Abstract
Fibroblast Growth Factors (FGFs) have been implicated in malignant transformation, tumor mitogenesis, angiogenesis and chemoresistance. The aim of this study was to determine which FGFs and FGFRs play functional roles in epithelial ovarian cancer. Restriction enzyme analysis of mRNA revealed that transformation was associated with a switch in FGFR2 and FGFR3, from the IIIc to the IIIb isoform. There was widespread expression of FGFs, including FGF7, in all tissues but, FGF3 and FGF19 were expressed by malignant cell lines and cancer tissue but were not present in normal tissue. Using FGFR-specific shRNAi we demonstrated that reductions in FGFR2 inhibited proliferation of ovarian cancer cell lines in vitro (>50%, p < 0.006) and reduced cisplatin IC50 (>60%, p < 0.0001). Cell cycle analysis revealed increased cisplatin sensitivity was associated with increased G2/M arrest and increased apoptosis. FGFR2 shRNAi reduced growth rates of ovarian tumor xenografts by 20% (p < 0.006) and when combined with cisplatin caused a 40% reduction in proliferation rates (p < 0.007). In contrast, RNAi-induced reductions in FGFR1 increased SKOV3 cell numbers, with associated changes in cell cycle but had no effect on ES 2 cells. However, the cisplatin IC50 was reduced (>50%, p < 0.0001) by FGFR1 shRNAi in both cell lines and there was increased apoptosis (46–50%) compared with control cells (35%) (p < 0.004). Together our data suggest that combining FGFR2 inhibitors with platinum-containing cytotoxic agents for the treatment of epithelial ovarian cancer may yield increased antitumor activity. However, data on the inhibition of FGFR1 suggest that broad spectrum FGFR inhibitors may have unexpected effects on proliferation.
Key words: ovarian cancer, fibroblast growth factor, fibroblast growth factor receptor, shRNAi, cisplatin
Introduction
Ovarian cancer remains the commonest cause of gynaecological cancer death, despite advances in treatment over the last 40 years.1 The limited efficacy of cytotoxic chemotherapy remains a key obstacle for the treatment of patients with advanced ovarian cancer.
The Fibroblast Growth Factors (FGFs) have been implicated in malignant transformation, tumor mitogenesis, angiogenesis and chemoresistance. To date 23 FGFs have been isolated.2 Signal transduction is mediated through five FGF receptors (FGFRs). FGFR-1, -2 and -3 exist in a number of different isoforms, IIIa, IIIb and IIIc, generated through alternative splicing. The IIIb and IIIc isoforms are generated by the differential inclusion of one of two exons at the -COOH terminal half of Ig domain III, which confers specificity of FGF binding.3
There have been a number of studies of the FGF-FGFR family in ovarian cancer. PCR studies showed that the FGF3 gene was amplified in 20% of ovarian cancer samples and that this was significantly associated with FigO stage but not with overall survival.4–6 Normal ovarian surface epithelium does not express the mRNA for FGFR2 IIIb but does express FGF1 and FGF7. In contrast, FGFR2 IIIb is expressed in 80% of epithelial ovarian carcinomas, whereas FGF1 and FGF7 were only expressed in 20% and 60% of epithelial ovarian carcinomas, respectively.7 An immortalized normal ovarian surface epithelial cell line did not respond to the addition of FGF 1, 7 or 10, whereas a significant increase in cell viability was observed for a number of ovarian cancer cell lines when exposed to all three ligands.8 The fact that the ovarian cancer cell lines responded to FGF7 suggests that they have an active FGFR2 IIIb isoform as this is the only receptor that mediates FGF7 signaling.9 Other studies have demonstrated that several other members of the FGF-FGFR family are present and, that in some cases they may have prognostic significance.10–12 However, these studies have evaluated only selected FGFs and FGFRs. Here we present the first comprehensive observational and functional studies of the FGF-FGFR family in epithelial ovarian cancer.
Results
FGF and FGF receptor RNA expression.
FGF receptor expression was studied using in situ hybridization (10 serous ovarian carcinomas and 5 normal ovaries) and immunohistochemistry (40 serous ovarian carcinomas and 10 normal ovaries) to preserve the architecture of the tissues. We demonstrated that the mRNA and protein for all the FGF receptors was expressed in the tumors (only FGFR2 shown Fig. 1A and C). FGFR2 and FGF7 (Fig. 1G) protein expression was mainly in the tumor however there was some expression in the stroma, whereas FGF3 (Fig. 1E) was expressed only in the tumor nucleus.
Figure 1.
Distribution of FGFR2, FGF 3 and FGF7 mRNA and protein. FGFR2 ISH antisense probe [positive] showed widespread deep purple staining which indicated that tumor cells expressed FGFR2 mRNA (A). FGFR2 ISH sense [negative] probe showed minimal staining (B). The protein distribution of FGFR2, FGF3 and FGF7 was also investigated in ovarian tumors using DAB+ (brown). For these experiments, the nuclei were counter-stained with haematoxylin [blue]. FGFR2 IHC showed widespread deep brown staining which indicated that tumor cells expressed FGFR2 protein (C). FGFR2 IHC antibody isotype negative control (D). FGF3 IHC showed widespread deep brown staining in the tumor cell nuclei (E). FGF3 IHC antibody isotype negative control (F). FGF7 IHC showed widespread deep brown staining in the tumor (G). FGF7 IHC antibody isotype negative control (H). Serous ovarian carcinomas were used for staining with 10 separate tumors for ISH and 40 tumors for IHC. Five normal ovaries for ISH and 10 normal ovary for IHC were also used.
This approach did not distinguish between the different FGF receptor isoforms and so the differential sensitivity of cDNA to restriction enzymes was examined (Fig. 2A). Ovarian tumor material from patients with serous ovarian carcinoma and normal ovary expressed FGFR1 IIIc, FGFR4 and FGFR5 mRNA. However, an isoform switch from FGFR2 IIIc and FGFR3 IIIc in normal ovarian RNA to the IIIb isoforms in ovarian tumors and ovarian carcinoma cell lines (CAOV3, ES2, OVCAR3 and SKOV3) was detected (Fig. 2B). The FGFR2 isoform switch endows ovarian carcinoma with the ability to respond to FGFs 3, 7 and 10, therefore the response to these cytokines was investigated in vitro. When grown in 0.2% serum ovarian carcinoma cell lines (ES2 and SKOV3) proliferate in response to FGF3 and 7 (10–100 ng/ml) (Fig. 3). FGF7 generally stimulates more proliferation as compared with FGF3 in both cell lines which may be due to FGF7 being a more potent stimulant of the FGFR2 IIIb receptor than FGF3 as seen in BaF3 cells.9 FGF3 and FGF7 induce more proliferation in ES2 cells than in SKOV3 cells which may be due to the sole expression of the FGFR2 IIIb isoform, which these growth factors stimulate, in ES2 cells whereas SKOV3 cells express both FGFR2 IIIb and IIIc isoforms. No differences in proliferation were observed in response to addition of endogenous FGF10 or inhibition of FGF10 by shRNAi in ES2 cells (data not shown).
Figure 2.
Expression of FGFR isoforms and their ligands FGF3, 10 and 19. (A) An example of RT-PCR and restriction enzyme digest to establish which isoform of FGFR2 is present in normal ovary and ovarian tumor. Digestion of the FGFR2 PCR product by HincII but not AvaI shows expression of FGFR2 IIIc isoform as shown in normal ovary (N) (1–3) where 1. 210 bp RT-PCR product alone, 2. HincII digestion yielded 38, 53 and 119 bp products and 3. AvaI did not digest the 210 bp RT-PCR product. Digestion of the FGFR2 RT-PCR product by AvaI but not HincII shows expression of FGFR2 isoform IIIb as shown in ovarian tumor (T) (serous ovarian carcinoma) (4–6) where 4. 210 bp RT-PCR product alone, 5. HincII did not cut the 210 bp RT-PCR product and 6. AvaI digestion yielded 26 and 184 bp products. The DNA markers shown are 500, 400, 300, 200 and 100 bp. (B) RT-PCR and subsequent restriction enzyme digest identified the presence and isoform type of FGFR1, 2 and 3 mRNAs from 5 commercially available normal ovary RNA, 4 ovarian cancer cell lines (CAOV3, ES2, OVCAR3 and SKOV3) and 8 ovarian tumors (serous ovarian carcinomas). In the table, B indicates the presence of the IIIb isoform and C indicates the presence of the IIIc isoform and ◆ indicates that one of the cell lines, SKOV-3, expressed both B and C isoforms of FGFR2 and FGFR3; in all other cases the mRNAs contained the isoform type stated. FGFR4 and 5 mRNA was found to be present in all normal ovaries, cell lines and ovarian tumors investigated; this is indicated by + in the table.
Figure 3.
Effect of FGF3 and FGF7 on ovarian cancer cell proliferation. FGF3 (diamond-solid line) and FGF7 (square-dashed line) increase cell proliferation of ES 2 (A) and SKOV3 (B) cell lines. Cells were plated (2,000 cells/well) in 24 well plates in 10% serum media, after 24 hours media was changed to 0.2% serum media with addition of varying concentrations of FGF3 or FGF7 (10–100 ng/ml). Cell numbers were determined 96 hours after the addition of growth factors. Values shown are mean ± SE M of triplicates (n = 3). *p < 0.02.
FGF10 mRNA was detected in 4 of 5 normal ovarian samples but only in 2 of 8 ovarian cancers, although this was not statistically significant (Table 1). FGF3 mRNA was present in 7 of 8 (88%) malignant ovarian tumors but not in any normal ovarian samples (Fisher's exact test, p < 0.01) (Table 1). Similarly, FGF19 mRNA was recorded in all 8 of the malignant ovarian tumors but only in 1 of 5 normal samples (p < 0.01, Fisher's exact test) (Table 1). The expression of the other FGFs in normal and ovarian cancer is shown in Table 1.
Table 1.
Expression of FGFs in ovarian samples
| Normal ovary (n = 5) | Ovarian cancer cell lines (n = 4) | Ovarian cancer tissues (n = 8) | |
| 4, 6, 21, 23 | Low | Low | Low |
| 8, 20 | Low | High | Low |
| 10 | High | Low | Low |
| 3, 19 | Low | High* | High* |
| 1, 2, 5, 7, 9, 16, 17, 18, 22 | High | High | High |
p < 0.01 compared to normal ovary. Low, >20% of specimens expressing the growth factor; High, >60% specimens expressing the growth factor.
Taken together the results demonstrate that ovarian epithelial transformation is associated with an FGF receptor isoform switch that endows the cells with the ability to respond to 2 cytokines that are expressed at high prevalence by ovarian cancer cells and which induce ovarian cancer cell proliferation, in vitro. The fact that a concentration-response effect was observed with exogenous FGF3 and 7 implies that there is a membrane-bound receptor with capacity to support the autocrine circuit described above.
Effect of FGF and FGF receptors on proliferation.
The function of the cytokine-receptor (FGFR-FGF) axes using specific shRNAi and neutralizing antibody approaches was analysed using two ovarian cancer cell lines.
Reduction in the expression of FGFR2, FGFR3, FGFR4, FGF3 and FGF7 using shRNAi significantly inhibited ES2 and SKOV3 ovarian cancer cell line proliferation by 40–80% compared with cells transfected with negative control (NC) shRNAi (p < 0.03) (Fig. 4A and B). The NC shRNAi did not impact on cell proliferation when compared with parental cell lines (data not shown).
Figure 4.
Effect of FGFRs and FGFs on proliferation. Effect of reducing FGFR1-4 (FR1-4) or FGF3, 7 (F3, F7), using shRNAi (3 separate shRNAi represented as ■, ◆ and ▴) on proliferation of ES2 (A) and SKOV3 (B). Cell number was determined 120 hours after plating 2,000 cells/well in a 24 well plate and shown as a percentage of negative control shRNAi cells. Values shown are mean ± SE M of triplicates (n = 3). Representative blots for the knockdown of the FGFRs or FGFs are shown for ES 2 (A) and SKOV3 (B) where C corresponds to the negative control shRNAi cell line. Cells were immunoprecipitated with antibodies against FR1-FR4, F3 and F7 then IB for FR1–FR4, F3 and F7 as described in materials and methods to determine knockdown of protein by shRNAi. *p < 0.0005, †p < 0.03 and ‡p < 0.005 when compared with number of shRNAi cells after 120 hours.
Interestingly, reducing FGFR1 by 60–80%, through shRNAi, caused a slight increase in cell numbers after 5 days in ES2 cells (p = 0.1439) whereas in SKOV3 cells reducing FGFR1 caused a 2–3 fold increase in cell numbers after 5 days compared with NC shRNAi cells (p = 0.0014).
Effect of FGF and FGF receptors on cisplatin sensitivity.
The contribution of FGF/FGFRs to platinum sensitivity in vitro was investigated using shRNAi. There was no significant difference in cisplatin sensitivity between parental and NC shRNAi cell lines (p > 0.2) (Table 2). Overall, reducing FGFR3 and FGFR4 had no effect on cisplatin sensitivity (p > 0.17) (Table 2) in either of the cell lines; similarly reduction of FGF10 had no effect on cisplatin sensitivity in ES2 cells (p = 0.75). Reducing FGFR1 (>60%), FGFR2 (>80%), FGF3 (>75%) and FGF7 (85%) separately caused statistically significant decreases (p < 0.0001) in cisplatin IC50 in both cell lines when compared with NC shRNAi (Table 1).
Table 2.
Contribution of FGFs and FGF receptors to platinum sensitivity in vitro
| ES2 IC50 µM | ES2 % change | SKOV3 IC50 µM | SKOV3 % change | |
| Parental | 1.86 ± 0.34 | - | 2.18 ± 0.24 | - |
| -ve control shRNAi | 1.72 ± 0.30 | 8 | 2.08 ± 0.30 | 5 |
| FGFR1 shRNAi | 0.66 ± 0.02* | 65 | 0.70 ± 0.03* | 68 |
| FGFR2 shRNAi | 0.53 ± 0.02* | 72 | 0.51 ± 0.04* | 77 |
| FGFR3 shRNAi | 1.30 ± 0.17 (2) | 30 | 1.95 ± 0.21 (2) | 11 |
| FGFR4 shRNAi | 1.36 ± 0.11 | 27 | 2.06 ± 0.13 | 5 |
| FGF3 shRNAi | 0.78 ± 0.03* | 58 | 0.66 ± 0.04* | 70 |
| FGF7 shRNAi | 0.71 ± 0.02* (2) | 62 | 0.83 ± 0.04* | 62 |
| FGF10 shRNAi | 1.73 ± 0.14 (2) | 7 |
p < 0.0001 compared with negative control shRNAi. Parentheses is number of separate shRNAi used if not three.
Negative control shRNAi- and FGFR2 shRNAi-modified ES2 cell lines were used to determine if reducing exogenous FGF3 and FGF7 using neutralizing antibodies had any further effect on cisplatin sensitivity (Fig. 5). Reducing endogenous FGF7 increases cisplatin sensitivity of NC and FGFR2 shRNAi cells when treated with 1 µM cisplatin (p < 0.0001 and p < 0.001, respectively). Inhibition of FGF7 also caused a decrease in proliferation of the NC and FGFR2 shRNAi cells (p < 0.0001 and p < 0.001, respectively). Whereas reducing exogenous FGF3 using anti-FGF3 antibody did not impact on cisplatin sensitivity or proliferation (data not shown).
Figure 5.
Effect of reducing exogenous FGF7 on cell growth and cisplatin sensitivity. ES 2 negative control shRNAi (C) and FR2 shRNAi (FR2) cell lines were plated in 24 well plates at 2,000 cells/well. After 24 hours they were treated with (cis) or without (0) cisplatin 1 µM in the presence of no antibody (black bar), IgG control antibody (white bar) or neutralizing F7 antibody (grey bar). Cell numbers were determined 96 hrs post-treatment and shown as a percentage of the number of cells in the absence of cisplatin or antibody. Values shown are mean ± SE M of triplicates (n = 3). *p < 0.0001, †p < 0.001.
Altogether these results show that the effect of FGF7 on ovarian cancer cell proliferation and cisplatin sensitivity is mediated through the FGFR2-IIIb receptor, as the neutralizing FGF7 antibody had the same impact on proliferation and cisplatin sensitivity as FGF7 shRNAi whilst augmenting the effects of the FGFR2 shRNAi. In contrast, FGF3 seems to be active within cells as FGF3 shRNAi reduced proliferation and enhanced cisplatin sensitivity but the neutralizing FGF3 antibody had no effect.
Effect of FGFR2 shRNAi on proliferation and response to cisplatin of ovarian carcinoma in vivo.
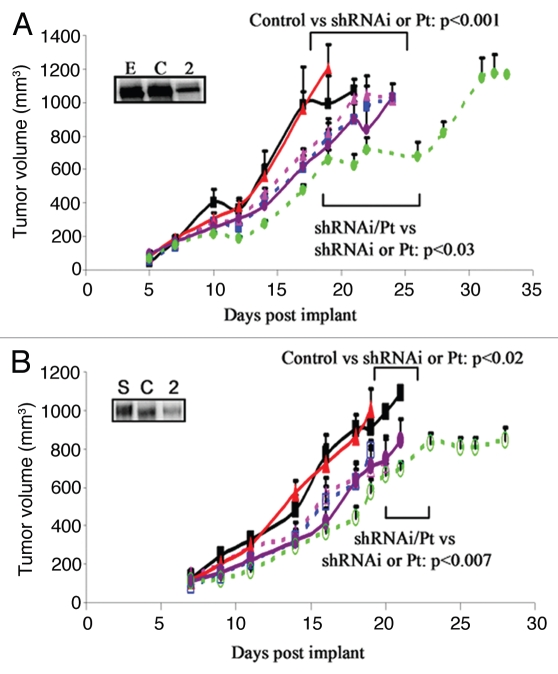
The parental, NC shRNAi and FGFR2 shRNAi transfected ES2 (Fig. 6A) and SKOV3 (Fig. 6B) ovarian carcinoma cell lines were grown as xenografts in Balb/c nude mice, which were treated with cisplatin (5 mg/kg) weekly for 3 weeks once they had reached a volume of 150–200 mm3 (Fig. 6). Both cell lines grew rapidly as tumors and there was no significant difference in the growth of parental and NC shRNAi tumors (p > 0.7). Reduction of FGFR2 within the ES2 and SKOV3 ovarian xenografts caused more than a 20% reduction in proliferation compared with NC shRNAi tumors (p < 0.006). Addition of cisplatin caused a 20% reduction in proliferation of parental and NC shRNAi tumors (p < 0.02). Growth of parental and NC shRNAi tumors in the presence of cisplatin was similar to the growth of FGFR2 shRNAi tumors in the absence of cisplatin (p > 0.5). Cisplatin caused a further 20% reduction in tumor growth of FGFR2 shRNAi tumors (p < 0.007), so that the combined effect of cisplatin and FGFR2 inhibition resulted in a 40% reduction in tumor growth (p < 0.0001) (Fig. 6). These data suggest that cisplatin had a clear impact on ovarian cancer cell growth but that inhibition of FGFR2 significantly augmented the effect of this cytotoxic agent. As the shRNAi did not completely remove the FGFR2 protein, a more effective inhibitor of FGFR2 might further enhance cisplatin sensitivity.
Figure 6.
Effect of reducing FR2 on growth and cisplatin sensitivity in ovarian cancer xenografts. Parental (triangle), control shRNAi (square) or FR2 shRNAi (circle) cells of ES2 (A) or SKOV3 (B) origin were subcutaneously implanted into the right flank of Female Balb/c-NUDE mice. When tumors had reached 150 mm3 they were treated with cisplatin 5 mg/kg once weekly for 3 weeks (dashed line) or saline control (solid line). Downregulation of the protein is represented by western blot for the appropriate protein after immunoprecipation where E or S is the parental cell line, C is control shRNAi and 2 is the FGFR2 shRNAi cell line. For each treatment group 8 mice were used and the values represent the mean ± SEM.
Effect of FGF receptor 1 and 2 shRNAi on the cell cycle and apoptosis in ovarian cancer cell lines treated with cisplatin.
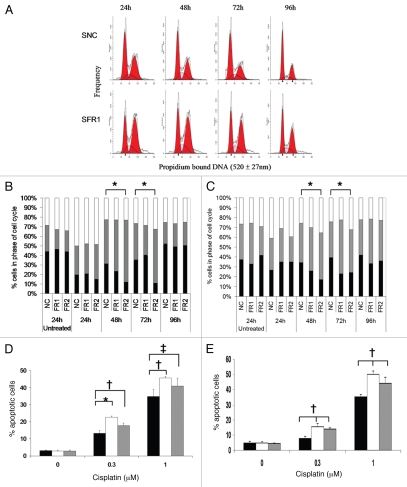
We investigated the impact of shRNAi-mediated reductions in FGFR1 and 2 in ES2 and SKOV3 cells on the cell cycle and apoptosis with or without cisplatin over 96 h (Fig. 7). Knockdown of FGFR1 in SKOV3 cells caused an increase in G2/M with an associated decrease in G1 compared with SKOV3 NC at all time points (24–96 h) (Fig. 7A). The SKOV3 NC cells have a doubling time of around 23 h whereas the SKOV3 FGFR1 knockdown cells are slightly faster with a doubling of around 17 hours. Therefore the increased percentage of SKOV3 FGFR1 shRNAi cells in G2/M may represent more cells going through mitosis as more cells were observed and there was <5% apoptosis as measured by annexin V staining at 96 h (Fig. 7E). In contrast, knockdown of FGFR1 in ES2 cells had no effect on cell cycle or apoptosis (Fig. 7B and D). Also the knockdown of FGFR2 in both cell lines had no effect on cell cycle or on apoptosis in either of the cell lines in the absence of cisplatin (Fig. 7B–E).
Figure 7.
Effects of knockdown of FGFR1 (FR1) and FR2 using shRNAi on cell cycle and apoptosis with or without cisplatin (1 µM) in ES 2 and SKOV3 cell lines. (A) Representative histogram of cell cycle distributions of SKOV3 NC shRNAi (SNC) and SKOV3 FGFR1 shRNAi (SFR1) 24, 48, 72 and 96 h after plating. Cell cycle distribution (G1-black bars, G2/M-grey bars and S phase-white bars) of NC shRNAi (NC), FGFR1 shRNAi (FR1) and FGFR2 shRNAi (FR2), untreated (24 h), 24, 48, 72 and 96 h after cisplatin treatment in ES 2 (B) and SKOV3 (C) cells. The significant differences between the negative control shRNAi and FGFR2 shRNAi in the G2/M phase of the cell cycle are represented by (*p < 0.006). Values shown are mean representatives of three separate experiments. The percentage of apoptotic ES2 (D) and SKOV3 (E) shRNAi cells as determined by 7-AAD and Annexin V staining. Black bars = NC shRNAi, white bars = FGFR1 shRNAi, grey bars = FGFR2 shRNAi. *p < 0.0001, †p < 0.004 and ‡p < 0.025. Values shown are mean ± SEM of three separate experiments.
Twenty four hours after cisplatin (1 µM) treatment there was an increase in the percentage of cells in S phase (25–34% to 31–50%) in all transfected cell lines compared with untreated controls after 24 hours (Fig. 7B and C). Forty eight hours after cisplatin (1 µM) treatment cells accumulated in G2/M compared with untreated cells, however there was a significantly (p < 0.006) higher percentage of FGFR2 shRNAi cells in G2/M (65 ± 5 or 48 ± 3%) compared with NC shRNAi (46 ± 1 or 40 ± 1) ES2 and SKOV3 cells, respectively (Fig. 7B and C). G2/M accumulation in the FGFR2 shRNAi cells was maintained for a further 24 hours whereas the NC shRNAi cells were no longer accumulating in G2/M and the cells were more evenly distributed throughout the cell cycle. Ninety six hours after cisplatin the FGFR2 shRNAi cells were returning to a cell cycle profile more akin to control untreated, whilst there was ∼35% apoptosis in the NC shRNAi cells there was significantly increased apoptosis in the FGFR1 and FGFR2 shRNAi cells, 46 or 41% (p < 0.004 and p < 0.025), respectively (Fig. 7D and E). The cell cycle of the ES2 FGFR1 shRNAi cells followed a similar pattern to the NC shRNAi cells except for a greater G2/M accumulation (p < 0.025) 48 h after cisplatin treatment. In the SKOV3 FGFR1 shRNAi cells the G2/M accumulation was maintained at all time points but in the absence of cisplatin this cell population had a higher percentage of cells in G2/M compared with NC shRNAi. These results suggest that knockdown of FGFR2 enhances cisplatin sensitivity by G2/M arrest with resultant increased cell death. Whereas enhanced cisplatin sensitivity in the FGFR1 shRNAi cells seems to be due to increased cell death.
Discussion
This study describes the first comprehensive investigation of the expression of the FGFs and FGFRs in epithelial ovarian cancer. The data showed that transformation is associated with a receptor isoform switch in FGFR2 and FGFR3 and also expression of FGF3 and FGF19. Further analysis highlight that transformation is associated with the establishment of an autocrine circuit consisting of FGF3 and 7 acting through FGFR2IIIb. Correspondingly, as normal ovarian epithelium lacks the b isoform, it cannot respond to either FGF 3 or 7.9 Previous studies have shown an increased expression of FGF3 DNA amplification in ovarian carcinoma.5 Here FGF3 mRNA and protein are demonstrated as present and functional in ovarian carcinoma suggesting that increased FGF3 expression may be relevant to the malignant phenotype. In addition, our data demonstrate that the inhibition of intracellular FGF3 impacts on proliferation; an observation that corresponds with the proposed intracellular mode of action of the cytokine. Other studies have suggested that FGF1 is of prognostic significance in the disease, this difference may in part be due to inter- and intra-tumor heterogeneity or case selection since Birrer et al. used laser capture to extract adenocarcinoma cells from high grade, advanced stage, serous ovarian cancer.11 Nevertheless, these data are still compatible to some extent with our findings as FGF1 is able to activate all FGF receptors including, FGFR2 IIIb.
Functional investigation showed inhibition of FGFR2 and/or FGF3 and 7 impacted significantly on ES2 and SKOV3 proliferation and reduced significantly the IC50 of cisplatin in vitro. In vivo evaluation demonstrated that this axis had functional significance in that inhibition of FGFR2 slowed tumor growth and augmented the cytotoxic effect of cisplatin. Cisplatin is an alkylating agent that forms DNA adducts, activating damage recognition proteins and resulting in the activation of several signal transduction pathways that can lead to apoptosis.13 Previous studies have shown that the FGFR inhibitor PD173074 potentiated the effects of cisplatin in small cell lung cancer thus showing by a different mechanism that inhibition of FGFRs can augment the effects of cisplatin.14 Our cell cycle analysis of the NC shRNAi cells shows that cisplatin increased apoptosis and G2/M arrest. We also show that apoptosis is augmented when FGFR1 expression is decreased thus explaining the increased cisplatin sensitivity in these cell lines. Although knockdown of FGFR2 expression was associated with increased apoptosis in the presence of cisplatin, the predominant effect was on the cell cycle causing sustained G2/M arrest (Fig. 7B and C). The underlying mechanisms of these differences in cellular fate between SKOV3 and ES2 cells needs to be further investigated but show that FGF receptors can function in contrasting ways. Intuitively, the anti-proliferative effects of inhibiting FGF3/7-FGFR2IIIb would seem to be at odds with the reduction in IC50 observed with the cell cycle-specific effects of cisplatin. However, in previous studies using NIH 3T3 6-1 cells that have an FGF2 autocrine circuit and in bladder cancer and renal cancer cell lines, in vitro and in vivo, the impact of FGF modulation on platinum sensitivity is independent of its effects on proliferation.15–17
Reducing FGFR1 expression in the ES2 cell line had no effect on proliferation, whereas in the SKOV3 cell line it was associated with a 2–3 fold increase in proliferation. This effect of modulating FGFR1 expression needs to be investigated further. On the other hand shRNAi-mediated reductions in the expression of FGFR1 did enhance platinum sensitivity in both cell lines, again emphasizing a distinction between the impact of FGF on proliferation and its effects on resistance to platinum chemotherapy in ovarian cancer. Interestingly, immunoprecipitation studies demonstrated that the SKOV3 cell line expresses more FGFR1 than the ES2 cell line suggesting that this might account for the different magnitude of effect seen in both cell lines. However, the data also highlight that broad spectrum inhibition of the FGF receptor system might not have the desired therapeutic effects.
This study has demonstrated that ovarian epithelial transformation is associated with the establishment of an autocrine circuit consisting of FGF3/7 and FGFR2IIIb. Inhibition of this axis at the cytokine or receptor level impacts on proliferation and platinum sensitivity in vitro and in vivo, thus highlighting FGFR2 and it cytokines, FGF3 and 7, as potential new targets for the treatment of ovarian cancer. On a more general level and in conjunction with other recent publications the data highlight the contribution of FGFs, in terms of angiogenesis, proliferation and chemosensitivity, to epithelial ovarian cancer.11
Materials and Methods
Ovarian cancer samples.
Ovarian cancer tissue was collected at the time of primary surgery (St. Mary's Hospital, Manchester, UK) from patients who had given informed consent. Tissue was immediately frozen in liquid nitrogen and subsequently stored at −80°C. Additional ovarian cancer tissue that had been embedded in paraffin was also studied (St. Mary's Hospital, Manchester, UK). The research had been approved by the South Manchester Local Research Ethics Committee (REC reference number: SOU/00/018).
Cell culture.
Human ovarian carcinoma cell lines CAOV3, ES2, OVCAR3 and SKOV3 (ATCC) were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (Invitrogen Ltd., Paisley, UK). The shRNAi cells were maintained in RPMI 1640 plus 10% FBS supplemented with 1 g/L G418 (Sigma, Gillingham, UK).
Reverse transcription-PCR.
mRNA was extracted from frozen tumor tissue and cell lines using the RNeasy Mini Kit according to the manufacturer's protocol (Qiagen Ltd., West Sussex, UK). Reverse transcription-PCR was performed on human ovarian tumor RNA (n = 8), tissue cultured human ovarian cancer cell RNA (n = 4, CAOV3, ES2, OVCAR3 and SKOV3) and normal human ovarian RNA (Ambion, Texas, USA; Biochain, Oxon, UK; OriGene, Rockville, USA; Panomics, Italy and Stratagene, California, USA) (n = 5) using the QIAGEN OneStep RT-PCR kit according to the manufacturer's protocol (Qiagen Ltd., West Sussex, UK). Products were run on a 1% agarose-ethidium bromide gel and DNA extracted using the QIAquick Gel Extraction Kit according to manufacturer's instructions (Qiagen Ltd., Paisley, UK). Sequencing was performed by the Molecular Biology Core Facility DNA sequencing service at The Paterson Institute for Cancer Research using an ABI 3100 Genetic Analyzer (Applied Biosystems Inc., California, USA).
FGF receptor subtype.
FGFR1, 2 and 3 receptor subtypes were determined using a method described by Tartaglia et al. 2001. Briefly, RT-PCR products for FGFR1, 2 and 3 were digested using 2 specific restriction endonucleases and separated using agarose gel electrophoresis.18
Riboprobe preparation.
Primers were designed to allow amplification, of a specific region (≥150 bp) of FGFR2, by RT-PCR from normal human ovary RNA (Ambion). The RT-PCR product was cloned into the polylinker site of the transcription vector pSPT19 (Roche). Primers are available on request. The construct (1 µg) was linearized by enzyme digestion and rendered RNAse-free by phenol chloroform isomyl alcohol purification. The riboprobe was DIG labeled using the DIG RNA labeling Kit (Roche) according to the manufacturer's instructions. A sense and antisense probe were prepared and quantification of probe labeling determined using the DIG nucleic acid detection kit and test strips (Roche).
In situ hybridization.
Paraffin-embedded tissues were cut into 4 µm sections and mounted on charged Superfrost Plus microscope slides (BDH, Poole, UK). The sections were dewaxed in xylene, rehydrated in IMS, soaked for 20 minutes in 0.2 M HCl, rinsed briefly in 2x SSC, then 0.05 M Tris HCl pH 7.4 before being treated with 5 µg/ml Proteinase K in 0.05 M Tris HCl pH 7.4 at 37°C for 30 minutes. Washes were carried out in 0.2% glycine in PBS, then PBS alone before immersing in cold 0.4% paraformaldehyde in PBS for 20 minutes. Geneframes (Thermo Electron Corp., London, UK) were applied to each slide to create the in situ reaction chamber around the tissue section. 600 ng–1,800 ng/µl of riboprobe (antisense-positive and sense-negative) was added to 125 µl hybridization solution (Sigma, Gillingham, UK) containing 5 µl herring sperm (Sigma, Gillingham, UK). The hybridization mix was denatured prior to use and hybridization was carried out at 50°C overnight. The slides were washed in 4x SSC for 30 minutes at room temperature, 37°C in 2x SSC for 10 minutes, 70°C in 0.2% SSC for 1 hour then in TBS at room temperature for 5 minutes. 10% normal goat serum (Dako Ltd., Ely, UK) in TBS was dropped onto the slides and left for 1 hour at room temperature. The anti-DIG alkaline phosphatase antibody (Roche Ltd., Basel, Switzerland) (1/1,500 dilution) with 1% goat serum in TBS was dropped onto the sections and incubated overnight at room temperature. The slides were then washed twice in TBS and equilibrated in BCIP/NBT buffer (0.1 M Tris pH 9.5, 0.1 M NaCl, 50 mM MgCl2). The color reaction substrate [4.5 µl/ml NBT (Roche Ltd., Basel, Switzerland), 3.5 µl/ml BCIP (Roche Ltd., Basel, Switzerland) and 25 µl levamisole (Dako Ltd., Ely, UK) in 1 ml BCIP/NBT buffer], was dropped onto the slides and incubated at room temperature until the color reaction developed. The slides were then fixed in an aqueous mountant (Dako Ltd., Ely, UK) and examined with an AxioSkop microscope (Zeiss, Welwyn Garden City, UK) using a x40 plan neofluar, 1.35 NA, oil immersion objective lens. Visualization was carried out utilizing a Progress C14 camera (Jenoptik, Jena, Germany) and Adobe Photoshop 7.
Immunohistochemistry.
Formalin-fixed 4 µm sections from paraffin-embedded blocks were dewaxed using xylene and serial ethanol dilutions, and subsequently pretreated by heat in a microwave oven for antigen retrieval (4 × 5 min) in citrate buffer (10 mM, pH 6.0). Peroxidase Block solution was added for 5 mins followed by 10% goat serum in TBS for 20 minutes. This was followed by sequential incubation of primary antibodies for 30 min [FGFR2 (Sigma), FGF3 (abcam), FGF7 (RnD Systems) or negative control antibodies (Dako)], secondary antibodies for 30 min and incubation with DAB for 5 min. The processed sections were examined using an Olympus BX51 microscope and the resulting images captured using the AnalySIS program.
shRNA plasmid construction and transfection.
Two complimentary oligonucleotides with 5′BamH1 3′XbaI overhangs and a HINDIII site in the loop were designed in duplicate or triplicate for FGFR1, FGFR2, FGFR3, FGFR4, FGF3, FGF7 and FGF10 (Table 1) using the NCBI blast program so that there was no cross reaction with other genes. These were then made by VHBio Ltd., (Gateshead, UK). The complimentary strands (1 µg) were annealed using 5 µl 10x Annealing buffer (500 mM NaCl, 100 mM Tris-HCl, pH 8.0 and 1 mM EDTA) and 43 µl water, heated to 93°C for 3 minutes on a hot block and left to cool on the hot block after which 200 µl water was added. The pGE-1 vector (Stratagene, Amsterdam, Netherlands) was linearized first with XbaI and then BamH1. One hundred nanograms of vector was added to 3 µl double stranded oligonucleotide, 1 µl ligase buffer and 1 µl T4 DNA ligase (Promega UK, Southampton, UK) in a final volume of 10 µl, then incubated at 16°C for 18 hours. Five microlitres vector was transformed into SCS1 supercompetent cells according to manufacturers instructions (Stratagene, Amsterdam, Netherlands); these cells were then smeared on Kanamycin/agar plates and placed at 37°C overnight. Colonies were picked and placed in Kanamycin/LB broth at 37°C overnight in a shaking incubator. DNA was extracted using the Qiagen miniprep kit (Qiagen Ltd., West Sussex, UK). A HINDIII restriction enzyme digest was performed on 1 µl of DNA and eluted on an agarose gel to determine linearization of DNA. Linearized DNA was sequenced and those vectors containing the relevant oligonucleotides were used for transfection.
Cells were transfected 24 h after plating with 10 µg vector containing oligonucleotides plus 10 µg Transfast (Promega UK, Southampton, UK) for 1 hr. After 24 hours cells were grown in RPMI 1640 supplemented with 10% FBS containing 1 g/L G418 (Sigma) for 2–4 weeks. Colonies were then picked and immunoprecipitation and immunoblotting were used to identify reduced levels of specific FGFs and FGF receptors. A pGe-1 negative control vector was used (Stratagene, Amsterdam, Netherlands).
Immunoprecipitation and immunoblotting.
Cell pellets were lysed in modified RIPA buffer (PBS, 1% NP40, 1% sodium deoxycholate and 0.1% SDS, 100 µg/ml PMSF, 1 mmol/L sodium orthovanadate and 1 protease inhibitor tablet/10 ml). FGF or FGF receptors were precipitated from 250 µg of protein using 3 µg specific antibodies: FGFR1 and FGF3 (Abcam, Cambridge, UK), FGFR2, FGFR3, FGFR4, FGF7 and FGF10 (R & D systems, Minneapolis, USA) at 4°C on orbital shaker overnight. One hundred microlitres of Protein A Agarose (Upstate, NY, USA) was added for 1 hour and the beads were collected. Samples were separated by SDS polyacrylamide gel electrophoresis, then electro-blotted to nitrocellulose membranes using semi-dry blotting apparatus (Sigma, Gillingham, UK). Membranes were probed using the following antibodies: FGFR1, 2 and 3 (Sigma, Gillingham, UK) FGFR4, FGF3, FGF7 and FGF10 (R & D systems, Minneapolis, MN, USA), with the antigen being detected with chemluminescence (PerkinElmer, Bucks, UK) and exposed to X-ray film (Sigma, Gillingham, UK).
Cell growth and cytotoxicity assays.
For all the following in vitro studies transfected cell lines were plated at 2,000 cells/well in 24 well plates in RPMI 1640 + 10% FBS.
For growth assays cell numbers were measured with a Coulter counter every 24 hours.
For cisplatin sensitivity the cells were treated with cisplatin (0.001–10 µM) (Sigma, Gillingham, UK) ± FGF3 or FGF7 neutralizing antibodies (5 µg/ml) (R & D systems, Minneapolis, MN, USA) 24 h after plating. After a further 96 hours cell numbers were determined using a Coulter counter.
For studying the effects of FGF3 and FGF7 the cells were placed in 0.2% serum with varying concentrations of FGF3 or FGF7 (10–100 ng/ml) (R & D systems, Minneapolis, MN, USA) 24 h after plating. After a further 96 hours cell numbers were determined using a Coulter counter.
Cell cycle analysis.
Twenty four hours after plating cells were treated with cisplatin (0 and 1 µM). Cells were collected every 24 hours by centrifugation and resuspended at 1 × 106 cells/ml in propidium iodide (PI) staining buffer (0.1% sodium citrate, 0.1% Triton-X 100 and 50 µg/ml PI) and were treated with 1 µg/mL RNase at room temperature for 30 minutes. Cell cycle histograms were generated after analysis of PI-stained cells with excitation at 488 nm and emission at 520 nm by fluorescenceactivated cell sorting (FACS) with a Becton Dickinson FACScan. For each sample, at least 1 × 104 events were recorded. Histograms generated by FACS were analyzed by ModFit Cell Cycle Analysis Software (Verity, Topsham, ME, USA) to determine the percentage of cells, in each phase (G1, S and G2/M).
Apoptosis analysis.
Cells were treated with 0, 0.3 or 1 µM cisplatin after 3 hours of plating. After 96 hours media and attached cells were collected and the cell pellet was resuspended in 200 µl PBS and placed in 96 well round bottom plate. After centrifugation, the pellet was placed in 100 µl Annexin V binding buffer containing 5 µl Annexin V-APC and 2 µl 7-AAD. The plate was incubated in the dark at room temperature for 15 mins then 100 µl of Annexin V binding buffer was added to each well and the plate was read on a BD FACSArray Bioanalyzer with laser turned to excitation at 635 nm for APC and 532 nm for 7-AAD with emission at 660 and 695 nm, respectively.
In vivo tumor studies.
Female Balb/c-NUDE mice (PICR, Manchester, UK) were housed in an individually ventilated caging system on a 12 hour light/dark environment maintained at constant temperature and humidity. Mice were fed a standard diet of irradiated feed (Harlan-Teklad, WI, USA) and allowed water ad libitum. Cells were grown as subcutaneous xenografts in groups of 8 mice following the injection of 1 × 107 cells. When the tumor volume reached 150–200 mm3, mice were randomized for treatment with 5 mg/kg cisplatin or saline control once weekly for 3 weeks. Tumor volume (defined as (height × width2)/2) was measured three times a week and mice were sacrificed before tumors reached 1,250 mm3. Tumors were halved and snap-frozen for subsequent FGFR2 analysis or fixed in paraffin for future studies. All procedures were carried out in accordance with UKCCCR guidelines 1999 by approved protocol (Home Office Project license no. 40-2804).
Statistics.
Statistical significance was tested using standard ANOVA methods and defined as p < 0.05, unless stated otherwise.
Acknowledgements
This work was supported by Cancer Research UK and the MRC.
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/12585
References
- 1.McGuire WP, Markman M. Primary ovarian cancer chemotherapy: current standards of care. Br J Cancer. 2003;89:3–8. doi: 10.1038/sj.bjc.6601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:1–12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orr-Urtreger A, Bedford MT, Burakova T, Arman E, Zimmer Y, Yayon A, et al. Developmental localization of the splicing alternatives of fibroblast growth factor receptor-2 (FGFR2) Dev Biol. 1993;158:475–486. doi: 10.1006/dbio.1993.1205. [DOI] [PubMed] [Google Scholar]
- 4.Hruza C, Dobianer K, Beck A, Czerwenka K, Hanak H, Klein M, et al. HER-2 and INT-2 amplification estimated by quantitative PCR in paraffin-embedded ovarian cancer tissue samples. Eur J Cancer. 1993;29:1593–1597. doi: 10.1016/0959-8049(93)90301-u. [DOI] [PubMed] [Google Scholar]
- 5.Rosen A, Sevelda P, Klein M, Dobianer K, Hruza C, Czerwenka K, et al. First experience with FGF-3 (INT-2) amplification in women with epithelial ovarian cancer. Br J Cancer. 1993;67:1122–1125. doi: 10.1038/bjc.1993.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seki A, Yoshinouchi M, Seki N, Kodama J, Miyagi Y, Kudo T. Detection of c-erbB-2 and FGF-3 (INT-2) gene amplification in epithelial ovarian cancer. Int J Oncol. 2000;17:103–106. doi: 10.3892/ijo.17.1.103. [DOI] [PubMed] [Google Scholar]
- 7.Steele IA, Edmondson RJ, Bulmer JN, Bolger BS, Leung HY, Davies BR. Induction of FGF receptor 2-IIIb expression and response to its ligands in epithelial ovarian cancer. Oncogene. 2001;20:5878–5887. doi: 10.1038/sj.onc.1204755. [DOI] [PubMed] [Google Scholar]
- 8.Steele IA, Edmondson RJ, Leung HY, Davies BR. Ligands to FGF receptor 2-IIIb induce proliferation, motility, protection from cell death and cytoskeletal rearrangements in epithelial ovarian cancer cell lines. Growth Factors. 2006;24:45–53. doi: 10.1080/08977190500361697. [DOI] [PubMed] [Google Scholar]
- 9.Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, et al. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 10.Crickard K, Gross JL, Crickard U, Yoonessi M, Lele S, Herblin WF, et al. Basic fibroblast growth factor and receptor expression in human ovarian cancer. Gynecol Oncol. 1994;55:277–284. doi: 10.1006/gyno.1994.1290. [DOI] [PubMed] [Google Scholar]
- 11.Birrer MJ, Johnson ME, Hao K, Wong KK, Park DC, Bell A, et al. Whole genome oligonucleotide-based array comparative genomic hybridization analysis identified fibroblast growth factor 1 as a prognostic marker for advanced-stage serous ovarian adenocarcinomas. J Clin Oncol. 2007;25:2281–2287. doi: 10.1200/JCO.2006.09.0795. [DOI] [PubMed] [Google Scholar]
- 12.Sahadevan K, Darby S, Leung HY, Mathers ME, Robson CN, Gnanapragasam VJ. Selective overexpression of fibroblast growth factor receptors 1 and 4 in clinical prostate cancer. J Pathol. 2007;213:82–90. doi: 10.1002/path.2205. [DOI] [PubMed] [Google Scholar]
- 13.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 14.Pardo OE, Latigo J, Jeffery RE, Nye E, Poulsom R, Spencer-Dene B, et al. The fibroblast growth factor receptor inhibitor PD173074 blocks small cell lung cancer growth in vitro and in vivo. Cancer Res. 2009;69:8645–8651. doi: 10.1158/0008-5472.CAN-09-1576. [DOI] [PubMed] [Google Scholar]
- 15.Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther. 2002;302:8–17. doi: 10.1124/jpet.302.1.8. [DOI] [PubMed] [Google Scholar]
- 16.Shaulian E, Resnitzky D, Shifman O, Blandino G, Amsterdam A, Yayon A, Oren M. Induction of Mdm2 and enhancement of cell survival by bFGF. Oncogene. 1997;15:2717–2725. doi: 10.1038/sj.onc.1201453. [DOI] [PubMed] [Google Scholar]
- 17.Miyake H, Hara I, Gohji K, Yamanaka K, Arakawa S, Kamidono S. Enhancement of chemosensitivity in human bladder cancer cells by adenoviral-mediated p53 gene transfer. Anticancer Res. 1998;18:3087–3092. [PubMed] [Google Scholar]
- 18.Tartaglia M, Fragale A, Battaglia PA. A competitive PRC-based method to measure human fibroblast growth factor receptor 1–4 (FGFR1–4) gene expression. DNA Cell Biol. 2001;20:367–379. doi: 10.1089/10445490152122488. [DOI] [PubMed] [Google Scholar]