M, Furuichi Y. DNA helicase activity in Werner's syndrome gene product synthesized in a baculovirus system. Nucleic Acids Res. 1997;25:2973–2978. doi: 10.1093/nar/25.15.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S, Li B, Gray MD, Oshima J, Mian IS, Campisi J. The premature ageing syndrome protein, WRN, is a 3′→5′ exonuclease. Nat Genet. 1998;20:114–116. doi: 10.1038/2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salk D. Werner's syndrome: a review of recent research with an analysis of connective tissue metabolism, growth control of cultured cells and chromosomal aberrations. Hum Genet. 1982;62:1–5. doi: 10.1007/BF00295598. [DOI] [PubMed] [Google Scholar]
- 16.Yu CE, Oshima J, Fu YH, Wijsman EM, Hisama F, Alisch R, et al. Positional cloning of the Werner's syndrome gene. Science. 1996;272:258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- 17.Goto M, Miller RW, Ishikawa Y, Sugano H. Excess of rare cancers in Werner syndrome (adult progeria) Cancer Epidemiol Biomarkers Prev. 1996;5:239–246. [PubMed] [Google Scholar]
- 18.Yu CE, Oshima J, Wijsman EM, Nakura J, Miki T, Piussan C, et al. Mutations in the consensus helicase domains of the Werner syndrome gene. Werner's Syndrome Collaborative Group. Am J Hum Genet. 1997;60:330–341. [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto T, Imamura O, Yamabe Y, Kuromitsu J, Tokutake Y, Shimamoto A. Mutation and haplotype analyses of the Werner's syndrome gene based on its genomic structure: genetic epidemiology in the Japanese population. Hum Genet. 1997;100:123–130. doi: 10.1007/s004390050477. [DOI] [PubMed] [Google Scholar]
- 20.Hickson ID. RecQ helicases: caretakers of the genome. Nat Rev Cancer. 2003;3:169–178. doi: 10.1038/nrc1012. [DOI] [PubMed] [Google Scholar]
- 21.Thweatt R, Goldstein S. Werner syndrome and biological ageing: a molecular genetic hypothesis. Bioessays. 1993;15:421–426. doi: 10.1002/bies.950150609. [DOI] [PubMed] [Google Scholar]
- 22.Ozgenc A, Loeb LA. Werner Syndrome, Aging and Cancer. Genome Dyn. 2006;1:206–217. doi: 10.1159/000092509. [DOI] [PubMed] [Google Scholar]
- 23.Toyoshima H. A case of Werner's syndrome with pancreatic carcinoma. Nishinihon J Dermatol. 1986;49:157. [Google Scholar]
- 24.Shimaoka Y, Hatamochi A, Hamasaki Y, Yamazaki S, Hiraishi H, Hattori Y, et al. Case of Werner's syndrome with pancreatic carcinoma. J Dermatol. 2007;34:674–676. doi: 10.1111/j.1346-8138.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 25.Chun SG, Yee NS, Holland JM, Shohet RV, Palalay MP, Bryant-Greenwood PK. Pancreatic adenocarcinoma associated with Werner's syndrome (adult-onset progeria) Gastrointest Cancer Res. 2010 In press. [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh M, Imai M, Sugimoto M, Goto M, Furuichi Y. Prevalence of Werner's syndrome heterozygotes in Japan. Lancet. 1999;353:1766. doi: 10.1016/S0140-6736(98)05869-3. [DOI] [PubMed] [Google Scholar]
- 27.Gold EB, Goldin SB. Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am. 1998;7:67–91. [PubMed] [Google Scholar]
- 28.Lüttges J, Stigge C, Pacena M, Klöppel G. Rare ductal adenocarcinoma of the pancreas in patients younger than age 40 years. Cancer. 2004;100:173–182. doi: 10.1002/cncr.11860. [DOI] [PubMed] [Google Scholar]
- 29.Sidorova JM, Li N, Folch A, Monnat RJ., Jr The RecQ helicase WRN is required for normal replication fork progression after DNA damage or replication fork arrest. Cell Cycle. 2008;7:796–807. doi: 10.4161/cc.7.6.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen JC, Lao Y, Kamath-Loeb A, Wold MS, Loeb LA. The N-terminal domain of the large subunit of human replication protein A binds to Werner syndrome protein and stimulates helicase activity. Mech Ageing Dev. 2003;124:921–930. doi: 10.1016/s0047-6374(03)00164-7. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Yook JS, Han SM, Koo HS. A Werner syndrome protein homolog affects C. elegans development, growth rate, life span and sensitivity to DNA damage by acting at a DNA damage checkpoint. Development. 2004;131:2565–2575. doi: 10.1242/dev.01136. [DOI] [PubMed] [Google Scholar]
- 32.Blander G, Kipnis J, Leal JF, Yu CE, Schellenberg GD, Oren M. Physical and functional interaction between p53 and the Werner's syndrome protein. J Biol Chem. 1999;274:29463–29469. doi: 10.1074/jbc.274.41.29463. [DOI] [PubMed] [Google Scholar]
- 33.Martin GM, Sprague CA, Epstein CJ. Replicative lifespan of cultivated human cells. Effects of donor's age, tissue and genotype. Lab Invest. 1970;23:86–92. [PubMed] [Google Scholar]
- 34.Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 35.Opresko PL. Telomere ResQue and preservation—roles for the Werner syndrome protein and other RecQ helicases. Mech Ageing Dev. 2008;129:79–90. doi: 10.1016/j.mad.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Opresko PL, Calvo JP, von Kobbe C. Role for the Werner syndrome protein in the promotion of tumor cell growth. Mech Ageing Dev. 2007;128:423–436. doi: 10.1016/j.mad.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Agrelo R, Cheng WH, Setien F, Ropero S, Espada J, Fraga MF, et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc Natl Acad Sci USA. 2006;103:8822–8827. doi: 10.1073/pnas.0600645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chughtai SA, Crundwell MC, Cruickshank NR, Affie E, Armstrong S, Knowles MA, et al. Two novel regions of interstitial deletion on chromosome 8p in colorectal cancer. Oncogene. 1999;18:657–665. doi: 10.1038/sj.onc.1202340. [DOI] [PubMed] [Google Scholar]
- 39.Armes JE, Hammet F, de Silva M, Ciciulla J, Ramus SJ, Soo WK, et al. Candidate tumor-suppressor genes on chromosome arm 8p in early-onset and high-grade breast cancers. Oncogene. 2004;23:5697–5702. doi: 10.1038/sj.onc.1207740. [DOI] [PubMed] [Google Scholar]
- 40.Turaga RVN, Paquet ER, Sild M, Vignard J, Garand C, Johnson FB, et al. The Werner syndrome protein affects the expression of genes involved in adipogenesis and inflammation in addition to cell cycle and DNA damage responses. Cell Cycle. 2009;8:2080–2092. doi: 10.4161/cc.8.13.8925. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, El-Naggar S, Clem B, Chesney J, Dean DC. The Rb/E2F pathway and Ras activation regulate RecQ helicase gene expression. Biochem J. 2008;412:299–306. doi: 10.1042/BJ20070975. [DOI] [PubMed] [Google Scholar]
- 42.Zhou B, Ikejima T, Watanabe T, Iwakoshi K, Idei Y, Tanuma S, Uchiumi F. The effect of 2-deoxy-D-glucose on Werner syndrome RecQ helicase gene. FEBS Lett. 2009;583:1331–1336. doi: 10.1016/j.febslet.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Von Kobbe C, May A, Grandori C, Bohr VA. Werner syndrome cells escape hydrogen peroxide-induced cell proliferation arrest. FASEB J. 2004;18:1970–1972. doi: 10.1096/fj.04-1895fje. [DOI] [PubMed] [Google Scholar]
- 44.Coleman MC, Asbury CR, Daniels D, Du J, Aykin-Burns N, Smith BJ, et al. 2-deoxy-D-glucose causes cytotoxicity, oxidative stress and radiosensitization in pancreatic cancer. Free Radic Biol Med. 2008;44:322–331. doi: 10.1016/j.freeradbiomed.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 45.Gosselin K, Martien S, Pourtier A, Vercamer C, Ostoich P, Morat L, et al. Senescence-associated oxidative DNA damage promotes the generation of neoplastic cells. Cancer Res. 2009;69:7917–7925. doi: 10.1158/0008-5472.CAN-08-2510. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Oshima J. Werner Syndrome. J Biomed Biotechnol. 2002;2:46–54. doi: 10.1155/S1110724302201011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alter BP. Cancer in Fanconi anemia 1927–2001. Cancer. 2003;97:425–440. doi: 10.1002/cncr.11046. [DOI] [PubMed] [Google Scholar]
- 48.Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA. 2006;296:1507–1517. doi: 10.1001/jama.296.12.1507. [DOI] [PubMed] [Google Scholar]
- 49.Hollander MC, Philburn RT, Patterson AD, Velasco-Miguel S, Friedberg EC, et al. Deletion of XPC leads to lung tumors in mice and is associated with early events in human lung carcinogenesis. Proc Natl Acad Sci USA. 2005;102:13200–13205. doi: 10.1073/pnas.0503133102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rapin I, Lindenbaum Y, Dickson DW, Kraemer KH, Robbins JH. Cockayne syndrome and xeroderma pigmentosum. Neurology. 2000;55:1442–1449. doi: 10.1212/wnl.55.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Sanctis C, Cacchione A. L' Idiozia xerodermica. Riv Sperm Freniatr. 1932;56:269–292. [Google Scholar]
- 52.van der Horst GT, van Steeg H, Berg RJ, van Gool AJ, de Wit J, Weeda G, et al. Defective transcription-coupled repair in Cockayne syndrome B mice is associated with skin cancer predisposition. Cell. 1997;89:425–435. doi: 10.1016/s0092-8674(00)80223-8. [DOI] [PubMed] [Google Scholar]
- 53.Hoeijmakers JH. DNA damage, aging and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 54.German J. Bloom's syndrome. XX. The first 100 cancers. Cancer Genet Cytogenet. 1997;93:100–106. doi: 10.1016/s0165-4608(96)00336-6. [DOI] [PubMed] [Google Scholar]
- 55.Kellermayer R. The versatile RECQL4. Genet Med. 2006;8:213–216. doi: 10.1097/01.gim.0000214457.58378.1a. [DOI] [PubMed] [Google Scholar]
- 56.Kudlow BA, Kennedy BK, Monnat RJ., Jr Werner and Hutchinson-Gilford progeria syndromes: mechanistic basis of human progeroid diseases. Nat Rev Mol Cell Biol. 2007;8:394–404. doi: 10.1038/nrm2161. [DOI] [PubMed] [Google Scholar]
- 57.Stefanini M, Botta E, Lanzafame M, Orioli D. Trichothiodystrophy: from basic mechanisms to clinical implications. DNA Repair (Amst) 2010;9:2–10. doi: 10.1016/j.dnarep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 59.Ryu B, Song J, Sohn T, Hruban RH, Kern SE. Frequent germline deletion polymorphism of chromosomal region 8p12-p21 identified as a recurrent homozygous deletion in human tumors. Genomics. 2001;72:108–112. doi: 10.1006/geno.2000.6449. [DOI] [PubMed] [Google Scholar]
- 60.Fuchs CS, Colditz GA, Stampfer MJ, Giovannucci EL, Hunter DJ, Rimm EB, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156:2255–2260. [PubMed] [Google Scholar]
- 61.Schenk M, Schwartz AG, O'Neal E, Kinnard M, Greenson JK, Fryzek JP, et al. Familial risk of pancreatic cancer. J Natl Cancer Inst. 2001;93:640–644. doi: 10.1093/jnci/93.8.640. [DOI] [PubMed] [Google Scholar]
- 62.Nyunoya T, Monick MM, Klingelhutz AL, Glaser H, Cagley JR, Brown CO, et al. Cigarette smoke induces cellular senescence via Werner's syndrome protein downregulation. Am J Respir Crit Care Med. 2009;179:279–287. doi: 10.1164/rccm.200802-320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hegi ME, Sciuscio D, Murat A, Levivier M, Stupp R. Epigenetic deregulation of DNA repair and its potential for therapy. Clin Cancer Res. 2009;15:5026–5031. doi: 10.1158/1078-0432.CCR-08-1169. [DOI] [PubMed] [Google Scholar]
- 64.Dong YP, Seki M, Yoshimura A, Inoue E, Furukawa S, Tada S, Enomoto T. WRN functions in a RAD18dependent damage avoidance pathway. Biol Pharm Bull. 2007;30:1080–1083. doi: 10.1248/bpb.30.1080. [DOI] [PubMed] [Google Scholar]
- 65.Li K, Wang R, Lozada E, Fan W, Orren DK, Luo J. Acetylation of WRN protein regulates its stability by inhibiting ubiquitination. PLoS One. 2010;5:10341. doi: 10.1371/journal.pone.0010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 67.Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK, Jr, Perrault J, Whitcomb DC. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 68.Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–170. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 69.Vasen HF, Gruis NA, Frants RR, van Der Velden PA, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden) Int J Cancer. 2000;87:809–811. [PubMed] [Google Scholar]
- 70.Landi S. Genetic predisposition and environmental risk factors to pancreatic cancer: A review of the literature. Mutat Res. 2009;681:299–307. doi: 10.1016/j.mrrev.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Lefrou L, Godart B, de Muret A, Scotto B, Dorval E. Germline tp53 neomutation in a patient with Li-Fraumeni syndrome and pancreatic adenocarcinoma. Gastroenterol Clin Biol. 2006;30:484–486. doi: 10.1016/s0399-8320(06)73212-2. [DOI] [PubMed] [Google Scholar]
- 72.Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis and medicolegal ramifications. Clin Genet. 2009;76:1–18. doi: 10.1111/j.1399-0004.2009.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swift M, Sholman L, Perry M, Chase C. Malignant neoplasms in the families of patients with ataxiatelangiectasia. Cancer Res. 1976;36:209–215. [PubMed] [Google Scholar]
- 74.Lynch HT, Deters CA, Lynch JF, Brand RE. Familial pancreatic carcinoma in Jews. Fam Cancer. 2004;3:233–240. doi: 10.1007/s10689-004-9549-8. [DOI] [PubMed] [Google Scholar]
- 75.Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996;56:5360–5364. [PubMed] [Google Scholar]
- 76.Lal G, Liu G, Schmocker B, Kaurah P, Ozcelik H, Narod SA, et al. Inherited predisposition to pancreatic adenocarcinoma: role of family history and germ-line p16, BRCA1 and BRCA2 mutations. Cancer Res. 2000;60:409–416. [PubMed] [Google Scholar]
- 77.Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–3793. [PubMed] [Google Scholar]
- 78.Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214–221. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 79.Neglia JP, FitzSimmons SC, Maisonneuve P, Schöni MH, Schöni-Affolter F, Corey M, Lowenfels AB. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N Engl J Med. 1995;332:494–499. doi: 10.1056/NEJM199502233320803. [DOI] [PubMed] [Google Scholar]
- 80.Maisonneuve P, Marshall BC, Lowenfels AB. Risk of pancreatic cancer in patients with cystic fibrosis. Gut. 2007;56:1327–1328. doi: 10.1136/gut.2007.125278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 82.Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394–1396. doi: 10.1136/gut.34.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eberle MA, Pfützer R, Pogue-Geile KL, Bronner MP, Crispin D, Kimmey MB, et al. A new susceptibility locus for autosomal dominant pancreatic cancer maps to chromosome 4q32-4. Am J Hum Genet. 2002;70:1044–1048. doi: 10.1086/339692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Earl J, Yan L, Vitone LJ, Risk J, Kemp SJ, McFaul C, et al. Evaluation of the 4q32-34 locus in European familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1948–1955. doi: 10.1158/1055-9965.EPI-06-0376. [DOI] [PubMed] [Google Scholar]