Abstract
Fatty acids may integrate into cell membranes to change physical properties of cell membranes, and subsequently alter cell functions in an unsaturation number-dependent manner. To address the roles of fatty acid unsaturation numbers in cellular pathways of Alzheimer's disease (AD), we systematically investigated the effects of fatty acids on cell membrane fluidity and α-secretase-cleaved soluble amyloid precursor protein (sAPPα) secretion in relations to unsaturation numbers using stearic acid (SA, 18:0), oleic acid (OA, 18:1), linoleic acid (LA, 18:2), α-linolenic acid (ALA, 18:3), arachidonic acid (AA, 20:4), eicosapentaenoic acid (EPA, 20:5), and docosahexaenoic acid (DHA, 22:6). Treatments of differentiated human neuroblastoma (SH-SY5Y cells) with AA, EPA and DHA for 24 h increased sAPPα secretion and membrane fluidity, whereas those treatments with SA, OA, LA and ALA did not. Treatments with AA and DHA did not alter the total expressions of amyloid precursor protein (APP) and α-secretases in SH-SY5Y cells. These results suggested that not all unsaturated fatty acids but only those with 4 or more double bonds, such as AA, EPA and DHA, are able to increase membrane fluidity and lead to increase in sAPPα secretion. This study provides insights into dietary strategies for the prevention of AD.
Keywords: Fatty acids, membrane fluidity, sAPPα, Alzheimer's disease
1. Introduction
The accumulation of neurotoxic amyloid-β peptide (Aβ) is a pathologically profound characteristic of Alzheimer's disease (AD) (McGeer et al., 1987; Perlmutter et al., 1990; Frautschy et al., 1998; Dickson, 1999; Stalder et al., 1999; Selkoe, 2000). Aβ is derived from amyloid precursor protein (APP) processing through the amyloidogenic pathway, in which APP is cleaved sequentially by β- and γ-secretases (Vassar, 2004). BACE1 is the major β-secretase for generation of Aβ by neurons (Cai et al., 2001). Alternatively, in the non-amyloidogenic pathway, APP is cleaved by α-secretases between amino acids 16 and 17 within the Aβ domain to produce sAPPα. α-secretases are members of ADAMs (a disintegrin and metalloprotease), including ADAM9, 10, 17 and 19. sAPPα is neurotrophic and neuroprotective (Thornton et al., 2006) and enhancing APP processing by α-secretases has been suggested as a potential therapeutic strategy for AD (Cheng et al., 2007). Since APP, α-, β- and γ-secretases are membrane protein molecules, APP processing should be governed by the local membrane environment. For example, the activity of β-secretase takes place preferentially in highly molecularly-ordered lipid rafts which are cholesterol, saturated phospholipids and sphingolipids-enriched microdomains (Tun et al., 2002; Cordy et al., 2003; Ehehalt et al., 2003; Marlow et al., 2003; Kaether and Haass, 2004; Vetrivel et al., 2004), while the activity of α-secretase is favorable in non-raft domains (Reid et al., 2007). Therefore, APP processing can be manipulated by changing the contents of membrane components, such as cholesterol and sphingolipids (Simons et al., 1998; Kojro et al., 2001; Sawamura et al., 2004; von Arnim et al., 2008).
Since fatty acids are capable of modulating membrane organization and functions (Yehuda et al., 2002; Stillwell et al., 2005; Shaikh and Edidin, 2006; Pepe, 2007; Shaikh and Edidin, 2008), we hypothesized that the effects of fatty acids (e.g. AA) on membrane fluidity and sAPPα secretion are dependent on their unsaturation numbers (i.e. the number of double bonds in the hydrocarbon chains). In this study, we systematically examined the effects of fatty acids with unsaturation numbers ranging from 0 to 6 double bonds including stearic acid (SA, 18:0), oleic acid (OA, 18:1), linoleic acid (LA, 18:2), α-linolenic acid (ALA, 18:3), arachidonic acid (AA, 20:4), eicosapentaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6) on membrane fluidity and sAPPα secretion in differentiated SH-SY5Y cells. Since these fatty acids are ingredients in daily food, information derived from this study should provide potential dietary strategy for prevention of AD.
2. Materials and Methods
2.1. Chemicals and Reagents
Dulbecco's modified Eagle's medium (DMEM)/F12 medium (1:1) and fetal bovine serum (FBS) and 5-hexadecanoylaminofluorescein were from Invitrogen (Carlsbad, CA). Stearic acid (SA, 18:0), oleic acid (OA, 18:1), linoleic acid (LA, 18:2), α-linolenic acid (ALA, 18:3), arachidonic acid (AA, 20:4), eicosapentaenoic acid (EPA, 20:5), docosahexaenoic acid (DHA, 22:6), cis-parinaric acid, Albumin from bovine serum (BSA), phorbol 12-myristate 13-acetate (PMA), dimethyl sulfoxide (DMSO) and all-trans retinoic acid (RA) were from Sigma-Aldrich (St. Louis, MO). Cis-2-eicosenoic acid and cis-5, 8, 11-eicosatrienoic acid were from Cayman (Ann Arbor, MI). Farnesyl-(2-carboxy-2-cyanovinyl)-julolidine (FCVJ) was from Dr. Haidekker's Laboratory (Univerisity of Georgia) (Nipper et al., 2008).
2.2. Cell culture
Human neuroblastoma SH-SY5Y cells (1.0×106 cells/dish) were seeded into 60 mm dishes and were cultured in DMEM/F12 medium (1:1) containing 10% FBS. For differentiation, SH-SY5Y cells were exposed to 10 μM RA for 6 days. Culture medium was replaced by fresh culture medium every other day. Treatments of cells with different fatty acids including SA, OA, LA, ALA, AA, EPA, DHA, cis-2-eicosenoic acid and cis-5, 8, 11-eicosatrienoic acid were in DMEM/F12 medium (1:1) containing 1% BSA for 24 h. All cells were maintained at 37°C in a 5% CO2 humidified incubator.
2.3. Fluorescent fatty acids labeled differentiated SH-SY5Y cells
Cells were incubated with 1μM 5-hexadecanoylaminofluorescein or cis-parinaric acid for 40 min and excess fluorescent fatty acids were then removed by washing cells with PBS three times. Fluorescent images were obtained at room temperature using a Nikon TE-2000 U fluorescence microscope with an oil immersion 60× objective lens. Images were acquired using a CCD camera controlled by a computer running a MetaVue imaging software (Universal Imaging, PA).
2.4. Characterization of membrane fluidity by fluorescence microscopy of FCVJ-labeled cells
A fluorescent molecular rotor, farnesyl-(2-carboxy-2-cyanovinyl)-julolidine (FCVJ), was used to measure the relative membrane fluidity in differentiated SH-SY5Y cells. FCVJ was designed to be a more membrane-compatible fluorescent molecular rotor (Haidekker et al., 2001) with the quantum yield strongly dependent on the local free volume. A higher fluorescent intensity of FCVJ reflects the intramolecular-rotational motions being restricted by a smaller local free volume, indicating a more viscous membrane. The hydrocarbon chain of FCVJ has longer to help improve plasma membrane localization and reduce migration into the inner compartments of the cell, cytosolic staining and background fluorescence (Haidekker et al., 2001). Previously, we have verified the application of FCVJ for measuring membrane viscosity by comparing the results obtained using FCVJ with those from the technique of fluorescence recovery after photobleaching (FRAP) (Nipper et al., 2008). In this study, we adapted the protocol from Haidekker et al. (2001) to fluorescently label cells with FCVJ. Briefly, after undergoing different treatments, e.g. SA, OA, LA, ALA, AA, EPA and DHA, SH-SY5Y cells were washed with PBS and incubated in DMEM containing 20% FBS and 1μM FCVJ for 20 minutes. Excess FCVJ was removed by washing cells with PBS three times. Fluorescent intensity measurements were performed at room temperature using a Nikon TE-2000 U fluorescence microscope with an oil immersion 60× objesctive lens. Images were acquired using a CCD camera controlled by a computer running a MetaVue imaging software (Universal Imaging, PA). The fluorescent intensities of FCVJ per cell were measured. Background subtraction was done for all images prior to data analysis.
2.5. Western blot analysis of sAPPα released from differentiated SH-SY5Y cells
After different treatments, e.g. SA, OA, LA, ALA, AA, EPA and DHA, culture medium was collected and the same volume of the cell lysate from each sample was used for Western blot analysis using β-actin as internal standard. Medium was centrifuged at 12,000 × g for 5 min to remove cell debris, and the same volume of medium from each sample (e.g., 40 μl) was diluted with Laemmli buffer, boiled for 5 min, subjected to electrophoresis in 7.5% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with 5% (w/v) nonfat dry milk in Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBST) and were incubated overnight at 4°C in 3% (w/v) bovine serum albumin (BSA) with 0.02% (w/v) sodium azide in TBST with a 6E10 monoclonal antibody (1:1000 dilution; Covance, Princeton, NJ) that recognizes residues 1–16 of the Aβ domain of sAPPα. Membranes were washed three times during a 15-min period with TBST and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG antibody (1:2000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) in 5% (w/v) nonfat dry milk in TBST at room temperature for 1 h. After washing with TBST for three times, the membrane was subjected to SuperSignal West Pico Chemiluminescent detection reagents from Pierce (Rockford, IL) to visualize bands. The protein bands detected on x-ray film were quantified using a computer-driven scanner and Quantity One software (Bio-Rad).
2.6. Western blot analysis of APP, ADAM9, ADAM10, ADAM17, ADAM19 and BACE1 in differentiated SH-SY5Y cells
After treatments, the protein concentration of the cell lysate was determined by BCA protein assay kit (Pierce Biotechnology, Rockford, IL) according to manufacture's instruction. Equivalent amounts of protein from each sample (e.g., 30 μg) was diluted with Laemmli buffer, boiled for 5 min, subjected to electrophoresis in 7.5% SDS-polyacrylamide gels, and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with 5% (w/v) nonfat dry milk in Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBST) and were incubated overnight at 4°C in 3% (w/v) BSA with 0.02% (w/v) sodium azide in TBST with 6E10 monoclonal antibody, anti-ADAM9 antibody or anti-ADAM19 antibody (1:1000 dilution; Abcam, Cambridge, MA), anti-ADAM10 antibody (1:1000 dilution; Millipore, Billerica, MA) or anti-ADAM17 antibody (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), anti-BACE1 antibody (1:1000 dilution; Sigma-Aldrich, St. Louis, MO). Membranes were washed three times during a 15-min period with TBST and incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG antibody (1:2000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) in 5% (w/v) nonfat dry milk in TBST at room temperature for 1 h. After washing with TBST for three times, the membrane was subjected to SuperSignal West Pico Chemiluminescent detection reagents from Pierce (Rockford, IL) to visualize bands. The protein bands detected on x-ray film were quantified using a computer-driven scanner and Quantity One software (Bio-Rad).
2.7. Quantification of Aβ1-42
After treatments, culture medium and cell lysates were collected, supplemented with protease inhibitor cocktail and centrifuged at 12,000 × g for 5 min at 4°C to remove cell debris. An aliquot (100 μl) of supernatant was used for Aβ1-42 quantification using an ELISA kit (Invitrogen, Carlsbad, CA) following manufacturer's recommendation. According to the instruction manual, substances including Aβ1–12, Aβ1–20, Aβ12–28, Aβ22–35, Aβ1–40, Aβ1–43, Aβ42–1 and APP have no cross-reactivity. The minimum detectable dose of Aβ1-42 is <1.0 pg/ml which is similar to a previous study (Prasanthi et al., 2009). The level of Aβ1-42 in each sample was measured in duplicates and expressed in pg/ml.
2.8. Statistical analysis
Data are presented as mean ± SD from at least three independent experiments. There are three trials in each experiment. Comparison between two groups was made with student's t test. Comparisons of more than two groups were made with one-way ANOVA, followed by Bonferroni's post hoc tests. Values of p< 0.05 are considered to be statistically significant.
3. Results
3.1. Exogenous fatty acids integrated into cellular membranes
In order to study the effects of fatty acids with different unsaturations on cellular membrane fluidity and sAPPα secretion, we first confirmed if fatty acids were able to integrate into membranes of SH-SY5Y cells. Therefore, we incubated differentiated SH-SY5Y cells for 40 min with 5-hexadecanoylaminofluorescein and cis-parinaric acid. Both 5-hexadecanoylaminofluorescein and cis-parinaric acid are fluorescent fatty acids representing saturated and unsaturated fatty acids, respectively. Fig. 1A and Fig. 1B showed that 5-hexadecanoylaminofluorescein and cis-parinaric acid were incorporated into cellular membranes of SH-SY5Y cells, suggesting that exogenous fatty acids used in this study are capable of integrating into cellular membranes in SH-SY5Y cells.
Figure 1.
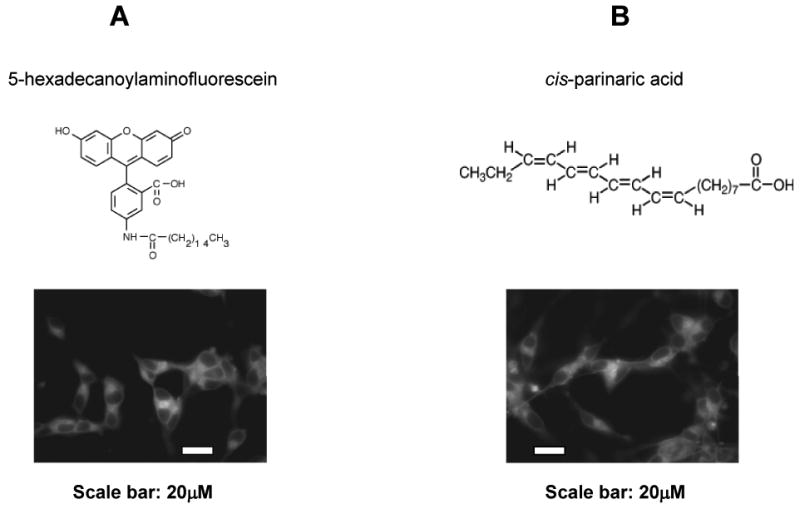
Fluorescently labeled fatty acids incorporated into membranes in differentiated SH-SY5Y cells. 5-hexadecanoylaminofluorescein (A) and cis-parinaric acid (B) represent saturated and unsaturated fatty acids, respectively. After incubation for 40 min, fluorescent images were acquired with an oil immersion 60× objective lens. Scale bar, 20 μm.
3.2. Fatty acids with 3 or less double bonds did not alter membrane fluidity and sAPPα secretion
To study the effects of fatty acids on membrane fluidity, we applied a fluorescent molecular rotor, FCVJ. As explained in the Materials and Methods section, FCVJ integrated into a highly fluidized membrane exhibits a lower quantum yield, as reflected by a lower fluorescent intensity. The application of this technique for the measurement of membrane fluidity has been validated (Nipper et al., 2008; Yang et al., 2010). FCVJ-labeled differentiated SH-SY5Y cells were exposed to SA, OA, LA and ALA for 24 h. Interestingly, cells did not exhibit a significant change in membrane fluidity as indicated by the unchanged fluorescent intensity of FCVJ as compared to control (Fig. 2A, B, C, D, right). These results suggest that SA, OA, LA and ALA do not alter membrane fluidity in SH-SY5Y cells.
Figure 2. SA, OA, LA and ALA on sAPPα secretion and membrane fluidity in differentiated SH-SY5Y cells.
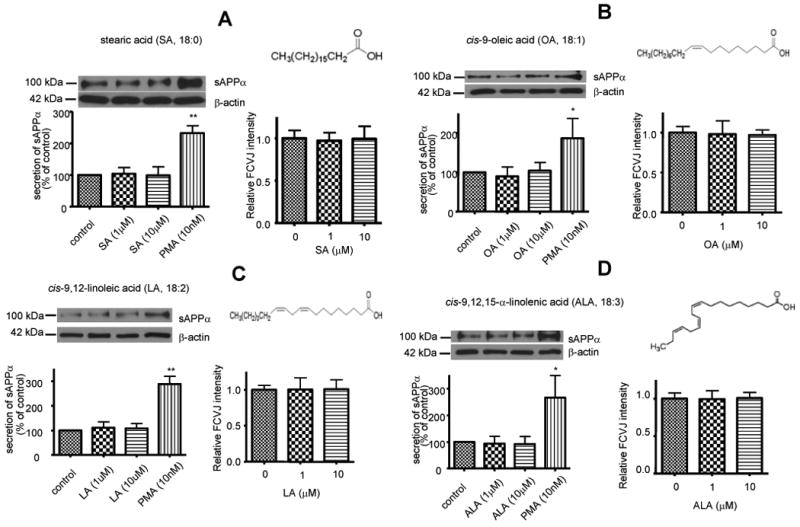
Western blot analysis of sAPPα shows that treatments of cells with SA (A, left), OA (B, left), LA (C, left) and ALA (D, left) did not affect sAPPα secretion to medium from SH-SY5Y cells. PMA treatment known to increase sAPPα secretion in cells was used as a positive control. SA (A, right), OA (B, right), LA (C, right) and ALA (D, right) did not affect membrane fluidity of cells, as the fluorescent intensity of FCVJ-labeled cells remained unchanged after treatments. Data are expressed as mean ± SD from at least three independent experiments (* p< 0.05, ** p< 0.01 compared with control).
To study the effects of SA, OA, LA and ALA on sAPPα secretion, cell culture medium was subjected to western blot analysis of sAPPα after the treatments of cells with SA, OA, LA and ALA. SA, OA, LA and ALA did not alter sAPPα secretion in SH-SY5Y cells (Fig. 2A, B, C, D, left). Since it has been reported that PMA, a PKC agonist, increases sAPPα secretion (Caporaso et al., 1992; Slack et al., 1993; Camden et al., 2005), treatment with PMA (10nM) was used as a positive control.
To test whether positions of double bonds plays a role in membrane fluidity and sAPPα secretion, differentiated SH-SY5Y cells were exposed to cis-2-eicosenoic acid and cis-5, 8, 11-eicosatrienoic acid, which have the same numbers of double bonds as OA and ALA respectively, but the double bonds are closer to the carboxylic groups of fatty acids. Results showed that these two fatty acids also had no effect on membrane fluidity and sAPPα secretion in SH-SY5Y cells, like OA and ALA (Fig. 3), suggesting that the positions of double bonds in fatty acids do not play a role in their effects on membrane fluidity and APP processing.
Figure 3. cis-2-Eicosenoic acid (20:1) and cis-5, 8, 11-Eicosatrienoic acid (20:3) on sAPPα secretion and membrane fluidity in differentiated SH-SY5Y cells.
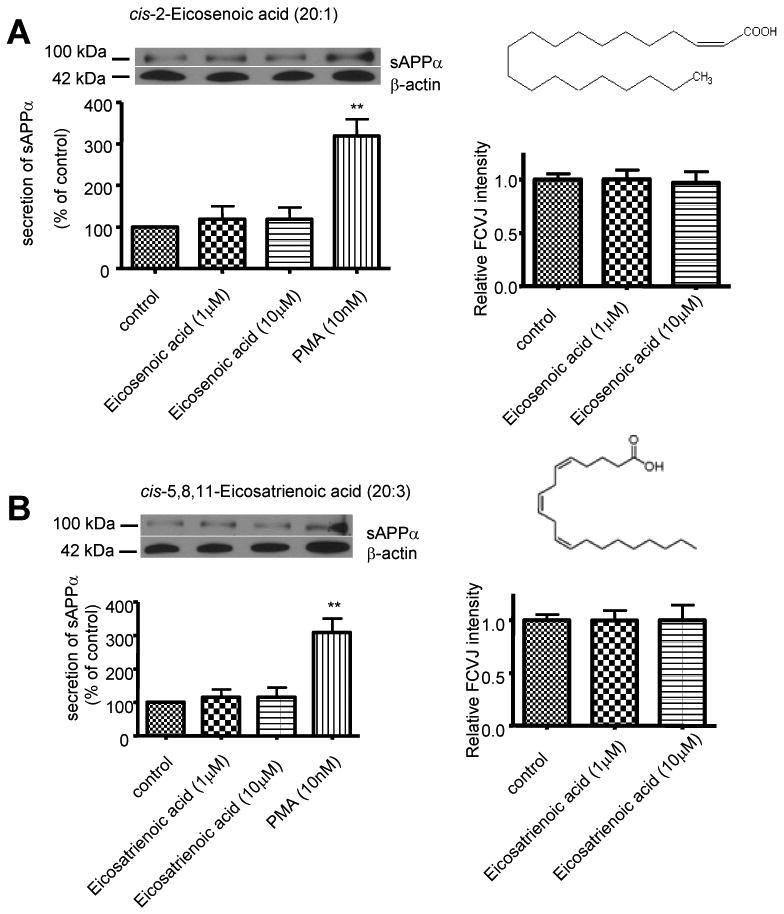
Western blot analysis of sAPPα shows that treatments of cells with cis-2-Eicosenoic acid (20:1) (A, left) and cis-5, 8, 11-Eicosatrienoic acid (20:3) (B, left) did not affect sAPPα secretion to medium from SH-SY5Y cells. PMA treatment was used as a positive control. cis-2-Eicosenoic acid (20:1) (A, right) and cis-5, 8, 11-Eicosatrienoic acid (20:3) (B, right) did not affect membrane fluidity of cells, as the fluorescent intensity of FCVJ-labeled cells remained unchanged after treatments. Data are expressed as mean ± SD from at least three independent experiments (** p< 0.01 compared with control).
3.3. Fatty acids with 4 or more double bonds increased membrane fluidity and sAPPα secretion
We then tested the effects of fatty acids with 4 or more double bonds including AA, EPA and DHA on membrane fluidity and sAPPα secretion. After treatment with AA, EPA and DHA, cells exhibited more fluidized membranes as indicated by lower fluorescent intensities of FCVJ compared to control (Fig. 4A, B, C, right). Western blot analysis showed that AA, EPA and DHA increased sAPPα secretion in differentiated SH-SY5Y cells (Fig. 4A, B, C, left).
Figure 4. AA, EPA and DHA increased the sAPPα secretion and membrane fluidity in differentiated SH-SY5Y cells.
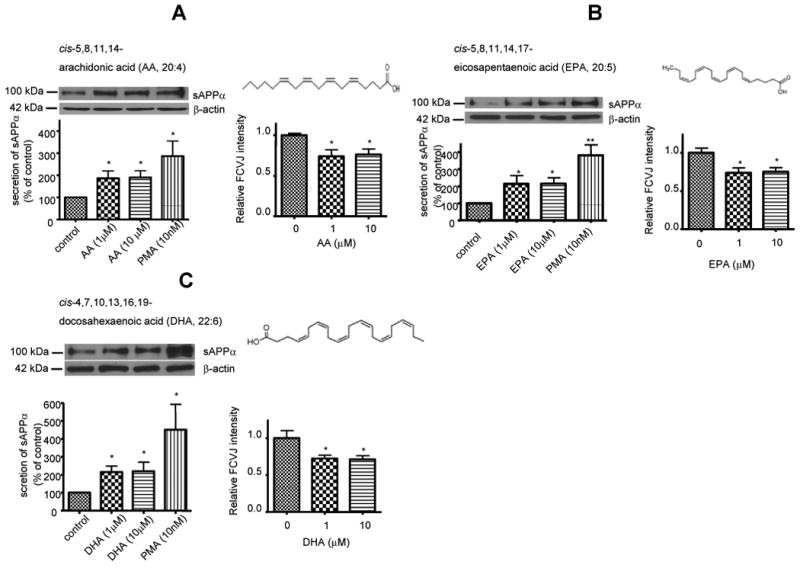
Western blot analysis of sAPPα shows that AA (A, left), EPA (B, left) and DHA (C, left) increased sAPPα secretion to medium from SH-SY5Y cells. PMA was used as a positive control. AA (A, right), EPA (B, right) and DHA (C, right) increased membrane fluidity in cells, as indicated by decreased fluorescent intensity of FCVJ-labeled cells. Data are expressed as mean ± SD from at least three independent experiments (* p< 0.05, ** p< 0.01 compared with control).
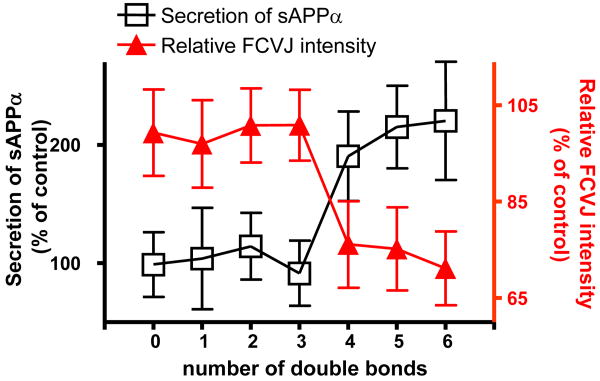
Together with results from SA, OA, LA and ALA treatments, these data showed that unsaturation numbers of fatty acids, but not the positions of double bonds, determined their effects on membrane fluidity and sAPPα secretion, as summarized in Fig. 5. These results suggested that fatty acids with 4 or more double bonds increased sAPPα secretion through increasing membrane fluidity in differentiated SH-SY5Y cells.
Figure 5. The effects of the number of double bonds in fatty acids on sAPPα secretion and membrane fluidity in differentiated SH-SY5Y cells.
Our data shows that not all unsaturated fatty acids, but only those with four or more double bonds, including AA, EPA and DHA, promoted sAPPα secretion and increased membrane fluidity. Data are expressed as percentages of control and mean ± SD from at least three independent experiments (* p< 0.05 compared with control).
3.4. AA and DHA did not alter the expressions of total APP and α-secretases in SH-SY5Y cells
To rule out the changes of sAPPα secretion possibly due to the changes in the expressions of total APP and α-secretases in cells, western blot analyses of APP and α-secretases were performed after the treatments of cells with AA and DHA for 24 h. Fig. 6 shows that AA and DHA did not alter the expressions of total APP and different isoforms of α-secretases including ADAM9, ADAM10, ADAM17 and ADAM19. Mature APP blots were shown in this study. These results suggested that AA, DHA and EPA do not alter α-secretases and APP expressions to contribute to the increase in sAPPα secretion.
Figure 6. Effects of AA and DHA on total APP and α-secretases expression in differentiated SH-SY5Y cells.
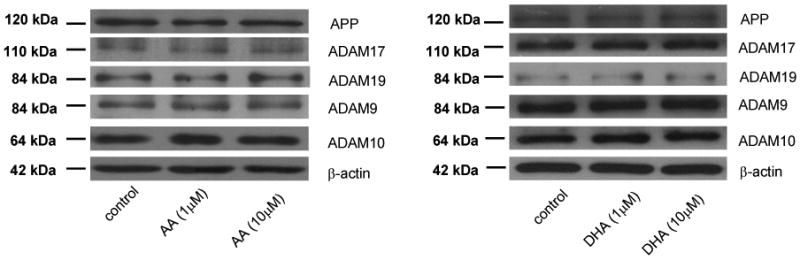
Western blot analysis of total APP and α-secretases shows that AA and DHA did not alter the expressions of total APP and different isoforms of α-secretases including ADAM9, ADAM 10, ADAM17 and ADAM 19. Data are representative blots from at least three independent experiments.
3.5. AA, EPA and DHA did not alter BACE1 expression, Aβ1-42 secretion and expression in SH-SY5Y cells
Since AA, EPA and DHA increase sAPPα secretion, they may alter expression of BACE1, the major β-secretase, and Aβ1-42 in SH-SY5Y cells. Western blot analysis showed that AA, EPA and DHA did not change BACE1 expression (Fig. 7A). ELISA measurement was used to quantify Aβ1-42 in culture medium. Fig. 7B showed that AA, EPA and DHA did not change the Aβ1-42 secretion and expression level in SH-SY5Y cells.
Figure 7. Effects of AA, EPA and DHA on BACE1 expression and Aβ1-42 from differentiated SH-SY5Y cells.
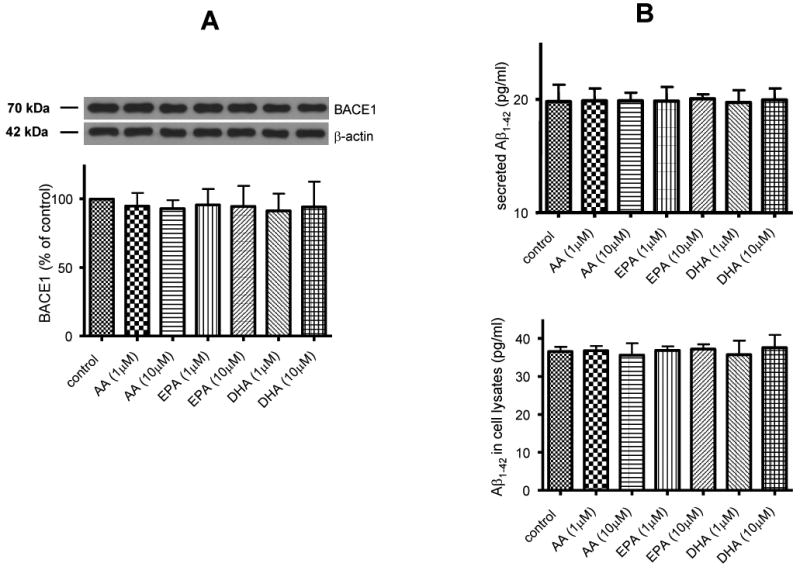
Treatments of AA, EPA and DHA did not change BACE1 expression in SH-SY5Y cells (A), Aβ1-42 secretion from SH-SY5Y cells (B, upper) and Aβ1-42 expression in SH-SY5Y cells (B, lower). Data are expressed as mean ± SD from at least three independent experiments.
4. Discussion
In this study, we have systematically examined how unsaturation numbers of fatty acids affected membrane fluidity and sAPPα secretion in differentiated SH-SY5Y cells. We found that not all unsaturated fatty acids, but only fatty acids with at least 4 or more double bonds increased membrane fluidity and sAPPα secretion (Fig. 5). The effects on membrane fluidity and sAPPα secretion were not dependent on the positions of double bonds in fatty acids. In addition, treatments of cells with fatty acids did not alter the expressions of APP and α-secretases in differentiated SH-SY5Y cells, which ruled out the effects of fatty acids on sAPPα secretion possibly due to the changes in APP and α-secretases expressions.
Studies have shown the roles of fatty acids on health and diseases (Bazan, 2007; Das, 2008; Hooijmans and Kiliaan, 2008). Fatty acids are important ingredients in various dietary sources (Connor, 2000; Chow, 2007). For example, ALA, an essential fatty acid, can be found in many vegetable oils (Mozaffarian, 2005), which has been reported to be neuroprotective in in vivo model of global ischemia (Lauritzen et al., 2000). SA is common in animal fats, and cocoa butter (Beare-Rogers et al., 2001). OA is a mono-unsaturated ω-9 fatty acid, which can be found in peanut oil and poppy seed oil (Untoro et al., 2006). DHA, mainly found in fishes, is also abundant in neuronal cell membranes, especially in synaptic membranes (Bazan and Scott, 1990) and myelin sheaths (Ansari and Shoeman, 1990). DHA is also essential for prenatal brain development and normal brain functions. Its levels in serum and brains are lower in AD patients compared with those in age-matched controls (Tully et al., 2003). Furthermore, greater consumption of DHA significantly reduced the likelihood of developing AD (Schaefer et al., 2006). AA is another abundant fatty acid in the brain. It is a second messenger (Khan et al., 1995) and a precursor for synthesis of eicosanoids (Zhou and Nilsson, 2001). It also helps maintain cell membrane fluidity (Fukaya et al., 2007). The disturbed metabolism of AA is associated with neurological disorder such as AD (Rapoport, 2008).
APP processing depends on the local membrane environment (Tun et al., 2002; Cordy et al., 2003; Ehehalt et al., 2003; Marlow et al., 2003; Kaether and Haass, 2004; Vetrivel et al., 2004; Reid et al., 2007) and can be altered by manipulating the membrane lipid composition (Simons et al., 1998; Kojro et al., 2001; Sawamura et al., 2004; von Arnim et al., 2008). Since fatty acids modulate membrane organization and functions (Yehuda et al., 2002; Stillwell et al., 2005; Shaikh and Edidin, 2006; Pepe, 2007; Shaikh and Edidin, 2008), they may affect APP processing. Therefore, we tested the hypothesis that the effects of fatty acids on membrane fluidity and sAPPα secretion depend on the number of double bonds (i.e. the unsaturation number) in the hydrocarbon chains of fatty acids.
To test the hypothesis, we investigated the effects of fatty acids with different unsaturation (from 0 to 6 double bonds) on membrane fluidity and sAPPα secretion in differentiated SH-SY5Y cells. Our data showed that fatty acids with 3 or less double bonds including SA, OA, LA and ALA had no effects on membrane fluidity and sAPPα secretion (Fig. 2). We also exposed SH-SY5Y cells to cis-2-eicosenoic acid and cis-5, 8, 11-eicosatrienoic acid, which has 1 or 3 double bonds as OA and ALA, respectively, but their double bonds are closer to the carboxylic group of fatty acids. Results showed that these two acids had no effect on membrane fluidity and sAPPα secretion in differentiated SH-SY5Y cells either, suggesting that the position of the double bonds may not play a role in altering membrane fluidity and APP processing (Fig. 3). On the other hand, fatty acids with 4 or more double bonds including AA, EPA and DHA increased membrane fluidity and sAPPα secretion (Fig. 4). Our data are consistent with other studies that AA increased membrane fluidity in hippocampal neurons in vivo (Fukaya et al., 2007) and cultured human umbilical vein, cerebral endothelial cells (Villacara et al., 1989; Beck et al., 1998). In addition, DHA has been shown to increase membrane fluidity and sAPPα secretion in HEK cells and SH-SY5Y cells overexpressing APP (Kogel et al., 2008). Our study demonstrated that not all the unsaturated fatty acids, but only fatty acids of unsaturation number ≥4, are able to increase membrane fluidity, and subsequently promote sAPPα secretion.
To explore if AA, EPA, DHA effects on membrane fluidity and secretion of sAPPα have dose dependency, we exposed SH-SY5Y cells to AA, EPA and DHA with lower doses at 10nM and 100nM. At lower doses, AA, EPA and DHA have no effect on membrane fluidity and sAPPα secretion (data not shown). Taken together, our data show that polyunsaturated fatty acids (PUFAs) only begin to have effects on membrane fluidity and APP processing at certain threshold value between 100nM and 1μM.
Membrane fluidity plays important roles in pathogenesis of AD. Hippocampal membranes of AD patients showed a significant lower fluidity compared with membranes from elderly non-demented controls (Eckert et al., 1999). Membrane fluidity in AD patients was correlated with abnormal APP processing and cognitive decline (Zainaghi et al., 2007). The clinical study by Croisile et al. (1993) indicated that long-term and high-dose treatment with piracetam may slow down the progression of AD and increased membrane fluidity (Eckert et al., 1999). DHA protection effect in Aβ-infused rats was associated with increased membrane fluidity (Hashimoto et al., 2006) which also provided oxidative stress resistance in hippocampal cells (Clement et al., 2010). In vivo, increased membrane fluidity in rat hippocampus improved memory formation (Muller et al., 1997; Scheuer et al., 1999), whereas reduced fluidity impaired memory (Schaeffer et al., 2005; Schaeffer and Gattaz, 2007). Furthermore, membrane fluidity affected not only APP processing (Kojro et al., 2001), but also Aβ aggregation size and hydrophobicity (Kremer et al., 2000), which is critical for the formation of Aβ plaques and downstream cellular pathways.
PUFAs play a central role in the normal development and functioning of brain (Schuchardt et al., 2010). Diets enriched in ω-3 PUFA increased membrane fluidity, affect signal transduction and modulate gene expression for brain function (Horrocks and Farooqui, 2004). Preclinical studies suggested that DHA maintains membrane fluidity, improved synaptic and neurotransmitter functioning, enhanced learning and memory performances and displayed neuroprotective properties (Carrie et al., 2009). Meanwhile, DHA decreased the amount of vascular Aβ deposition (Hooijmans et al., 2007) and reduced Aβ burden (Lim et al., 2005) in aged Alzheimer mouse model. In AD mouse model, DHA modulated APP processing by decreasing both α- and β-APP C-terminal fragment products and full-length APP (Lim et al., 2005), which is contradictory to the idea that higher membrane fluidity increased sAPPα production. More works are warrant to resolve this discrepancy. Despite favorable effects of DHA, it should be noted that DHA has a potential to increase oxidative stress (Meydani et al., 1991), resulting in lipid peroxidation (Song and Miyazawa, 2001), especially with long-term and high dose treatments (Grundt et al., 2003; Wang et al., 2003). Peroxidation of lipids and lipoproteins, especially low-density lipoprotein (LDL), was thought to lead to the development of atherosclerotic plaques (Wiklund et al., 1991). As such, although the impact of PUFAs on lipid peroxidation is controversial (Nenseter and Drevon, 1996), considerations should be taken when PUFAs are used for dietary supplementation.
Although we report that AA promoted sAPPα secretion, it is important to note that AA exerts both neurotrophic and neurotoxic effects (Farooqui and Horrocks, 2006). The concentration of AA (<10 μmol/kg) in the brain tissue is very low under normal conditions. AA performs a variety of functions, such as regulating activity of protein kinase A, protein kinase C, NADPH oxidase, choline acetyltransferase, and caspase-3 (Farooqui and Horrocks, 2006). 1 μM of AA also significantly potentiates the elongation of neurites in hippocampal cultures (Katsuki and Okuda, 1995). Here we report that 1 μM of AA increased membrane fluidity and promoted the secretion of sAPPα which is neurotrophic and neuroprotective. However, under pathological conditions, higher concentrations (∼ 0.5 mmol/kg) of AA are present in the brains (Farooqui and Horrocks, 2006). Higher concentrations of AA result in mitochondrial dysfunction (Schapira, 1996). AA also triggers nuclear factor-κB (NF-κB) and increases neuronal death (Toborek et al., 1999). AA can be converted to inflammatory mediators, prostaglandins and leukotrienes, by prostaglandin synthetases and lipoxygenases, respectively (Farooqui and Horrocks, 2006). Therefore, whether AA is beneficial to brains is highly concentration-dependent.
Other compounds capable of altering membrane fluidity can also modulate sAPPα secretion. For example, treatment with methyl-β-cyclodextrin (MβCD) or lovastatin to reduce cellular cholesterol resulted in increase in membrane fluidity and sAPPα secretion (Kojro et al., 2001). In addition, decreased sAPPα secretion by Pluronic F68 (PF68) is associated with decreased membrane fluidity, while increased sAPPα by benzyl alcohol (C6H5OH) is associated with increased membrane fluidity (Peters et al., 2009). These data suggested that sAPPα secretion can be altered by manipulating membrane fluidity (Wolozin, 2001). Taken together, our data and those from Kojro et al (2001) and Peter et al (2009) provide the explanation that fatty acids with 4 or more double bonds promote sAPPα secretion through increasing membrane fluidity in differentiated SH-SY5Y cells.
sAPPα has been shown to be neurotrophic. sAPPα enhanced neuronal survival and neurite extension in a dose-dependent manner with a maximum effect at approximately 100 nM in rat primary cerebral cortical neurons (Araki et al., 1991) and significantly increased neurite length and branching in pheochromocytoma PC12 cells (Milward et al., 1992) and stimulated neurite outgrowth of hippocampal neurons in an isoform-dependent manner (Qiu et al., 1995). ADAM10 over-expression, which led to increased sAPPα production, increased cortical synaptogenesis (Bell et al., 2008). sAPPα is also neuroprotective, although mechanisms are yet to be fully understood. For example, sAPPα enhanced basal glucose and glutamate transport and protected against oxidative impairment of glucose and glutamate transport in synaptosomes by a cyclic GMP-mediated mechanism (Mattson et al., 1999); sAPPα exerted neuroprotective effects through regulating intraneuronal calcium (Mattson et al., 1993); sAPPα antagonized dendritic degeneration and neuron death triggered by proteasomal stress (Copanaki et al., 2010), and this anti-apoptotic effect of sAPPα was associated with inhibition of the stress-triggered c-Jun N-terminal kinase (JNK)-signalling pathway; Statins, widely used cholesterol-lowering drugs, exerted neuroprotective effects through stimulating α-secretase cleavage of APP (Ma et al., 2009). Obviously, more efforts are needed to further understand the neurotrophic and neuroprotective mechanisms of sAPPα.
Information derived from this study should provide potential dietary strategies for prevention of AD.
Acknowledgments
This work was supported by NIH Grants 1P01 AG18357 and 1R21 NS052385.
Abbreviations used
- AA
arachidonic acid
- AD
Alzheimer's disease
- ADAM
a disintegrin and metalloprotease
- ALA
α-linolenic acid
- APP
amyloid precursor protein
- Aβ
amyloid-β peptide
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- DHA
docosahexaenoic acid
- DMEM
dulbecco's modified Eagle's medium
- DMSO
dimethyl sulfoxide
- EPA
eicosapentaenoic acid
- FBS
fetal bovine serum
- FCVJ
farnesyl-(2-carboxy-2-cyanovinyl)-julolidine
- FRAP
fluorescence recovery after photobleaching
- LA
linoleic acid
- MβCD
methyl-β-cyclodextrin
- NF-κB
nuclear factor-κB
- OA
oleic acid
- PF68
Pluronic F68
- PMA
phorbol 12-myristate 13-acetate
- PUFA
polyunsaturated fatty acid
- RA
all-trans retinoic acid
- SA
stearic acid
- sAPPα
α-secretase-cleaved soluble APP
- sPLA2-III
secretory phospholipase A2 type III
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ansari KA, Shoeman DW. Arachidonic and docosahexanoic acid content of bovine brain myelin: implications for the pathogenesis of multiple sclerosis. Neurochem Res. 1990;15:7–11. doi: 10.1007/BF00969177. [DOI] [PubMed] [Google Scholar]
- Araki W, Kitaguchi N, Tokushima Y, Ishii K, Aratake H, Shimohama S, Nakamura S, Kimura J. Trophic effect of beta-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun. 1991;181:265–271. doi: 10.1016/s0006-291x(05)81412-3. [DOI] [PubMed] [Google Scholar]
- Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr Opin Clin Nutr Metab Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- Bazan NG, Scott BL. Dietary omega-3 fatty acids and accumulation of docosahexaenoic acid in rod photoreceptor cells of the retina and at synapses. Ups J Med Sci Suppl. 1990;48:97–107. [PubMed] [Google Scholar]
- Beare-Rogers J, Dieffenbacher A, Holm JV. Lexicon of Lipid nutrition (IUPAC Technical Report) Pure Appl Chem. 2001;73:685–744. [Google Scholar]
- Beck R, Bertolino S, Abbot SE, Aaronson PI, Smirnov SV. Modulation of arachidonic acid release and membrane fluidity by albumin in vascular smooth muscle and endothelial cells. Circ Res. 1998;83:923–931. doi: 10.1161/01.res.83.9.923. [DOI] [PubMed] [Google Scholar]
- Bell KF, Zheng L, Fahrenholz F, Cuello AC. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging. 2008;29:554–565. doi: 10.1016/j.neurobiolaging.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Camden JM, Schrader AM, Camden RE, Gonzalez FA, Erb L, Seye CI, Weisman GA. P2Y2 nucleotide receptors enhance alpha-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280:18696–18702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- Caporaso GL, Gandy SE, Buxbaum JD, Ramabhadran TV, Greengard P. Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992;89:3055–3059. doi: 10.1073/pnas.89.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie I, Abellan Van Kan G, Rolland Y, Gillette-Guyonnet S, Vellas B. PUFA for prevention and treatment of dementia? Curr Pharm Des. 2009;15:4173–4185. doi: 10.2174/138161209789909764. [DOI] [PubMed] [Google Scholar]
- Cheng H, Vetrivel KS, Gong P, Meckler X, Parent A, Thinakaran G. Mechanisms of disease: new therapeutic strategies for Alzheimer's disease--targeting APP processing in lipid rafts. Nat Clin Pract Neurol. 2007;3:374–382. doi: 10.1038/ncpneuro0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CK, editor. Fatty acids in foods and their health implications. 3rd. Boca Raton, FL: Taylor & Francis Group; 2007. [Google Scholar]
- Clement AB, Gimpl G, Behl C. Oxidative stress resistance in hippocampal cells is associated with altered membrane fluidity and enhanced nonamyloidogenic cleavage of endogenous amyloid precursor protein. Free Radic Biol Med. 2010;48:1236–1241. doi: 10.1016/j.freeradbiomed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71:171S–175S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- Copanaki E, Chang S, Vlachos A, Tschape JA, Muller UC, Kogel D, Deller T. sAPPalpha antagonizes dendritic degeneration and neuron death triggered by proteasomal stress. Mol Cell Neurosci. 2010;44:386–393. doi: 10.1016/j.mcn.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisile B, Trillet M, Fondarai J, Laurent B, Mauguiere F, Billardon M. Long-term and high-dose piracetam treatment of Alzheimer's disease. Neurology. 1993;43:301–305. doi: 10.1212/wnl.43.2.301. [DOI] [PubMed] [Google Scholar]
- Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis. 2008;7:37. doi: 10.1186/1476-511X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Microglia in Alzheimer's disease and transgenic models. How close the fit? Am J Pathol. 1999;154:1627–1631. doi: 10.1016/S0002-9440(10)65416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert GP, Cairns NJ, Muller WE. Piracetam reverses hippocampal membrane alterations in Alzheimer's disease. J Neural Transm. 1999;106:757–761. doi: 10.1007/s007020050196. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12:245–260. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Fukaya T, Gondaira T, Kashiyae Y, Kotani S, Ishikura Y, Fujikawa S, Kiso Y, Sakakibara M. Arachidonic acid preserves hippocampal neuron membrane fluidity in senescent rats. Neurobiol Aging. 2007;28:1179–1186. doi: 10.1016/j.neurobiolaging.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Grundt H, Nilsen DW, Mansoor MA, Nordoy A. Increased lipid peroxidation during long-term intervention with high doses of n-3 fatty acids (PUFAs) following an acute myocardial infarction. Eur J Clin Nutr. 2003;57:793–800. doi: 10.1038/sj.ejcn.1601730. [DOI] [PubMed] [Google Scholar]
- Haidekker MA, Ling T, Anglo M, Stevens HY, Frangos JA, Theodorakis EA. New fluorescent probes for the measurement of cell membrane viscosity. Chem Biol. 2001;8:123–131. doi: 10.1016/s1074-5521(00)90061-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hossain S, Shimada T, Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33:934–939. doi: 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Kiliaan AJ. Fatty acids, lipid metabolism and Alzheimer pathology. Eur J Pharmacol. 2008;585:176–196. doi: 10.1016/j.ejphar.2007.11.081. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Tanila H, Kiliaan AJ. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD) Neurobiol Dis. 2007;28:16–29. doi: 10.1016/j.nbd.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Kaether C, Haass C. A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation. J Cell Biol. 2004;167:809–812. doi: 10.1083/jcb.200410090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H, Okuda S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog Neurobiol. 1995;46:607–636. doi: 10.1016/0301-0082(95)00016-o. [DOI] [PubMed] [Google Scholar]
- Khan WA, Blobe GC, Hannun YA. Arachidonic acid and free fatty acids as second messengers and the role of protein kinase C. Cell Signal. 1995;7:171–184. doi: 10.1016/0898-6568(94)00089-t. [DOI] [PubMed] [Google Scholar]
- Kogel D, Copanaki E, Hartig U, Bottner S, Peters I, Muller WE, Eckert G. Modulation of membrane fluidity by omega 3 fatty acids: Enhanced generation of sAPPalpha is required for the neuroprotective effects of DHA. The 38th annual meeting of the Society for Neuroscience.2008. [Google Scholar]
- Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JJ, Pallitto MM, Sklansky DJ, Murphy RM. Correlation of beta-amyloid aggregate size and hydrophobicity with decreased bilayer fluidity of model membranes. Biochemistry. 2000;39:10309–10318. doi: 10.1021/bi0001980. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Zhao Y, Kwak YD, Yang Z, Thompson R, Luo Z, Xu H, Liao FF. Statin's excitoprotection is mediated by sAPP and the subsequent attenuation of calpain-induced truncation events, likely via rho-ROCK signaling. J Neurosci. 2009;29:11226–11236. doi: 10.1523/JNEUROSCI.6150-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow L, Cain M, Pappolla MA, Sambamurti K. Beta-secretase processing of the Alzheimer's amyloid protein precursor (APP) J Mol Neurosci. 2003;20:233–239. doi: 10.1385/JMN:20:3:233. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Guo ZH, Geiger JD. Secreted form of amyloid precursor protein enhances basal glucose and glutamate transport and protects against oxidative impairment of glucose and glutamate transport in synaptosomes by a cyclic GMP-mediated mechanism. J Neurochem. 1999;73:532–537. doi: 10.1046/j.1471-4159.1999.0730532.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE. Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron. 1993;10:243–254. doi: 10.1016/0896-6273(93)90315-i. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- Meydani M, Natiello F, Goldin B, Free N, Woods M, Schaefer E, Blumberg JB, Gorbach SL. Effect of long-term fish oil supplementation on vitamin E status and lipid peroxidation in women. J Nutr. 1991;121:484–491. doi: 10.1093/jn/121.4.484. [DOI] [PubMed] [Google Scholar]
- Milward EA, Papadopoulos R, Fuller SJ, Moir RD, Small D, Beyreuther K, Masters CL. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9:129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med. 2005;11:24–30. quiz 31, 79. [PubMed] [Google Scholar]
- Muller WE, Koch S, Scheuer K, Rostock A, Bartsch R. Effects of piracetam on membrane fluidity in the aged mouse, rat, and human brain. Biochem Pharmacol. 1997;53:135–140. doi: 10.1016/s0006-2952(96)00463-7. [DOI] [PubMed] [Google Scholar]
- Nenseter MS, Drevon CA. Dietary polyunsaturates and peroxidation of low density lipoprotein. Curr Opin Lipidol. 1996;7:8–13. doi: 10.1097/00041433-199602000-00003. [DOI] [PubMed] [Google Scholar]
- Nipper ME, Majd S, Mayer M, Lee JC, Theodorakis EA, Haidekker MA. Characterization of changes in the viscosity of lipid membranes with the molecular rotor FCVJ. Biochim Biophys Acta. 2008;1778:1148–1153. doi: 10.1016/j.bbamem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Pepe S. Dietary polyunsaturated fatty acids and age-related membrane changes in the heart. Ann N Y Acad Sci. 2007;1114:381–388. doi: 10.1196/annals.1396.046. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS, Barron E, Chui HC. Morphologic association between microglia and senile plaque amyloid in Alzheimer's disease. Neurosci Lett. 1990;119:32–36. doi: 10.1016/0304-3940(90)90748-x. [DOI] [PubMed] [Google Scholar]
- Peters I, Igbavboa U, Schutt T, Haidari S, Hartig U, Rosello X, Bottner S, Copanaki E, Deller T, Kogel D, Wood WG, Muller WE, Eckert GP. The interaction of beta-amyloid protein with cellular membranes stimulates its own production. Biochim Biophys Acta. 2009;1788:964–972. doi: 10.1016/j.bbamem.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanthi JR, Huls A, Thomasson S, Thompson A, Schommer E, Ghribi O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on beta-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol Neurodegener. 2009;4:1. doi: 10.1186/1750-1326-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Ferreira A, Miller C, Koo EH, Selkoe DJ. Cell-surface beta-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner. J Neurosci. 1995;15:2157–2167. doi: 10.1523/JNEUROSCI.15-03-02157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI. Arachidonic acid and the brain. J Nutr. 2008;138:2515–2520. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid PC, Urano Y, Kodama T, Hamakubo T. Alzheimer's disease: cholesterol, membrane rafts, isoprenoids and statins. J Cell Mol Med. 2007;11:383–392. doi: 10.1111/j.1582-4934.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura N, Ko M, Yu W, Zou K, Hanada K, Suzuki T, Gong JS, Yanagisawa K, Michikawa M. Modulation of amyloid precursor protein cleavage by cellular sphingolipids. J Biol Chem. 2004;279:11984–11991. doi: 10.1074/jbc.M309832200. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- Schaeffer EL, Gattaz WF. Requirement of hippocampal phospholipase A2 activity for long-term memory retrieval in rats. J Neural Transm. 2007;114:379–385. doi: 10.1007/s00702-006-0585-4. [DOI] [PubMed] [Google Scholar]
- Schaeffer EL, Bassi F, Jr, Gattaz WF. Inhibition of phospholipase A2 activity reduces membrane fluidity in rat hippocampus. J Neural Transm. 2005;112:641–647. doi: 10.1007/s00702-005-0301-9. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Oxidative stress and mitochondrial dysfunction in neurodegeneration. Curr Opin Neurol. 1996;9:260–264. doi: 10.1097/00019052-199608000-00003. [DOI] [PubMed] [Google Scholar]
- Scheuer K, Rostock A, Bartsch R, Muller WE. Piracetam improves cognitive performance by restoring neurochemical deficits of the aged rat brain. Pharmacopsychiatry. 1999;32 1:10–16. doi: 10.1055/s-2007-979231. [DOI] [PubMed] [Google Scholar]
- Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr. 2010;169:149–164. doi: 10.1007/s00431-009-1035-8. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The origins of Alzheimer disease: a is for amyloid. JAMA. 2000;283:1615–1617. doi: 10.1001/jama.283.12.1615. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Edidin M. Polyunsaturated fatty acids, membrane organization, T cells, and antigen presentation. Am J Clin Nutr. 2006;84:1277–1289. doi: 10.1093/ajcn/84.6.1277. [DOI] [PubMed] [Google Scholar]
- Shaikh SR, Edidin M. Polyunsaturated fatty acids and membrane organization: elucidating mechanisms to balance immunotherapy and susceptibility to infection. Chem Phys Lipids. 2008;153:24–33. doi: 10.1016/j.chemphyslip.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack BE, Nitsch RM, Livneh E, Kunz GM, Jr, Eldar H, Wurtman RJ. Regulation of amyloid precursor protein release by protein kinase C in Swiss 3T3 fibroblasts. Ann N Y Acad Sci. 1993;695:128–131. doi: 10.1111/j.1749-6632.1993.tb23040.x. [DOI] [PubMed] [Google Scholar]
- Song JH, Miyazawa T. Enhanced level of n-3 fatty acid in membrane phospholipids induces lipid peroxidation in rats fed dietary docosahexaenoic acid oil. Atherosclerosis. 2001;155:9–18. doi: 10.1016/s0021-9150(00)00523-2. [DOI] [PubMed] [Google Scholar]
- Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am J Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45:559–579. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- Thornton E, Vink R, Blumbergs PC, Van Den Heuvel C. Soluble amyloid precursor protein alpha reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res. 2006;1094:38–46. doi: 10.1016/j.brainres.2006.03.107. [DOI] [PubMed] [Google Scholar]
- Toborek M, Malecki A, Garrido R, Mattson MP, Hennig B, Young B. Arachidonic acid-induced oxidative injury to cultured spinal cord neurons. J Neurochem. 1999;73:684–692. doi: 10.1046/j.1471-4159.1999.0730684.x. [DOI] [PubMed] [Google Scholar]
- Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr. 2003;89:483–489. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- Tun H, Marlow L, Pinnix I, Kinsey R, Sambamurti K. Lipid rafts play an important role in A beta biogenesis by regulating the beta-secretase pathway. J Mol Neurosci. 2002;19:31–35. doi: 10.1007/s12031-002-0007-5. [DOI] [PubMed] [Google Scholar]
- Untoro J, Schultink W, West CE, Gross R, Hautvast JG. Efficacy of oral iodized peanut oil is greater than that of iodized poppy seed oil smong Indonesian schoolchildren. The Amer J Clin Nutrit. 2006;84:1208–1214. doi: 10.1093/ajcn/84.5.1208. [DOI] [PubMed] [Google Scholar]
- Vassar R. BACE1: the beta-secretase enzyme in Alzheimer's disease. J Mol Neurosci. 2004;23:105–114. doi: 10.1385/JMN:23:1-2:105. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacara A, Spatz M, Dodson RF, Corn C, Bembry J. Effect of arachidonic acid on cultured cerebromicrovascular endothelium: permeability, lipid peroxidation and membrane “fluidity”. Acta Neuropathol. 1989;78:310–316. doi: 10.1007/BF00687761. [DOI] [PubMed] [Google Scholar]
- von Arnim CA, von Einem B, Weber P, Wagner M, Schwanzar D, Spoelgen R, Strauss WL, Schneckenburger H. Impact of cholesterol level upon APP and BACE proximity and APP cleavage. Biochem Biophys Res Commun. 2008;370:207–212. doi: 10.1016/j.bbrc.2008.03.047. [DOI] [PubMed] [Google Scholar]
- Wang JY, Sekine S, Saito M. Effect of docosahexaenoic acid and ascorbate on peroxidation of retinal membranes of ODS rats. Free Radic Res. 2003;37:419–424. doi: 10.1080/1071576031000070084. [DOI] [PubMed] [Google Scholar]
- Wiklund O, Mattsson L, Bjornheden T, Camejo G, Bondjers G. Uptake and degradation of low density lipoproteins in atherosclerotic rabbit aorta: role of local LDL modification. J Lipid Res. 1991;32:55–62. [PubMed] [Google Scholar]
- Wolozin B. A fluid connection: cholesterol and Abeta. Proc Natl Acad Sci U S A. 2001;98:5371–5373. doi: 10.1073/pnas.101123198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Sheng W, He Y, Cui J, Haidekker MA, Sun GY, Lee JC. Secretory phospholipase A2 type III enhances alpha-secretase-dependent amyloid precursor protein processing through alterations in membrane fluidity. J Lipid Res. 2010;51:957–966. doi: 10.1194/jlr.M002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging. 2002;23:843–853. doi: 10.1016/s0197-4580(02)00074-x. [DOI] [PubMed] [Google Scholar]
- Zainaghi IA, Forlenza OV, Gattaz WF. Abnormal APP processing in platelets of patients with Alzheimer's disease: correlations with membrane fluidity and cognitive decline. Psychopharmacology (Berl) 2007;192:547–553. doi: 10.1007/s00213-007-0748-5. [DOI] [PubMed] [Google Scholar]
- Zhou L, Nilsson A. Sources of eicosanoid precursor fatty acid pools in tissues. J Lipid Res. 2001;42:1521–1542. [PubMed] [Google Scholar]