Abstract
Background
Daily controller medication use is recommended for children with persistent asthma to achieve asthma control.
Objective
To examine patterns of inhaled corticosteroid (ICS) use and asthma control in an observational study of children and adolescents with mild-to-moderate asthma (the Childhood Asthma Management Program Continuation Study).
Methods
We assessed patterns of ICS use during a 12-month period (consistent, intermittent, and none) and asthma control (well controlled vs poorly controlled). Multivariate logistic regression examined the association between pattern of ICS use and asthma control.
Results
Of 914 patients enrolled, 425 were recommended to continue receiving ICS therapy in the Childhood Asthma Management Program Continuation Study. Of these patients, 46% reported consistent ICS use and 20% reported no ICS use during year 1. By year 4, consistent ICS use decreased to 20%, whereas no ICS use increased to 57%; poorly controlled asthma was reported in 18% of encounters. In multivariate models controlling for age, sex, forced expiratory volume in 1 second, and asthma severity assessment, patients reporting consistent ICS use during a 12-month period were more likely to report poor asthma control (odds ratio, 1.6; 95% confidence interval, 1.2–2.1) compared with those reporting no ICS use.
Conclusions
In this observational study of children and adolescents with mild-to-moderate asthma, most did not report continued use of ICS. Patients recommended to continue receiving ICS therapy and reporting consistent ICS use were less likely to report well-controlled asthma even after controlling for markers of asthma severity. Although residual confounding by severity cannot be ruled out, many children and adolescents may not achieve well-controlled asthma despite consistent use of ICS.
Introduction
Inhaled corticosteroids (ICSs) are recommended as first-line controller therapy for children and adolescents with persistent asthma.1 Consistent ICS use has been shown to improve asthma symptoms and lessen the frequency of asthma-related emergency department visits and hospitalizations.2 In contrast, underuse of ICSs has been associated with worse asthma outcomes.3 Intermittent use of ICSs and other asthma controller medications by patients is common, often because of patient or caregiver nonadherence to physician recommendations. However, in some situations, health care providers may be recommending intermittent controller medication use, particularly in children with milder disease or in children who have seasonally variable asthma.4 Indeed, the recent revisions of the National Heart, Lung, and Blood Institute asthma guidelines state that daily treatment only during specific periods of risk may be considered for some children.1
Whether intermittent asthma controller medication use leads to differential health outcomes compared with consistent use is unclear. A randomized trial5 of intermittent ICS use in adults with mild-to-persistent asthma showed that peak expiratory flow can be maintained successfully with intermittent symptom-based courses of ICS, yet other outcomes (eg, symptom days) are not well controlled. A similar study in children has not yet been completed. In young children with recurrent wheeze, one study6 of intermittent ICS therapy showed no effect on disease progression and no short-term benefit during episodes of wheezing.
Asthma treatment guidelines also recommend the systematic monitoring of asthma control in children to guide appropriate asthma therapy.7 Asthma control, which reflects the current symptom status of a child with asthma regardless of underlying disease severity, can be evaluated via several validated questionnaires, including the Asthma Control Test, the Asthma Therapy Evaluation Questionnaire, and the Asthma Control Questionnaire.8–11 Multiple studies8,12–15 have shown that poor asthma control is associated with increased asthma-related hospitalizations and health care use. However, the relationship between patterns of asthma controller medication use, particularly intermittent controller medication use, and asthma control in children remains unclear.
We examined asthma controller medication use patterns within a cohort of children and adolescents with mild-to-moderate asthma who were enrolled in the Childhood Asthma Management Program Continuation Study (CAMPCS), an observational follow-up study to the original CAMP trial. We sought to determine the frequency of different patterns of asthma controller medication use and asthma control over time. We hypothesized that the pattern of asthma controller medication use, particularly consistent use, would be associated with improved patient-reported asthma control over time.
Methods
Periods of Study
CAMP was a 5-year, randomized, placebo-controlled trial of the safety and efficacy of 2 inhaled anti-inflammatory therapies for children with mild-to-moderate asthma. Characteristics of the patients, results of the trial, and treatment effects have been published previously.2,16 At the completion of the clinical trial, participants were invited to enroll in CAMPCS, a 4.5-year, observational, follow-up study (1999–2004) to determine the effects of long-term treatment early in childhood on asthma outcomes and physical growth and development. All procedures for the observational study were reviewed by local institutional review boards, and written informed consent or assent was obtained from participants at enrollment. Of 1041 participants, 941 (90.4%) re-enrolled in CAMPCS. During the transition phase from CAMP to CAMPCS, study medication was withdrawn and children and adolescents were monitored for lung function, airway hyperresponsiveness, growth, and asthma control. At the end of the medication washout, care for asthma was transferred to the physician who cared for the child before enrollment in CAMP. The CAMP staff provided the family and the treating physician with a summary of the child's clinical course and treatment during CAMP, as well as recommendations for continued asthma care, including a recommendation on whether the child should continue to receive ICS therapy. For this analysis, we stratified patients into 2 groups based on whether a treatment recommendation for ICS was made by a CAMP clinician at the end of the CAMP clinical trial.
During CAMPCS, patients had in-person clinical visits at 6-month intervals, with telephone contacts between the 6-months visits (ie, there was a patient encounter every 3 months). Spirometry was performed at each clinical visit to calculate forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio. Height and weight were evaluated at each study visit. Information on environmental exposures (self-report by interview) was collected annually. Allergy skin testing was performed 96 months after CAMP randomization, and serum IgE and blood eosinophil levels were measured 84 and 108 months after CAMP randomization. Data, including information on asthma symptoms and asthma medication use in the past 7 days, were collected at each contact.
Timing of Data Presented
For this analysis, the follow-up time during CAMPCS was divided into study years, broken down into four 12-month periods. During this period, each patient could have had up to 16 clinical encounters, with evaluation of his or her current asthma medication use and questions directed at the level of asthma control occurring at each in-person or telephone encounter. We excluded 27 patients who had not completed at least 1 full 12-month period, resulting in 914 children and adolescents for analysis.
Outcome Measures
The primary outcome measure for this analysis was asthma control. Asthma control has recently been operationalized via validated questionnaires, such as the Asthma Control Test, the Asthma Therapy Evaluation Questionnaire, or the Asthma Control Questionnaire.8,9,11 Because these questionnaires were developed subsequent to the initiation of data collection in CAMPCS, we created a similar metric of asthma control based on 3 structured questions asked at each patient encounter. These questions evaluated the reported rates of nighttime awakenings, activity limitations, and use of β-agonists in the 7 days before the interview. The same 3 questions were asked during both in-person and telephone encounters. Asthma was considered not well controlled if a participant reported 1 or more nocturnal awakenings, 3 or more days with activity limitations, or 3 or more days with use of β-agonists during the preceding 7 days.
The primary predictor of interest was the reported pattern of ICS use during 12 months of continuous observation spanning 5 consecutive CAMPCS clinical encounters. Patterns of ICS use were classified as consistent (4 of 4 clinical encounters), variable (2–3 of 4 clinical encounters), intermittent (1 of 4 clinical encounters), or none (0 of 4 clinical encounters).
Statistical Analysis
Multivariate logistic regression analyses, using generalized estimating equations to control for the correlation between repeated measurements among individuals, were used to model the odds of not well-controlled asthma. The reported pattern of ICS use of more than 4 encounters was the primary independent variable, with level of asthma control (well controlled vs not well controlled) as the dependent variable. Only encounters with complete data for 4 preceding encounters were included in the analysis. The models were adjusted for demographic and disease severity markers, including FEV1 (percent predicted prebronchodilator) within 15 months of the outcome period, clinician-rated asthma severity during the final CAMP washout visit, age, and sex. All analyses were performed using SAS statistical software, version 9.1 (SAS Institute, Chicago, Illinois).
Results
During the CAMPCS transition encounter, 425 (46.5%) of the 914 children and adolescents received a recommendation to continue ICS therapy for asthma treatment. Children who received a recommendation to use ICSs were more likely to be rated with moderate-to-severe asthma; were more likely to have a lower baseline FEV1, a lower FEV1/FVC ratio, and a higher mean serum IgE level; and exhibited more airway responsiveness (mean ln PC20, 0.7 vs 1.6) (Table 1). Also, more boys (65% vs 43%) received an ICS recommendation. There was no difference in age or race/ethnicity based on the transition visit treatment recommendation.
Table 1. Patient Demographics at the Initiation of the CAMP Posttrial Observation Perioda.
| Characteristic | ICS therapy | |
|---|---|---|
| Recommended (n=425) | Not recommended (n=489) | |
| Age, yb | 13.5 (2.3) | 13.7 (2.2) |
| Race | ||
| Black | 62 (14.6) | 64 (13.1) |
| Hispanic | 36 (8.5) | 47 (9.6) |
| Other | 35 (8.2) | 37 (7.6) |
| White | 292 (68.7) | 341 (69.7) |
| Sex | ||
| Male | 276 (64.9)c | 277 (56.6) |
| Female | 149 (35.1) | 212 (43.4) |
| Pulmonary function test resultsb | ||
| FEV1, % predicted | 92 (13)c | 99 (13) |
| FVC, % predicted | 105 (13) | 107 (12) |
| FEV1/FVC ratio | 76 (9)c | 81 (8) |
| Serum IgE level, U/mLd | 634c | 391 |
| No. of positive allergy skin test resultsd | 4 | 3 |
| Asthma status rated at CAMPCS transitione | ||
| In remission (mild) | 217 (51.1)c | 414 (84.7) |
| Moderate to severe | 190 (44.7) | 32 (6.5) |
Abbreviations: CAMP, Childhood Asthma Management Program; CAMPCS, CAMP Continuation Study; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid.
Data are given as number (percentage) of each group unless otherwise indicated.
Data are given as mean (SD).
P < .05 using the χ2 test for categorical variables and the t test for continuous variables.
Data are given as the median.
Percentages do not total 100 because 61 children did not have data available on the reclassification of severity at the time of the transition.
Asthma controller medication use reported in the entire cohort during the first 4 years of follow-up is shown in Figure 1. During year 1, 406 (44.0%) of 922 participants reported any asthma controller medication use. Also, 43% reported using ICSs during at least 1 of their encounters during the year; 28% reported only ICS use during that year, and 15% reported ICS use along with an additional asthma controller medication, such as a leukotriene receptor antagonist (LTRA) (7%) or a long-acting β-agonist (11%). During year 4, the number reporting controller medication use had decreased to 31%, with the number reporting ICS use decreasing to 24%. During the observation period, the reported use of LTRA monotherapy increased from 2% in year 1 to 6% in year 4.
Figure 1.
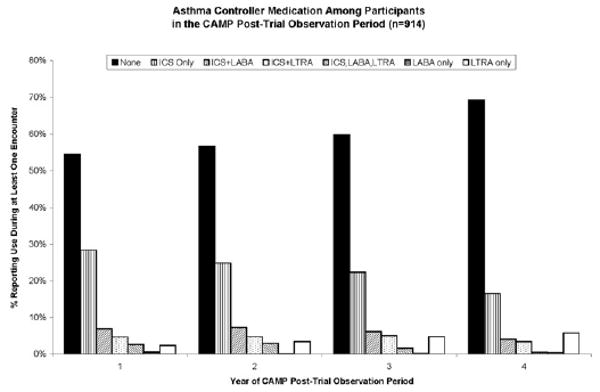
Asthma controller medication use reported in the Childhood Asthma Management Program posttrial observation period. The bars represent the percentage of participants (N = 914) who reported using any of 3 classes of asthma controller medication, either alone or in any combination during at least 1 of the 4 encounters during each follow-up year. ICS indicates inhaled corticosteroid; LABA, long-acting β-agonist; LTRA, leukotriene receptor antagonist.
Inhaled corticosteroid use was very uncommon among children who did not receive a recommendation to take an ICS; only 9% reported any ICS use during the first year of follow-up. Thus, our analysis focused on patterns of ICS use among the 425 participants who received a recommendation to continue receiving ICS therapy. During the first year of follow-up, 46% of these participants reported consistent ICS use, defined as reporting ICS use at all 4 encounters during the year, and 20% reported not using ICSs during any encounter (Fig 2). During year 1, children reporting consistent ICS use were more likely to be female (42% vs 31%), were more likely to have been rated as having moderate-to-severe asthma (57% vs 23%), and had a higher median serum IgE level (760 vs 529 U/mL) than those reporting no ICS use (P < .05). There were no significant differences in the age, FEV1, FEV1/FVC ratio, or median number of positive allergy skin test results among the ICS pattern groups in year 1 of follow-up. Within the ICS-recommended group over time, only 36 (8.5%) of 425 participants reported ICS use during all 8 clinic visits and all 8 interim telephone contacts during the 4-year observation period. During the 4 years of follow-up, the number of children reporting consistent ICS use decreased to 20% and the number reporting no ICS use increased to 57% (Fig 2).
Figure 2.
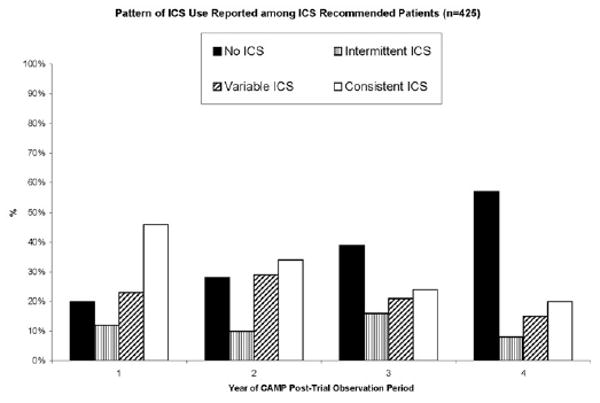
Patterns of inhaled corticosteroid (ICS) use among ICS-recommended participants in the Childhood Asthma Management Program posttrial observation period. The bars represent the patterns of ICS use reported by the ICS-recommended participants (n = 425). Each bar represents a distinct pattern of ICS use: none (0 of 4 encounters), intermittent (1 of 4 encounters), variable (2–3 of 4 encounters), or consistent (4 of 4 encounters).
Among the ICS-recommended group, there were 5,456 clinical encounters in which asthma control was evaluated. Asthma control at these encounters was the primary outcome in our multivariable model. Asthma that was not well controlled was reported during 1,002 (18.4%) of these encounters. Patients with asthma that was evaluated as moderate to severe were significantly more likely to report not well-controlled asthma compared with patients with asthma that was evaluated as mild (Table 2). In unadjusted analyses (Table 2), age, female sex, and having moderate-to-severe asthma were significantly associated with reporting not well-controlled asthma. Pulmonary function, as measured by FEV1, was not associated with the level of reported asthma control. The pattern of ICS use (intermittent, variable, or consistent) was significantly associated with not well-controlled asthma. In a multivariate generalized mixed regression model adjusting for clinician-rated asthma severity, age, sex, and pulmonary function, reporting either variable or consistent ICS use remained significantly associated with higher odds of reporting not well-controlled asthma (Table 2). The results did not change when different measures of pulmonary function (FEV1/FVC ratio or bronchial responsiveness as measured by PC20) were included separately or in combination in the models (data not shown).
Table 2. Unadjusted Analyses of Poor Asthma Control During the CAMPCS Encountersa.
| Predictor | Odds ratio (95% confidence interval) | |
|---|---|---|
| Unadjusted | Adjustedb | |
| ICS use pattern | ||
| None | Referent | Referent |
| Intermittent | 1.5 (1.1–2.0) | 1.4 (1.0–1.9) |
| Variable | 1.9 (1.6–2.9) | 1.8 (1.4–2.4) |
| Consistent | 1.6 (1.3–2.5) | 1.6 (1.2–2.1) |
| Asthma severity | ||
| Mild | Referent | Referent |
| Moderate to severe | 2.4 (1.8–3.2) | 2.0 (1.8–2.4) |
| Female sex | 1.8 (1.3–2.4) | 1.7 (1.5–2.0) |
| Age | 1.1 (1.0–1.2) | 1.1 (1.0–1.1) |
| FEV1c | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) |
Abbreviations: CAMPCS, Childhood Asthma Management Program Continuation Study; FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid.
Poor asthma control is defined as reporting 1 or more nocturnal awakenings, 3 or more days with activity limitations, or 3 or more days with use of β-agonists during the preceding 7 days.
Model adjusted for FEV1, age, sex, and asthma severity.
Pulmonary function data were obtained from the closest preceding encounter 3 to 15 months before the outcome encounter.
Discussion
Our analysis has characterized asthma controller medication use over time among children and adolescents with asthma enrolled in CAMPCS. We found that most participants reported well-controlled asthma symptoms and that no asthma controller medication was used during a 4-year observational period. Inhaled corticosteroid use continued to decrease during the study period and was not countered by a significant increased use of alternate asthma controller medications, such as LTRA. Among a subgroup of participants who were recommended to continue receiving ICS therapy at the outset of the observational period, reported rates of ICS use were higher but also diminished over time.
Our results illustrate trends in asthma medication use over time in a large cohort of participants with mild-to-moderate asthma. We observed a decline in the proportion of patients using ICS over time, with many patients reporting no use of an asthma controller medication during the 4-year observational period. Such a trend is not entirely surprising, because the cohort was mainly composed of adolescent patients. One explanation may be the natural history of asthma, demonstrated in previous epidemiologic studies, which has shown remission in asthma symptoms over time17–19; only a few children who report minimal symptoms during adolescence continue to have symptoms into adulthood.20,21 Even among patients without true remission, symptoms may improve over time. Patients and health care providers likely make decisions about asthma controller medication use based on their current symptoms. In the setting of well-controlled asthma, they choose to discontinue controller therapy. This is illustrated by the subgroup recommended to continue ICS therapy at the outset of the CAMPCS follow-up period. Among this subgroup, rates of reported ICS use decreased over time and because these participants reported well-controlled asthma symptoms during most of the clinical encounters, we can speculate that they or their treating clinicians made the decision to discontinue use of controller therapy. Such decisions are consistent with asthma treatment guidelines; a step-down approach to therapy is recommended for patients whose asthma symptoms are well controlled.1
In our analysis of asthma control over time, we found that female sex and clinician-evaluated asthma severity were independently associated with not well-controlled asthma. Such sex differences in asthma outcomes have been seen in other studies, with adolescent girls demonstrating worse asthma outcomes.22–24 Airway responsiveness is more severe in postpubertal females with asthma than in males25 and may contribute to increased asthma severity, increased episodes of poor control, and asthma medication use. An association between level of control and asthma severity is not surprising and has also been reported.26,27 In contrast, our findings revealed that participant age was unrelated to poor asthma control. Finally, contrary to our initial hypothesis that consistent ICS use would be associated with well-controlled asthma, participants who reported consistent ICS use were more likely to report not well-controlled asthma over time. This suggests that there are individuals who report not well-controlled asthma despite seemingly appropriate therapy.
In the multivariate analysis, we controlled for several accepted markers of asthma severity, including pulmonary function and clinician-rated severity evaluation, yet the association of increased medication use and not well-controlled asthma persisted. This may be the result of residual confounding by asthma severity, and the reported use of asthma medications would thus be interpreted as an additional marker of asthma disease severity.
However, other explanations should be considered, including patient nonadherence or other potential patient comorbidities. Depression has been shown to affect both asthma severity and control,28 and self-report of medication adherence tends to result in overestimating the patient's adherence. Patients or their parents often try to please their physicians by reporting what they think their physician wants to hear. This is supported by evidence from clinical trials that the mean number of doses taken, as recorded in diaries, is significantly higher than the number actually taken, as recorded by the monitoring devices, and most patients overestimate use of medication.29,30 In a 6-month ancillary study31 focusing on adherence among the CAMP posttrial observation cohort, monthly telephone encounters were conducted by nurses who had no prior relationship and no subsequent contact with the participants. In this study, low ICS adherence was associated with more frequent asthma symptoms. However, our data did not include other measures of medication adherence, and participants who were reporting consistent ICS use and poor control during their encounters may have been poorly adherent despite reporting taking ICS as prescribed.
An alternative explanation for our findings may be an inherent variability in response to asthma therapy, particularly the existence of relative steroid resistance. Variability in response to ICS has been demonstrated in other studies,32,33 and high rates of uncontrolled asthma have been identified in other populations in the setting of standard asthma medication use.34,35 In the current analysis, we have identified a subgroup of children and adolescents who did not achieve asthma control despite seemingly appropriate therapy. This subgroup may have relative steroid resistance, possibly as the result of genetic factors such as steroid receptor polymorphisms.36–38 A recent analysis of clinical data from the CAMP trial has examined a cohort with persistently poor response to ICSs, as defined by lung function response and exacerbations.39 Our current study supports the concept of steroid resistance, not just in the clinical trial setting but longitudinally in cohorts as well. In fact, given that many children with asthma improve their asthma control to the level that they are either asymptomatic or need only intermittent reliever therapy, our findings illustrate the natural history of a subgroup of asthmatic patients who may be predisposed to steroid resistance. Future work is needed to better characterize such individuals who do not achieve asthma control despite ICS or other appropriate controller therapy.
Our analysis has several limitations. Because CAMPCS is an observational study, and not a randomized trial of differing strategies for ICS use, the determinants of the different patterns of ICS use among patients are unclear. We did not have access to data on whether asthma medication was being prescribed by each participant's physician. We also did not have data on ICS dose regimens; therefore, it is possible that some participants were not receiving sufficient ICS doses to achieve asthma control.
In conclusion, we found low reported use of ICS and other asthma controller medications during the 4-year observational period of children and adolescents with mild-to-moderate asthma in the CAMP posttrial observation period, even among participants who received a recommendation to continue receiving ICS therapy at the end of the CAMP trial. In general, these children continued to have well-controlled asthma, suggesting that patients and clinicians are stepping down therapy in an appropriate manner based on current symptoms. However, we also identified a subgroup of children and adolescents who are not achieving well-controlled asthma despite reporting consistent use of ICSs. Medication nonadherence or the coexistence of asthma-related comorbidities may explain these findings and should be investigated in children and adolescents with difficult to control asthma. However, further study of the role of relative steroid resistance and its impact on the management of patients may help to better understand the relationship between medication use and asthma control.
Acknowledgments
We thank Kelan Tantisira, MD, for his critical review of and input in the manuscript.
Funding Sources: This study was supported by an unrestricted research grant from Merck Inc. CAMP is supported by contracts NO1-HR-16044, NO1-HR-16045, NO1-HR-16046, NO1-HR-16047, NO1-HR-16048, NO1-HR-16049, NO1-HR-16050, NO1-HR-16051, and NO1-HR-16052 from the National Heart, Lung, and Blood Institute and General Clinical, Research Center grants M01RR00051, M01RR0099718–24, M01RR02719–14, and RR00036 from the National Center for Research Resources.
Footnotes
Disclosures: Dr Fuhlbrigge has served as a consultant to GlaxoSmith-Kline, Merck, Novartis, and Sepracor (for epidemiologic studies); has received unrestricted research support from GlaxoSmithKline, Merck, and Boehringer Ingelheim; has served on an advisory board and been a member of a speakers bureau for GlaxoSmithKline and Merck; and has served as a member of the DSMB for an industry-sponsored clinical trial (Sepracor).
References
- 1.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma—Summary Report 2007. J Allergy Clin Immunol. 2007;120(suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.The Childhood Asthma Management Program Research Group Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 3.Lozano P, Finkelstein JA, Hecht J, Shulruff R, Weiss KB. Asthma medication use and disease burden in children in a primary care population. Arch Pediatr Adolesc Med. 2003;157:81–88. doi: 10.1001/archpedi.157.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Sawicki GS, Smith L, Bokhour B, et al. Periodic use of inhaled steroids in children with mild persistent asthma: what are pediatricians recommending? Clin Pediatr. 2008;47:446–451. doi: 10.1177/0009922807312184. [DOI] [PubMed] [Google Scholar]
- 5.Boushey HA, Sorkness CA, King TS, et al. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352:1519–1528. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 6.Bisgaard H, Hermansen MN, Loland L, Halkjaer LB, Buchvald F. Intermittent inhaled corticosteroids in infants with episodic wheezing. N Engl J Med. 2006;354:1998–2005. doi: 10.1056/NEJMoa054692. [DOI] [PubMed] [Google Scholar]
- 7.National Asthma Education and Prevention Program Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma Update on Selected Topics—2002. J Allergy Clin Immunol. 2002;110(suppl):S141–S219. [PubMed] [Google Scholar]
- 8.Vollmer WM, Markson LE, O'Connor E, et al. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med. 1999;160(pt 1):1647–1652. doi: 10.1164/ajrccm.160.5.9902098. [DOI] [PubMed] [Google Scholar]
- 9.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu AH, Zeiger R, Sorkness C, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 11.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the Asthma Control Test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Peters D, Chen C, Markson LE, Allen-Ramey FC, Vollmer WM. Using an asthma control questionnaire and administrative data to predict health-care utilization. Chest. 2006;129:918–924. doi: 10.1378/chest.129.4.918. [DOI] [PubMed] [Google Scholar]
- 13.Patel PH, Welsh C, Foggs MB. Improved asthma outcomes using a coordinated care approach in a large medical group. Dis Manag. 2004;7:102–111. doi: 10.1089/1093507041253235. [DOI] [PubMed] [Google Scholar]
- 14.Schatz M, Mosen D, Apter AJ, et al. Relationship of validated psychometric tools to subsequent medical utilization for asthma. J Allergy Clin Immunol. 2005;115:564–570. doi: 10.1016/j.jaci.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Schatz M, Zeiger RS, Drane A, et al. Reliability and predictive validity of the Asthma Control Test administered by telephone calls using speech recognition technology. J Allergy Clin Immunol. 2007;119:336–343. doi: 10.1016/j.jaci.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Childhood Asthma Management Program Research Group The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Control Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 17.Vonk JM, Postma DS, Boezen HM, et al. Childhood factors associated with asthma remission after 30 year follow up. Thorax. 2004;59:925–929. doi: 10.1136/thx.2003.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FD. Asthma treatment and asthma prevention: a tale of 2 parallel pathways. J Allergy Clin Immunol. 2007;119:30–33. doi: 10.1016/j.jaci.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Ernst P, Cai B, Blais L, Suissa S. The early course of newly diagnosed asthma. Am J Med. 2002;112:44–48. doi: 10.1016/s0002-9343(01)01033-6. [DOI] [PubMed] [Google Scholar]
- 20.Horak E, Lanigan A, Roberts M, et al. Longitudinal study of childhood wheezy bronchitis and asthma: outcome at age 42. Br Med J (Clin Res Ed) 2003;326:422–423. doi: 10.1136/bmj.326.7386.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oswald H, Phelan PD, Lanigan A, Hibbert M, Bowes G, Olinsky A. Outcome of childhood asthma in mid-adult life. Br Med J (Clin Res Ed) 1994;309:95–96. doi: 10.1136/bmj.309.6947.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 23.Debley JS, Redding GJ, Critchlow CW. Impact of adolescence and gender on asthma hospitalization: a population-based birth cohort study. Pediatr Pulmonol. 2004;38:443–450. doi: 10.1002/ppul.20108. [DOI] [PubMed] [Google Scholar]
- 24.Wright AL, Stern DA, Kauffmann F, Martinez FD. Factors influencing gender differences in the diagnosis and treatment of asthma in childhood: the Tucson Children's Respiratory Study. Pediatr Pulmonol. 2006;41:318–325. doi: 10.1002/ppul.20373. [DOI] [PubMed] [Google Scholar]
- 25.Tantisira KG, Colvin R, Tonascia J, Strunk RC, Weiss ST, Fuhlbrigge AL. Airway responsiveness in mild to moderate childhood asthma: sex influences on the natural history. Am J Respir Crit Care Med. 2008;178:325–331. doi: 10.1164/rccm.200708-1174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonicelli L, Bucca C, Neri M, et al. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23:723–729. doi: 10.1183/09031936.04.00004904. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Gould MK, Blanc PD, et al. Asthma control, severity, and quality of life: quantifying the effect of uncontrolled disease. J Allergy Clin Immunol. 2007;120:396–402. doi: 10.1016/j.jaci.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 28.Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. J Allergy Clin Immunol. 2008;122:490–495. doi: 10.1016/j.jaci.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Jonasson G, Carlsen KH, Sodal A, Jonasson C, Mowinckel P. Patient compliance in a clinical trial with inhaled budesonide in children with mild asthma. Eur Respir J. 1999;14:150–154. doi: 10.1034/j.1399-3003.1999.14a25.x. [DOI] [PubMed] [Google Scholar]
- 30.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98(pt 1):1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 31.Bender B, Rankin A, Tran ZV, Wamboldt FS. Brief interval telephone surveys of medication adherence and asthma symptoms in the Childhood Asthma Management Program Continuation Study. Ann Allergy Asthma Immunol. 2008;101:382–386. doi: 10.1016/S1081-1206(10)60314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szefler SJ, Martin RJ, King TS, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 33.Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007;119:1454–1461. doi: 10.1016/j.jaci.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Cazzoletti L, Marcon A, Janson C, et al. Asthma control in Europe: a real-world evaluation based on an international population-based study. J Allergy Clin Immunol. 2007;120:1360–1367. doi: 10.1016/j.jaci.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Tantisira KG, Lake S, Silverman ES, et al. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004;13:1353–1359. doi: 10.1093/hmg/ddh149. [DOI] [PubMed] [Google Scholar]
- 37.Weiss ST, Lake SL, Silverman ES, et al. Asthma steroid pharmacogenetics: a study strategy to identify replicated treatment responses. Proc Am Thorac Soc. 2004;1:364–367. doi: 10.1513/pats.200409-043MS. [DOI] [PubMed] [Google Scholar]
- 38.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers AJ, Tantisira KG, Fuhlbrigge AL, et al. Predictors of poor response during asthma therapy differ with definition of outcome. Pharmacogenomics. 2009;10:1231–1242. doi: 10.2217/PGS.09.86. [DOI] [PMC free article] [PubMed] [Google Scholar]


