Abstract
Introduction
Relatively few studies have interrogated gene-by sedentary behavior interactions on obesity phenotypes. Increasing sedentary behaviors (e.g., screen time: television, video, and computer use), have paralleled declines in physical activity in the United States and may play an important role in weight gain. Our purpose in this paper is to examine the possible influence of screen time during adolescence on the additive genetic variance of body mass change during a critical point in the lifecycle, the transition from adolescence to young adulthood.
Methods
In the National Longitudinal Study of Adolescent Health, siblings and twin pairs (16.5±1.7 y) were followed into young adulthood (22.4±1.8 y). Self-reported screen time (hrs/wk) and body mass index (BMI kg/m2), calculated from measured height and weight at adolescence and at young adulthood, was available for 3795 participants. We employed a variance component approach to estimate the interaction between genotype and screen time for body mass change. Additive genotype-by-screen time interactions were assessed using likelihood-ratio tests. Models were adjusted for race, age, sex, and age-by-sex interaction.
Results
The genetic variation in body mass change was significantly larger in individuals with low (σG=27.59±1.58) compared to high (σG=18.76 ± 2.59) levels of screen time (P<0.003) during adolescence.
Conclusions
Our findings demonstrate that sedentary behaviors during adolescence may interact with genetic factors to influence body mass change between adolescence and young adulthood. Accounting for obesity-related behaviors may improve current understanding of the genetic variation in body mass change.
Keywords: Body mass change, gene by environment interaction, screen time, adolescence, obesity
INTRODUCTION
The complex interplay between genes and environmental factors in relation to changes in body mass index (BMI) across the lifecycle is not well understood. There is a substantial heritability of BMI and BMI change demonstrated by twin, adoption and family studies [1–5], and we are incrementally making progress in the identification of susceptibility alleles (i.e., ,FTO, MC4R, NEGR1, etc) associated with obesity related traits[6–7]. However, very few studies have addressed the complexity of the obesity phenotype by incorporating both genetic and environmental effects. Indeed, a wealth of research supports a role for behavioral and environmental factors in weight gain [8–9]. Thus, the interaction of behavioral factors related to energy balance (i.e. diet and physical activity) with genetic susceptibility are likely important and understudied.
The transition from adolescence to young adulthood is accompanied by substantial weight gain, declining physical activity, and increasing sedentary behaviors, such as screen time (i.e. TV, video, and computer use) [10–11]. Some but not all studies have demonstrated that sedentary behaviors increase the risk for weight gain [11–13]. Intervention research, including randomized controlled trials, has shown declines in BMI in children with reductions in screen time [14–15].
Previous studies in twins demonstrated that physical activity reduced the influence of genetic factors on the development of obesity [16–18], suggesting that the individuals at greatest genetic risk for obesity would benefit the most from physical activity. On the other hand, whether sedentary behaviors modify genetic susceptibility to weight gain has not yet been studied. The transition between adolescence and adulthood offers important opportunities for understanding genetic and environmental influences on weight gain. Understanding how differences in sedentary behavior interact with genetic factors during adolescence to further influence changes in body mass can help inform appropriate intervention programs. Screen time habits are particularly relevant as a modifiable factor shown to be related to obesity, independent of physical activity [19–21].
Gene-environment interactions are understudied because environmental data are difficult to quantify and the estimation of interactive effects requires large sample sizes and accurate phenotypic and genotypic measures. Data from the National Longitudinal Study of Adolescent Health (Add Health) includes descriptions of sibling relationships (i.e. twins, full siblings, cousins, etc) and genotypic measures (used to determine twin zygosity) and longitudinal measures of physical activity, sedentary behaviors, and BMI. Therefore, we used these measures to examine interactions among sedentary behaviors and genetic factors with body mass change.
We previously calculated a heritability of 0.43±0.05 (p<1×10-7) for body mass change across adolescence into young adulthood in the Add Health cohort[22]. Furthermore, we found evidence that factors in the shared household environment were relatively more important during adolescence versus young adulthood in terms of their significant influence on BMI change.
The purpose of this study was to examine the possible influence of screen time during adolescence on the additive genetic variance of body mass change during the transition from adolescence to young adulthood. We hypothesized that we would find distinct additive genetic effects in individuals with low versus high levels of screen time during adolescence. We used a variance component approach that applies maximum likelihood estimation to test for the presence of additive genotype-by screen time interaction by comparing models with and without the interaction term.
METHODS AND PROCEDURES
Study population
The parent study, the National Longitudinal Study of Adolescent Health (Add Health) is a nationally representative, longitudinal survey of youths, grades 7 to 12 at baseline (Wave I), followed through adolescence (Wave II) and into young adulthood (Wave III). Survey procedures described elsewhere [23] were approved by the Institutional Review Board, University of North Carolina at Chapel Hill. The Add Health cohort included a Family Subsample that is the focus of the present analysis.
Family Subsample
Add Health oversampled respondents sharing a household with related (twins, full siblings, one-half siblings, cousins) and non-related adolescents. Of 4782 adolescents, ages13–20 years, 4588 were available in 2001 for followup as young adults, ages 18 to 27 years (see Figure 1). Non-twin sibships were classified by self-report. Twin zygosity was determined using DNA by matching 12 molecular genetic markers at Waves I or III (n = 808) [24] or by full agreement of self-report measures (n = 683) from a detailed list of questions (see under Section 38, questions 1–14 at http://www.cpc.unc.edu/projects/addhealth/codebooks/wave2). Using DNA extracted from buccal cell samples, zygosity was tested for twin pairs. DNA was genotyped at the Institute of Behavioral Genetics (http://ibgwww.colorado.edu/genotyping_lab), University of Colorado (Boulder, CO). Zygosity was not determined for 83 participants who were therefore excluded. In addition, we excluded 793 participants who were severely disabled (n = 100), pregnant (n=169), or missing primary exposure variables for adolescent and young adult BMI, and adolescent screen time (n=441). After exclusion criteria were met, our sample included 3795 individuals. In addition, 26 participants were excluded from these analyses because they were outliers for BMI change(see explanation below). For the current analysis, our final sample included 3769 participants, 1945 females and 1824 males.
Figure 1.
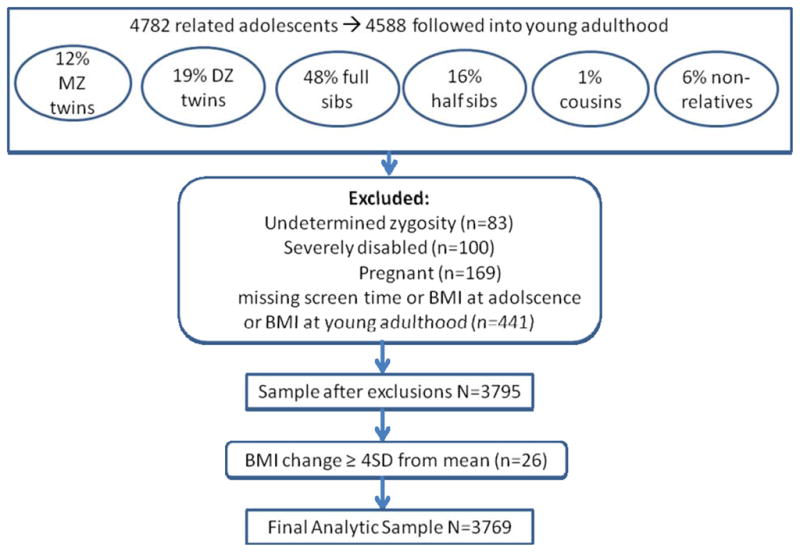
Study population included adolescents from among the Add Health family subsample who were followed into young adulthood. Exclusion criteria limited the sample to 3795 individuals, of whom 26 were considered outliers based on the outcome, BMI change between adolescence and young adulthood.
BMI
BMI (kilograms per meter squared) was calculated from measured height and weight, assessed at Waves II and III using standardized procedures. Self-reported height and weight were substituted for those refusing measurement and/or weighing more than the scale capacity (Wave II, n = 54; III, n = 155). Self-reported data have been shown to correctly classify a large proportion of the Add Health sample [10, 25]. Our main outcome measure, BMI change was calculated as the difference between BMI obtained during adolescence (Wave II) and young adulthood (Wave III). BMI change for 6 females and 20 males were considered outliers, based on a cutoff of 4 standard deviations from the mean, and thus these individuals were removed from further analyses. In addition, BMI change values were natural log transformed to improve the distribution of these data, and scaled by 10. Natural log transformation improved kurtosis for BMI change to below 1.0.
Screen time
Screen time (i.e., TV, video, and computer use) is a standard measure of sedentary behavior. Screen time during adolescence (Wave II) was ascertained using a standard, interview administered activity recall based on questionnaires validated in other epidemiologic studies [26]. The screen time items elicited information separately on hours of TV, video, and computer game use, which were summed to create a measure of screen time in hours per week.
We dichotomized screen time as high if the participant engaged in greater than 14 hours per week of TV, video, and computer game use, and low if screen time was 14 hours or less per week, based on the American Academy of Pediatrics recommendation [27]. We considered this dichotomized screen time variable at Wave II as the main exposure of interest in our quantitative genetic analyses. Previously, we determined that the influence of shared household environment during adolescence, which includes patterns of physical inactivity, had a significant association with BMI change between adolescence and young adulthood [22]. Thus, we used the adolescent screen time measure as our primary exposure.
Statistical methods
To examine the genetic architecture of BMI change as a function of screen time we tested for the presence of additive genotype-by screen time interaction using a variance component approach. In the univariate analysis, the covariance is given by:
where 2Φij σ2g is the additive genetic effect (Φ is the coefficient of kinship between two individuals) and Iσ2e is the residual environmental effect.
To test for gene-by-environment interaction, the univariate variance component model is extended to include the genetic covariance between relative pairs in two environments and the residual environmental variance between relative pairs in the two environments. These analyses use the information of all relative-pairs. The expected genetic covariance between relative pairs with low levels of screen time and relative pairs with high levels of screen time (i,j) is:
where the subscripts LST and HST refer to low levels and high levels of screen time, respectively, ρg(LST, HST) is the additive genetic correlation between the expressions of the trait in the two groups, and σgLST and σgHST are the genetic standard deviations for low levels and high levels of screen time, respectively. σeLST and σeHST are the residual environmental standard deviations for low levels and high levels of screen time, respectively. If there is additive genotype-by-screen time interaction, the genetic correlation between the groups will be significantly less than one (HA: ρG(LST, HST ) < 1.0) and/or the genetic standard deviations will not be equal between the groups (HA: σgLST ≠ σgHST). If there is a residual environment by screen time interaction, the residual environmental standard deviations will not be equal between the groups. Significance of an additive genotype-by-screen time interaction was assessed using likelihood-ratio tests (α = 0.05) that compare the likelihood of a model that includes a genotype-by-screen time interaction parameter against a restricted model that excludes the interaction [28–30]. When comparing models with standard deviations constrained to be equal, interpretation of significant differences were based on the assumption of an asymptotic distribution for the likelihood test statistic. A significant difference between the genetic standard deviations suggests that the magnitude of the genetic component for BMI change is different in high versus low screen time groups. A significant difference between the residual environmental standard deviations suggests that the magnitude of the residual environmental component for BMI change is different in high versus low screen time groups. However, for the model that restricted the genetic correlation to one, the genetic correlation was constrained to the upper boundary of the parameter space (ρG = 1.0), thus the test statistic is as a 1/2:1/2 mixture of a distribution and a point mass at zero [31]. Significant differences between the models would suggest distinct genetic effects that influence the change in BMI in individuals with high versus low screen time.
All models are summarized in Table 1. Models were adjusted for race, age, sex, and age-by-sex interaction in the context of the variance components analyses irrespective of statistical significance and all analyses were performed in SOLAR (Southwest Foundation for Biomedical Research, San Antonio, TX).
Table 1.
Description of estimates to determine the presence and interpretation of an additive genotype-by-screen time interaction.
| Description | Null Hypothesis | Tests | Results | Inference |
|---|---|---|---|---|
| Genetic standard deviations for the two environments are statistically different from one another | σgLST= σgHST | Compare the likelihood ratio of a model where σgLST = σgHST to the likelihood ratio of a model without such a constraint | σgLST ≠ σgHST | Magnitude of genetic influence is different in the two environments |
| Residual environmental standard deviations for the two environments are statistically different from one another | σeLST = σeHST | Compare the likelihood ratio of a model where σeLST = σeHST to the likelihood ratio of a model without such a constraint | σeLST ≠ σeHST | Magnitude of residual environmental influence is different in the two environments |
| Genetic correlation between relatives for BMI change in two environments is different than 1 (high versus low screen time) | ρG(LST, HST ) = 1.0 | Compare the likelihood ratio of a model with ρG(LST, HST ) = 1 to the likelihood ratio of a model without such a constraint | ρG (LST,HST) =1.0 | The proportion of the genetic variance in BMI change is similar between the two environments |
| ρG (LST,HST) < 1.0 | Significant proportion of genes that contribute additive genetic variance of BMI change is different between the two environments |
Only one estimate, the genetic standard deviations or the genetic correlation, are constrained at a time while the other is allowed to vary naturally.
RESULTS
During adolescence mean BMI was 22.91 ± 4.85 kg/m2 and increased to 26.2 ± 6.11 kg/m2 during young adulthood making an overall mean change in BMI of 3.21 ± 3.37 kg/m2 (Table 2). Females had a larger increase in BMI (3.25 ± 3.60 kg/m2) from adolescence to young adulthood than males (3.17± 3.12 kg/m2). Baseline (adolescent) screen time was divided into low and high screen time, resulting in mean screen time viewing of 36.49 ± 24.01 hours/week of screen time in the high screen time group versus 7.86 ± 3.75 hours/week in the low screen time group. BMI increases from adolescence to adulthood were larger in individuals with high (3.38 ± 3.14 kg/m2)versus low (3.19 ± 3.62 kg/m2) baseline screen time.
Table 2.
Mean ±SD for the total sample and stratified by gender and high or low screen time during adolescence for BMI and BMI Change in the transition from adolescence to young adulthood.
| Total (Mean ±SD) | Females (Mean ±SD) | Males (Mean ±SD) | High screen time group | Low screen time group | |
|---|---|---|---|---|---|
| N=37951 | N=19511 | N=18441 | N=20411 | N=17541 | |
| BMI during adolescence | 22.91 ± 4.85 | 22.85 ± 5.04 | 22.94 ± 4.65 | 23.01 ± 5.02 | 22.80 ± 4.66 |
| BMI during young adulthood | 26.2 ± 6.11 | 26.23 ± 6.62 | 26.16 ± 5.53 | 26.39 ± 6.24 | 25.98 ± 5.95 |
| BMI change1,2adolescence to young adulthood | 3.21 ± 3.37 | 3.25 ± 3.60 | 3.17 ± 3.12 | 3.38 ± 3.14 | 3.19 ± 3.62 |
BMI change for 6 females and 20 males were considered outliers and thus these individuals were removed from further analyses.
Total sample N=3769, Males N=1824, Females N=1945, High screen time group N=2025, Low screen time group N=1744
BMI change was calculated as the difference between BMI obtained during Wave II (adolescence) and Wave III (young adulthood).
Genetic architecture and screen time during adolescence
When considering levels of screen time during the adolescent period, the additive genetic standard deviation for BMI change was 27.59 ± 1.58 for the low screen time group and 18.76 ± 2.59 for the high screen time group (Table 3). The corresponding heritability for the low screen time was 64.7% and for high screen time 32.1%, respectively. Evidence that the magnitude of the genetic effect on BMI change was different based on level of screen time during the adolescent period was illustrated by the significantly poor model fit (P < 0.003) in which the genetic standard deviations in those with low and high levels of screen time were constrained to be equal (σgLST = σgHST)compared to the fit of the general model in which such interaction was allowed. The genetic correlation (ρG) for BMI change between high and low screen time groups (Table 3) was not significantly different from 1.0 [ρG (LST, HST) =1.0; P=0.08] suggesting that the proportion of the genetic variance in BMI change is similar between the two environments. In other words, it is likely that similar genes influence BMI change between adolescents with low compared to high screen time.
Table 3.
Maximum Likelihood Estimates of Variance Components from Analysis of Genotype-by-Environment Interaction for BMI Change*
| Screen time during Adolescence | |
|---|---|
| σgLST | N=1744 |
| 27.59 ± 1.58 | |
| σgHST | N=2025 |
| 18.76 ± 2.59a | |
| σeLST | N=1744 |
| 20.36 ± 1.63 | |
| σeHST | N=2025 |
| 27.28 ± 3.26b | |
| ρG | 0.67 ± 0.22c |
Based on BMI change values that were natural log transformed and scaled by 10. LST = low screen time group; HST = high screen time group;
σg = genetic standard deviation; σe = environmental standard deviation; ρG = genetic correlation
Test for difference in σg for model with BMI change for σgLST = σgHST to a model without such a constraint, P < 0.003.
Test for difference in σe for model with BMI change for σeLST = σeHST to a model without such a constraint, P < 0.05.
Test for ρG less than 1 for BMI change between LST and HST, P = 0.08.
To further examine the influence on BMI change from other unmeasured environmental factors and screen time interaction, we tested whether or not the residual environmental variances on BMI change between the two screen time groups were equal. Evidence that the magnitude of the residual environmental effect on BMI change was different based on level of screen time during the adolescent period was illustrated by the significantly poor model fit (P < 0.05) in which the residual environmental standard deviations in those with low and high levels of screen time were constrained to be equal (σeLST = σeHST)compared to the fit of the general model in which such interaction was allowed.
DISCUSSION
In the United States, the transition from adolescence to young adulthood is accompanied by increases in time spent in sedentary behaviors, such as screen time [11]. In this study, we identified important gene-by-sedentary behavior interactions on BMI change. In particular, we found evidence that genetic factors influencing BMI change were differentially expressed in individuals with high levels of screen time, compared to those individuals with low levels of screen time. These findings are important because the etiology of body mass change is complex with both genetic and environmental components, but with limited research that has tackled this complex relationship.
An increasing number of studies illustrate that genes as well as environmental factors are important in the etiology of BMI and BMI change. Many studies have examined the relationships between physical activity, genetic susceptibility, and obesity related measures [16–18]. However, the relationship between sedentary behaviors, such as screen time, and genetic susceptibility has been largely unexplored [32] and may be particularly crucial to understanding the increase in BMI with age as adolescents transition to young adulthood.
We found evidence that the variation in the genetic component of BMI change from adolescence to young adulthood was different among individuals with low versus high levels of baseline (adolescent) screen time. Among adolescents with low screen time (7.86 ± 3.75 hours/week), the additive influence from genes was larger than for the adolescents that reported high screen time (36.49 ± 24.01 hours/week). From these results we infer that behavioral factors can modify the genetic effects on BMI change. The genetic component of variance is larger for individuals with low versus high baseline (adolescent) screen time and the residual environmental component of variance is smaller for individuals with low versus high baseline (adolescent) screen time. Thus, for adolescents that often invest extensive time watching TV or videos, the time spent in sedentary pursuits has a substantial influence on body mass seemingly reducing the importance of genetic susceptibility. In conjunction with previous findings in young adult twins demonstrating that physical activity reduced the influence of genetic factors on the development of obesity [16–18], our results further suggest that genetic susceptibility for BMI gain is also lowest in individuals that are extremely sedentary. Thus, behaviors (be it those individuals that were apparently extremely sedentary or in other studies those persons that show extreme levels of physical activity), demonstrate a modification of the underlying genetic susceptibility for BMI gain (in both cases demonstrating lower heritabilities than their comparison groups).
Our findings illustrate that there may be a complex interplay between genes and environment affecting body mass change between the period of adolescence and young adulthood. We know little about how changes in BMI are affected by individual susceptibility to environmental contexts and individual behaviors, such as screen time. The transitional period between adolescence and young adulthood is a particularly important time to investigate such relationships, as it is a major lifecycle period of risk for weight gain. From the genetics perspective, studying periods of rapid weight gain provides focus on the maximal expression of phenotypes of interest [33]. From the environment perspective, it is well known that periods of rapid growth are also periods of heightened sensitivity to environmental inputs [34–36]. Clearly more research is needed on the dynamic and complex relationship between genes and environment over lifecycle periods of risk for weight gain.
We acknowledge important limitations to our study. First, it is limited by the self-reported information on sedentary behaviors which were obtained in an interview. However, questionnaires are the most feasible methodology for assessing such behaviors in a large, population-based study and have been shown to have moderate-high reliability [37]. Such questionnaires combining television viewing and computer game use fared best in terms of validity, but also showed television viewing to be under-reported [38]. Second, we used self-reported weight for individuals who exceeded scale capacity (Wave II, n =54; III, n = 155). In our sample, even in cases where obese individuals underestimated their weight, their self-report weights still put them in the obese category. Even if they were within the upper limits of the scale, their BMI values would still be within the tails of the distribution. Self reported values have been shown to correctly classify a large proportion of the Add Health sample [10, 25].
Third, heritability estimates obtained from sib-based data sets could be inflated because such data do not account for either shared or common environmental factors or dominance effects. In addition, it is important to note that this is a quantitative genetic analysis and no specific gene or set of genes were investigated.
In summary, our analyses suggest that behavioral factors (i.e. sedentary behavior) can modify the additive genetic effects on BMI change between adolescence and early adulthood. The genetic component of variance was smaller in those individuals that reported extreme levels of sedentary behavior in comparison to those that were not as sedentary. Thus, for adolescents who spend a considerable amount of time watching TV or videos, the time spent in sedentary pursuits may have a substantial influence on the expression of their inherit underlying genetic susceptibility. Our results could improve current understanding of gene-environment interaction and BMI change. In our case, we observed different influences from genes based on level of screen time. Our findings reinforce the importance of obesogenic behaviors, like screen time, in public health efforts to reduce weight gain.
Acknowledgments
This work was funded by NIH, R01HD057194. This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, funded by P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, CPC, 123 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth@unc.edu). No direct support was received from grant P01-HD31921 for this analysis. We thank Add Health participants, and the collaborators & staff of Add Health.
Footnotes
DISCLOSURE
The authors declare no conflict of interest.
References
- 1.Duncan A, et al. Genetic and environmental contributions to BMI in adolescent and young adult women. Obesity. 2009;17(5):1040–1043. doi: 10.1038/oby.2008.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox C, et al. Genomewide linkage analysis of weight change in the Framingham Heart Study. The Journal of Endocrinology & Metabolism. 2005;90:3197–3201. doi: 10.1210/jc.2004-1752. [DOI] [PubMed] [Google Scholar]
- 3.Maes HN, MC, Eaves L. Genetic and environmental factors in relative body weight and human adiposity. Behavioral Genetics. 1997;27:325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 4.Schousboe K, et al. Sex differences in heritability of BMI: A comparative study of results from twin studies in eight countries. Twin Research. 2003;6(5):409–421. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- 5.Hjelmborg JB, et al. Genetic influences on growth traits of BMI: A longitudinal study of adult twins. Obesity. 2008;16(4):847–852. doi: 10.1038/oby.2007.135. [DOI] [PubMed] [Google Scholar]
- 6.Calton MA, Vaisse C. Narrowing down the role of common variants in the genetic predisposition to obesity. Genome Med. 2009;1(3):31. doi: 10.1186/gm31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutch D, Clement K. Unraveling the genetics of human obesity. PLOS Genetics. 2006;2:e188. doi: 10.1371/journal.pgen.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson P. Reducing overweight and obesity: closing the gap between primary care and public health. Family Practice. 2008;25:i10–i16. doi: 10.1093/fampra/cmn060. [DOI] [PubMed] [Google Scholar]
- 9.Avenell A, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technology Assessment. 2004;8(21) doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 10.Gordon-Larsen P, et al. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. American Journal of Clinical Nutrition. 2004;80(3):569–575. doi: 10.1093/ajcn/80.3.569. [DOI] [PubMed] [Google Scholar]
- 11.Must A, Tybor DJ. Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. Int J Obes (Lond) 2005;29(Suppl 2):S84–96. doi: 10.1038/sj.ijo.0803064. [DOI] [PubMed] [Google Scholar]
- 12.Catenacci V, Wyatt H. The role of physical activity in producing and maintaining weight loss. Nature Clinical Practice Endocrinology & Metabolism. 2007;3(7):518–529. doi: 10.1038/ncpendmet0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson D, Madans J, Pamuk E. A prospective study of childbearing and 10-year weight gain in US white women 25 to 45 years of age. Int J Obes Relat Metab Disord. 1994;18:561–69. [PubMed] [Google Scholar]
- 14.Epstein LH, et al. A randomized trial of the effects of reducing television viewing and computer use on body mass index in young children. Arch Pediatr Adolesc Med. 2008;162(3):239–45. doi: 10.1001/archpediatrics.2007.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson TN. Reducing children’s television viewing to prevent obesity: a randomized controlled trial. JAMA. 1999;282(16):1561–7. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- 16.Heitmann BL, et al. Are genetic determinants of weight gain modified by leisure-time physical activity? A prospective study of Finnish twins. Am J Clin Nutr. 1997;66(3):672–8. doi: 10.1093/ajcn/66.3.672. [DOI] [PubMed] [Google Scholar]
- 17.Karnehed N, et al. Physical activity, diet and gene-environment interactions in relation to body mass index and waist circumference: the Swedish young male twins study. Public Health Nutr. 2006;9(7):851–8. doi: 10.1017/phn2005926. [DOI] [PubMed] [Google Scholar]
- 18.Mustelin L, et al. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes (Lond) 2009;33(1):29–36. doi: 10.1038/ijo.2008.258. [DOI] [PubMed] [Google Scholar]
- 19.Ching P, et al. Activity level and risk of overweight in male health professionals. Am J Public Health. 1996;86:25–30. doi: 10.2105/ajph.86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu F, et al. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 21.Jeffery R, French S. Epidemic obesity in the United States: are fast foods and television viewing contributing? Am J Public Health. 1998;88:277–280. doi: 10.2105/ajph.88.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.North KE, et al. Genetic epidemiology of BMI and body mass change from adolescence to young adulthood. Obesity (Silver Spring) 2010;18(7):1474–6. doi: 10.1038/oby.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popkin B, Udry J. Adolescent obesity increases signifiantly in second and third generation US immigrants. Journal of Nutrition. 1998;128:701–706. doi: 10.1093/jn/128.4.701. [DOI] [PubMed] [Google Scholar]
- 24.Rowe D, Jacobsen K, van den Oord E. Genetic and environmental influence on vocabulary IQ. Child Development. 1999;70:1151–1162. doi: 10.1111/1467-8624.00084. [DOI] [PubMed] [Google Scholar]
- 25.Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106(1 Pt 1):52–8. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- 26.Gordon-Larsen P, McMurray RG, Popkin BM. Determinants of adolescent physical activity and inactivity patterns. Pediatrics. 2000;105(6):E83. doi: 10.1542/peds.105.6.e83. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Pediatrics Committee on Public Education. Children, adolescents, and television. Pediatrics. 2001;107:423–426. doi: 10.1542/peds.107.2.423. [DOI] [PubMed] [Google Scholar]
- 28.Comuzzie A, et al. The quantiative geneics of sexual dimorphism in body fat measurements. American Journal of Human Biology. 1993;5:725–734. doi: 10.1002/ajhb.1310050616. [DOI] [PubMed] [Google Scholar]
- 29.Robertson A. The sampling variance of the genetic correlation coefficient. Biometrics. 1959;15:469–485. [Google Scholar]
- 30.Towne B, Blangero J, Mott G. Genetic analysis of sexual dimorphism in serum apo A1 and HDL-C concentrations in baboons. American Journal of Primatology. 1992;27:107–117. doi: 10.1002/ajp.1350270206. [DOI] [PubMed] [Google Scholar]
- 31.Eisen E, Legates J. Genotype-sex interaction and the genetic correlation between the sexes for body weight in Mus musculus. Genetics. 1966;54(2):611–623. doi: 10.1093/genetics/54.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owen N, et al. Environmental determinants of physical activity and sedentary behavior. Exerc Sport Sci Rev. 2000;28(4):153–8. [PubMed] [Google Scholar]
- 33.Strug L, Sun L, Corey M. The genetics of cross-sectional and longitudinal body mass index. BMC Genetics. 2003;4:S14–S19. doi: 10.1186/1471-2156-4-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adair LS, Prentice A. A critical evaluation of the fetal origins hypothesis and its implications for developing countries. J Nutr. 2004;134(1):191–193. doi: 10.1093/jn/134.1.191. [DOI] [PubMed] [Google Scholar]
- 35.Cameron N, Demerath EW. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol. 2002;(Suppl 35):159–84. doi: 10.1002/ajpa.10183. [DOI] [PubMed] [Google Scholar]
- 36.Dietz W. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- 37.Clark B, et al. Validity and reliability of measures of television viewing time and other non-occupational sedentary behaviour of adults: a review. Obesity Reviews. 2009;10(1):7–16. doi: 10.1111/j.1467-789X.2008.00508.x. [DOI] [PubMed] [Google Scholar]
- 38.Matton L, et al. Reliability and validity of the Flemish Physical Activity Computerized Questionnaire in adults. Res Q Exerc Sport. 2007;78:293–306. doi: 10.1080/02701367.2007.10599427. [DOI] [PubMed] [Google Scholar]


