Abstract
Preeclampsia is a major obstetrical complication affecting maternal and fetal health. While it is clear that there is a substantial placental contribution to preeclampsia pathogenesis, the maternal contribution is less well characterized. We therefore performed a genome wide transcriptome analysis to explore disease-associated changes in maternal gene expression patterns in peripheral blood mononuclear cells (PBMCs).
Methods
Preeclampsia was defined as gestational hypertension, proteinuria and hyperurecimia. Total RNA was isolated from PBMCs obtained from women with uncomplicated pregnancies (n=5) and women with preeclamptic pregnancies (n=5). Gene expression analysis was carried out using Agilent oligonucleotide microarrays. Biological pathway analysis was undertaken using Ingenuity Pathway Analysis software. Quantitative real time PCR (QRTPCR) was performed to validate the gene expression changes of selected genes in normotensive and preeclamptic patients (n=12 each).
Results
We identified a total of 368 genes that were differentially expressed in women with preeclampsia compared to normal controls with false discovery rate (FDR) controlled at 10%. In follow up experiments we further analyzed the expression levels of a number of genes that were identified as altered by the microarray data including survivin (BIRC5), caveolin (CAV1), GATA binding protein-1 (GATA1), signal tranducer and activator of transcription 1 (STAT1), E2F transcription factor-1 (E2F1), fibronectin-1 (FN1), interleukin-4 (IL-4), matrix metalloprotease-9 (MMP-9) and WAP four-disulfide domain protein (WFDC-1) by QRTPCR. Additionally we performed immunoblot analysis and zymography to verify some of these candidate genes at the protein level. Computational analysis of gene function identified an anti-proliferative and altered immune function cellular phenotype in severe preeclamptic samples.
Conclusions
We have characterized the genome-wide mRNA expression changes associated with preeclampsia-specific genes in circulating maternal blood cells at the time of delivery. In addition to providing information relating to the biological basis of the preeclampsia phenotype, our data provide a number of potential biomarkers for use in the further characterization of this disease.
Keywords: Preeclampsia, pregnancy, peripheral blood mononuclear cells (PBMCs), microarray
Introduction
Preeclampsia is a hypertensive disorder of pregnancy that affects 5–7% of all pregnancies (1) and is one of the major obstetrical complications resulting in maternal and fetal morbidity and mortality. Although its etiology is unknown at present, our understanding of the disease has greatly improved in the past few years. This is partly due to the widespread availability of high-throughput genomic technologies. For example, the placental transcriptome has been studied extensively using high-throughput microarray based approaches with the aim of characterizing the molecular basis of placental pathology. These studies have provided valuable information that has improved our understanding of the disease including the identification of soluble Flt-1 (FLT1) and endoglin (ENG), which encode proteins that are known to be involved in the pathobiology of preeclampsia(2–5). Such approaches have also been exploited to identify predictive biomarkers for the early detection and management of the disease(6).
The placenta, a fetal tissue, performs a multitude of functions and is a highly vascularized organ. Significantly, it has intervillous space that serves as a “reservoir” for maternal blood. Therefore, it is not unlikely that placental tissue samples used for transcriptome analysis will contain considerable numbers of maternal leukocytes genomes. Consequently, the resulting data may be confounded by a significant amount of genomic information that is derived from the mother. Therefore, it is challenging to distinguish between gene expression patterns that are maternal or placental in their origin.
Etiology of preeclampsia still remains unknown although oxidative stress and adaptations to the maternal immune system are the two major theories suggested (7, 8). There is sizable systemic inflammation during normal pregnancy that is further elevated in women with preeclampsia(9). Thus a better understanding of maternal gene expression changes, such as those identified by Okazaki et al. (2007) (10) and Sun et al., (2009) (11) could provide a biomarker for early detection or the management of the disease at later stage.
We hypothesized that considerable changes in maternal leukocyte gene expression occur in the context of preeclampsia. To test this hypothesis and further our understanding of gene regulation in preeclampsia we performed genome-wide transcriptome-profiling experiments to characterize gene expression changes in the peripheral blood mononuclear cells (PBMCs) of women with normotensive pregnancies and women with preeclampsia. Differences between PBMCs from normal and preeclamptic mothers identified by this approach provide new insight into the pathobiology of preeclampsia and justify future efforts to search for early gestational PBMC derived mRNA markers for non-invasive preeclampsia screening.
MATERIALS AND METHODS
Sample collection and processing
Peripheral venous blood samples were obtained at term, with informed written consent, from women with normal or preeclamptic pregnancies. The protocol was approved by the Institutional Review Board of Magee-Womens Hospital. Preeclampsia was defined using the criteria of gestational hypertension and proteinuria and reversal of hypertension and proteinuria after delivery(12). Gestational hypertension was defined as new onset systolic blood pressure >140 or diastolic pressure >90 mmHg after 20 weeks gestation. Proteinuria was defined as >300mg per 24 hour urine collection or >2+ (100mg/dL) on a voided specimen or > 1+ (30mg/dL) on a catheterized random urine specimen. All the preeclamptic women had hyperuricemia, which was defined as a uric acid concentration greater than one standard deviation above the mean for gestations age(13). Pregnancy controls were normotensive throughout gestation, did not have proteinuria, had an uncomplicated pregnancy and delivered at term. Preeclampsia was considered severe, if the women had very high blood pressure (greater than 160 mmHg systolic and 110 mmHg diastolic) and more than 5 gms of protein in 24hr urine. In addition the severe preeclamptic women exhibited elevated liver enzyme levels, thrombocytopenia, or severe headaches and fetal growth restriction. The clinical characteristics of the women who provided the blood used in the study are shown in Table 1.
Table 1.
Characteristics of the entire Study Population
| Normal Pregnancy (n=20) | Preeclampsia † (n=20) | |
|---|---|---|
| Maternal age (years) | 27.4 ± 1.4 | 29.2 ± 1.2 |
| Gestational weeks at delivery | 38.6 ± 0.6 | 34.0 ± 0.8* |
| Maternal race (n) | ||
| White | 15 | 14 |
| African-American | 3 | 5 |
| Others | 2 | 1 |
| Nulliparous (n) | 20 | 18 |
| Average Systolic BP at 20 wks (mm Hg) | 109 ± 14 | 122 ± 15* |
| Average Diastolic BP at 20 wks (mm Hg) | 67 ± 8 | 71 ± 12* |
| Average Systolic BP at delivery (mm Hg) | 124 ± 11 | 161 ± 11* |
| Average Diastolic BP at delivery (mmHg) | 78 ± 10 | 102± 9* |
| Proteinuria (%) | 0 | 100 |
| Uric acid (mg/dL) | ND | 6.7±3 |
Data presented as Mean ± Sd. ND = not determined.
Preeclampsia definition is based on the Working Group Report (2003) and hyperuricemia of 1SD above normal for gestational age.
<p=0.05 compared to normal pregnancy as determined by Fisher’s LSD. One patient had chronic hypertension with superimposed preeclampsia.
Peripheral blood mononuclear cells (PBMCs) isolation
PBMCs were prepared using Ficoll-Paque following manufacturer’s protocol (Amersham Biosciences Corporation, Piscataway, NJ, USA) within 2–3 hrs of collection. Briefly, 5 to 10 mL of the blood sample was overlaid on top of a 5 mL Ficoll gradient and centrifuged at 400 × g for 30 min. The top white interface containing PBMCs was collected and diluted 1:10 with PBS and centrifuged again for 10 min at 100 × g. PBMC cell pellets were saved in aliquots for further characterization.
RNA isolation
Total RNA was isolated from PBMCs by homogenizing in 20 volumes of RNAwiz (Ambion, Austin, TX) using a Tissuemizer (Tekmar, Cincinnati, OH) at speed 60 for two 30 s bursts. After 20 min incubation at room temperature (RT), 0.2 volumes of chloroform was added to the homogenate, mixed thoroughly and allowed to incubate for an additional 10 min. The mixture was then centrifuged at 10,000 × g for 10 min. The clear aqueous phase was collected in a clean tube and diluted with an equal volume of RNase free water. The RNA was precipitated by the addition of one volume of isopropanol. After a 10 min incubation at room temperature the RNA was recovered by centrifugation at 10,000 × g for 15 min at 4°C. The pellet was washed with 70% ethanol, allowed to air dry and was dissolved in minimal volume of RNase free water. The amount of RNA was estimated by spectrophotometry (A260).
Agilent Oligonucleotide Microarrays
Data sets were prepared according to the guidelines of minimum information about a microarray experiment (MIAME) and were deposited in the Gene Expression Omnibus (GEO) data base: http://www.ncbi.nlm.nih.gov/geo/.
For gene expression measurements we used Agilent 4 × 44K whole human genome microarray kits (G4112F; Agilent Technologies, Inc., Santa Clara, CA) according to the manufacturer’s protocol. Briefly, 500 ng of total RNA was amplified using Agilent Low Input Linear Amplification and labeling kit and resultant cRNA was labeled with cyanine-3 (cy-3, 10 mM; PerkinElmer Life and Analytical Sciences, Boston, MA). Cy-3 labeled probes were purified using Qiagen RNeasy Mini kit (Qiagen, Valencia, CA) per the manufacturer’s protocol. Sufficient yield and dye incorporation were confirmed using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE). Arrays were hybridized for 17 hours at 60°C under continuous rotation at ~ 20 RPM. The gasket slide cover slips were removed and the slides washed for one minute in Agilent Wash Buffer 1 (6x sodium chloride/sodium phosphate/EDTA (SSPE) + 0.005% N-laurylsarcosine). The slides were then washed in Agilent Wash Buffer 2 (0.06x SSPE + 0.005% N-laurylsarcosine) for 1 minute followed by 1 minute in acetonitrile and then 30 seconds Agilent Stabilization and Drying solution. Arrays were scanned using the Agilent DNA microarray scanner. DNA microarray feature intensities were measured using Agilent Feature Extraction software version 9.5.2. Background-subtracted signal intensities of the remaining arrays were log-base 2 transformed and then normalized across arrays by cyclic lowess in the R statistical package. Since array data often contains multiple (and variable numbers of) probes per gene, the probe intensities were averaged and combined into individual gene intensity values.
Statistical Analyses
Oligonucleotide microarray experiments were performed using total RNA isolated from PBMCs at term from normal and preeclamptic pregnancies (n= 5 each). After normalizing the data using cyclic loess method(14), we searched for genes differentially expressed between the normal and the preeclamptic samples using the Empirical Bayesian method controlling for false discovery rate (FDR) at 5 and 10%(15). Microarray data were clustered using the agglomerative hierarchical clustering method using the R statistical package. Each sample was treated as a 43,376-dimension vector (there are a total of 43,376 probes in an array). The Euclidean distance between samples was used as the distance metric. All non-microarray data are presented as mean ±SEM. Relative gene expression values were log transformed to make them closer to be normally distributed. Both t test and Wilcoxon’s rank sum test were used to test against the null hypotheses that the genes have equal expression levels in both preeclamptic and normal conditions. The two types of tests generally gave similar results, except for E2F. The p values of the tests were adjusted using Benjamini & Hochberg’s method(16) to control for false discovery rate.
Quantitative RTPCR
Taqman quantitative RTPCR (QRTPCR) was performed to validate a subset of genes that showed differential expression in PBMCs of preeclamptic women. The cases that were analyzed for real-time PCR consisted of both moderate and severe PE. Nine genes were selected based upon their fold differences and functional importance as indicated by literature search.
Each RNA sample was converted to cDNA using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA) per the manufacturer’s protocol. TaqMan gene expression assays for genes: IL-4 (Hs00174122_m1), FN1 (Hs01549980_g1), MMP9 (Hs00234579_m1), WFDC1 (Hs00221849_m1), BIRC5 (Hs00153353_m1), CAV1 (Hs00184697_m1), GATA1 (Hs00231112_m1), STAT1 (Hs01014002_m1), and E2F1 (Hs00153451_m1), as well as for the endogenous control GusB (Hs00939627_m1) were purchased from Applied Biosystems (Foster City, CA). For each real time PCR reaction, 1μL cDNA, 1μL gene expression assay, and 10μL TaqMan gene expression master mix were combined with water in a well on the reaction plate for a total volume of 20μL. Each reaction was run in duplicate, and each sample was also run against the endogenous control on the same reaction plate. This eliminated any differences in input DNA variation and allowed the data to be read as a relative quantity. Two plates were required for each gene, and to eliminate inter-plate variation, one sample that acted as a calibrator was run on both plates. For these experiments, a normal pregnant (NP) sample acted as the calibrator, and all sample values were recorded as relative to the calibrator. The real time PCR reactions were read and analyzed using the 7900HT Sequence Detection System (Applied Biosystems).
Biological Pathway Analysis
A list of statistically significant differential gene expression between normal and severe preeclampsia was generated. Each gene identifier was mapped to its corresponding gene object in the Ingenuity Pathways Knowledge Base(17). These genes, called focus genes, were overlaid onto a global molecular network developed from information contained in the Ingenuity Pathways Knowledge Base. Based on their functional connectivity, networks of these focus genes were generated by Ingenuity Pathways Analysis software.
Genes or gene products are represented as nodes, and the biological relationship between two nodes is represented as an edge (line). All edges are supported by at least one reference from the literature, from a textbook, or from canonical information stored in the Ingenuity Pathways Knowledge Base. The intensity of the node color indicates the degree of up- (red) or down- (green) regulation. Nodes are displayed using various shapes that represent the functional class of the gene product.
The Functional Analysis of a network identified the biological functions that were most significant to the genes in the network. The network genes associated with biological functions and/or diseases in the Ingenuity Pathways Knowledge Base were considered for the analysis. Fisher’s exact test was used to calculate a p-value determining the probability that each biological function assigned to that network is due to chance alone.
Canonical pathways analysis identified the pathways from the Ingenuity Pathways Analysis library of canonical pathways that were most significant to the data set. A data set of significantly up/down-regulated (expression) genes containing gene identifiers and corresponding expression values was uploaded into the application and associated with a canonical pathway in the Ingenuity Pathways Knowledge Base. The significance of the association between the data set and the canonical pathway was measured in two ways: 1) A ratio of the number of genes from the data set that map to the pathway divided by the total number of genes that map to the canonical pathway is displayed and 2) Fisher’s exact test was used to calculate a p-value determining the probability that the association between the genes in the dataset and the canonical pathway is explained by chance alone.
Western Blotting and Zymography
The cases that were analyzed for western analysis and zymography consisted of both moderate and severe PE. Total proteins were isolated from PBMCs and subjected to electrophoresis followed by Immuno blot analysis according to published protocols(18). A monoclonal antibody that recognizes Caveolin (sc-70516) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A goat polyclonal anti-WFDC1 antibody (NB100-57087) that recognizes a 75 kDa protein was purchased from Novus Biologicals (Littleton, CO) and a monoclonal antibody from Sigma was used to target actins.
Zymography was performed for the detection of MMP9 activity following manufacturer’s protocol (Invitrogen, Carlsbad, CA). Briefly 5 μg total protein was mixed with equal volume of Novex Tris-Glycine SDS sample buffer, electrophoresed on a premade 10% acrylamide gel containing SDS and gelatin and developed for zymography as detailed for Novex Zymogram gels (Invitrogen, Calsbad, CA).
RESULTS
Table 1 describes the general clinical characteristics of the study population. All the preeclamptic (PE) patients had significantly elevated blood pressures, proteinuria and hyperurecimia.
Statistical Analysis of Microarray Data Reveals Altered Expression in Severe Preeclampsia
We firstly compared PBMC gene expression patterns from patients with preeclampsia against those from normal pregnant controls. This analysis revealed only 10 differentially expressed genes that reached statistical significance when the false discovery rate (FDR) was controlled at 10%. These genes are listed in Table S1A. Given the fact that so few clear differences were observed between cases and normal pregnant controls, we subjected our microarray data to agglomerative hierarchical clustering analysis. The goal of this exercise was to identify sub groups of samples that might display distinct molecular phenotypes. This analysis included all the preeclampsia cases and pregnant controls. Each sample was treated as a 43,376-dimension vector (there are in total 43,376 probes on each array) and the Euclidean distance between samples was used as the distance metric. As shown in Figure 1, we identified a clear distinction between individuals whose phenotype meets the definition of “severe preeclampsia” (19) compared to those with either less profound disease or normal pregnancy. This finding suggests that there are subsets of molecular phenotypes in preeclampsia that display significant differences at the level of the PBMC transcriptome. We then searched for genes whose altered levels of expression defined these broadly based distinctive phenotypes. This analysis yielded a list of 368 genes at 10% FDR (Table S1B). A heat map displaying the most significant of these genes (adjusted P value <0.05) are presented in Figure 2.
Figure 1. Agglomerative Hierarchical Clustering of Microarray Data.
The Height axis represents the maximum Euclidean distance between the microarrays in two clusters, PE1, and PE2 and all the five normal form one cluster and SEVPE1 to 3 form another cluster. PE1, PE2 denote samples taken from subjects with mild preeclampsia; SEVPE1, SEVPE2, SEVPE3 denote samples taken from subjects with severe preeclampsia respectively.
Figure 2. Heat Map Showing Partial List of Genes Whose Expressions Were Significantly Altered (Adjusted P Value of <0.05) Between PBMCs Obtained from Individuals with Severe Preeclampsia Versus Normal Controls and Non-Severe Cases.
The entire list of 368 genes is presented in Table S1B. Green and red denote reduced and increased gene expression respectively.
Functional Classification of Differentially Transcribed Genes Reveals Altered Expression of Distinct Cellular Pathways in PBMCs
We next focused our attentions on the differences between the severe cases of preeclampsia and the rest of the cohort. Specifically, we used Ingenuity Pathways Analysis (IPA) software to perform a functional and network analysis of the data presented in Table S1B. This allowed us to identify gene classifiers and pathways that are significantly enriched between normal pregnant and severely preeclamptic PBMCs. The most striking differences identified were amongst genes whose products are involved in cell proliferation. For example, it can be seen in Figure 3 and Table S1B that numerous cell cycle and mitotic checkpoint genes are altered between normal and preeclamptic individuals. These include a number of factors whose expressions are elevated in preeclampsia and which are associated with cell cycle progression including CDK2, CCNA2, CCNB1, CCNB2, CDC45L, CDC25A, CDC2, E2F1, MYBL2, FOXM1 CDC20, CDCA8, CDCA2, CDCA3, NEK2, TPX2, CABLES1 and IRS1. Similarly, factors associated with reduced cell cycle progression such as SASH1, CDKN1C and STAT1 were found to be down regulated in preeclampsia cases.
Figure 3. Cell Cycle Related Differential Gene Expression.
Visual representation of statistically significant differential gene expression microarray data functionally classified through Ingenuity Pathways Analysis as related to cell-cycle regulation (3A). Solid lines between nodes indicate direct molecular interaction reported in the Ingenuity Pathways Knowledge Base between connected transcripts and/or gene products schematized per reported cellular localization; dotted lines between nodes indicate an indirect functional interaction between transcripts and/or gene products as reported in the Ingenuity Pathways Knowledge Base. In the heat map (3B), green denotes down-regulation (fold-ratio), whilst red denotes up-regulation (fold-ratio). Numerical fold ratio values are reported in the supplementary data. PE1, PE2 denote samples taken from subjects with mild preeclampsia; SEVPE1, SEVPE2, SEVPE3 denote samples taken from subjects with severe preeclampsia respectively.
Other related genes whose expressions are increased in severe preeclampsia include BIRC5, BIRC4BP and BCL2, which are involved in the inhibition of apoptosis, whereas the expressions of a number of proapoptotic genes such as TNFRSF8, TNFRSF16 and TRAF3 are reduced.
We also observed the altered expression of a number of genes involved in inflammation and the immune response (Table S1B). These include FCER1A, CLEC10A, IL15, IL4, IL1B, IL3RA, IL10RA, PSME1 and PSME2 that are reduced in severe preeclampsia cases. Interestingly, mRNAs encoded by the negative regulators of T-cell activation VSIG4 and SIGLEC7 genes (20, 21) are dramatically increased in severe preeclampsia cases.
We also found that the levels of expression of a number of angiogenesis associated factors to be reduced in PBMCs from severe preeclampsia cases. For example, ECGF1 and NRP1(22-24) were reduced while the anti-angiogenic factor TBXA2R was increased.
Validation of differential gene expression by real-time PCR
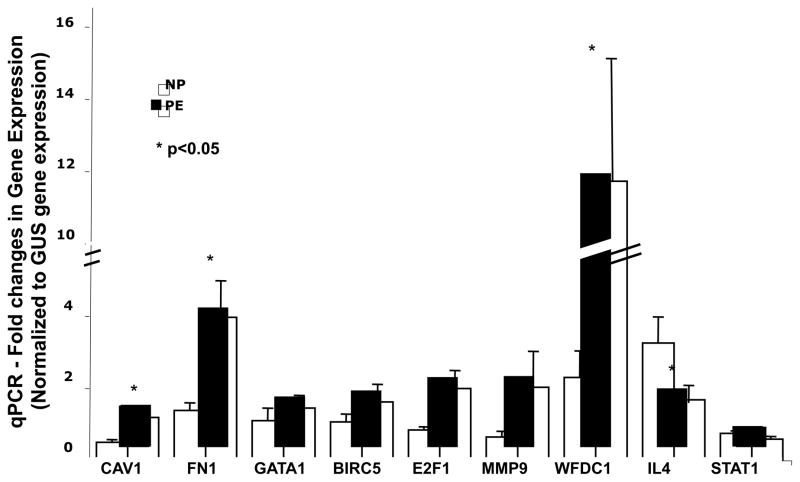
Microarray data were validated by QRTPCR using commercially available primer/probe sets from Applied Biosystems. Using this approach we were able to validate the altered expression of FN1, MMP9, WFDC1, BIRC5, CAV1, GATA1, and E2F1 whose expressions were increased in PBMCs from preeclampsia cases, and STAT1 and IL4 whose expressions were reduced in these same PBMCs. These data are presented in Figure 4.
Figure 4. QRTPCR Validation of Differentially Expressed Genes in PBMCs.
Selected differentially up-regulated and down-regulated genes were analyzed by real-time PCR to verify the DNA array results as explained in methods section. Total RNA isolated from normal pregnant women (NP, n=12) and women with preeclampsia (PE, n=12) were analyzed. Gene expression changes are considered significantly differentially expressed (marked by * in Figure 4) if the p values of both t test and Wilcoxon’s test are <=0.05, and the adjusted p values for both tests, using the Benjamini & Hochberg’s method, are <= 0.1.
Western Blot verification
Differences in mRNA levels were further substantiated using Western blot analysis and zymography at the protein level using monoclonal antibodies targeted towards select gene products. Total protein extracts were prepared from PBMCs obtained from normal pregnant women and women with preeclampsia and were subjected to Western blot analysis to verify the observed gene expression changes at the protein level. We chose WFDC1 and CAV1 gene products as candidates for Western analysis. In accordance with trends observed in the DNA array and QRTPCR data, these proteins were up regulated in PBMCs from women with preeclampsia. Likewise we analyzed levels of MMP9 gene product at the protein level by zymography and also found that the activity is increased in preeclampsia also (Figure 5).
Figure 5. Analysis of Protein Expression Levels for Selected Gene Products.
Total proteins (50μg) prepared from PBMCs of normal pregnant and preeclamptic women (n=6 each) were subjected to Western blot analysis for the identification of WFDC1 and Caveolin-1, as explained in methods section. MMP-9 activity was analyzed by zymography. Actin served as internal control and a Coomassie blue stained membrane with the total protein pattern is also shown.
DISCUSSION
We have performed comprehensive analysis of the maternal PBMC transcriptome in the context of preeclampsia. Our primary goal was to gain insight into the biological basis of preeclampsia by identifying disease-specific molecular changes in maternal peripheral blood cells. Our secondary aim was to establish proof of concept for the use of maternal PBMCs in the molecular classification of preeclampsia phenotypes with our long-term goal being the identification of early gestational predictive biomarkers of preeclampsia.
The study of preeclampsia is challenging because it involves multiple organs in both the fetal and maternal systems(25). Despite extensive efforts to the contrary, the etiology of preeclampsia still remains elusive. In recent years, however, there has been a rapid increase in our understanding of the pathobiology of preeclampsia and this has been driven to a significant degree by recent technological advancements in genomics and proteomics(26–28). However, many of the transcriptomic studies to date have been carried out using RNA derived from placental tissue samples obtained at term. Although these approaches have yielded highly significant data, they do not address biological changes that occur in a maternal context. Our study is therefore significant because it adds to our understanding of biological changes that occur in the maternal circulation in the context of preeclampsia. Caveats include the relatively low number of samples analyzed at the microarray stage and the fact that one of the PE patient samples used was obtained from an individual with chronic hypertension. However, the microarray data were confirmed at the single gene level in a larger cohort of cases and controls. Furthermore, the gene expression profiles of cases versus controls were clearly distinct both globally by microarray and in a single gene context.
One outcome of our study was the observation that the transcriptome of PBMCs from severe preeclampsia patients is distinct from that of their less severe counterparts and also from normal pregnant controls. These differences are manifested by coordinated alterations in the expressions of a variety of genes that play central roles in a number of related biological functions. These include cell proliferation, immune function, inflammation, apoptosis, angiogenesis and the regulation of blood pressure.
The fact that many of the upregulated genes encode proteins that drive cell proliferation suggests that individuals with severe preeclampsia have a proliferative PBMC phenotype. For example, E2F1 is a DNA binding transcription factor involved in the regulation of cell cycle progression and apoptosis (29) and is known to regulate and be regulated by a number of genes that, in our data, are altered at the level of transcription including CCNA2, CDK2 and MYBL2. The CCNA2 gene product binds and activates the CDC2- and CDK2-derived proteins, both of which are significantly altered at the level of transcription in our data, and thus promotes both cell cycle G1/S and G2/M transitions(30). Similarly, MYBL2 encoded protein is phosphorylated by CDK2 during the S-phase of the cell cycle and has been shown to activate the CDC2 and CCND1 genes products(31). Taken together, these observations suggest that E2F1 is centrally involved in the altered expression of cell cycle genes in PBMCs from individuals with severe preeclampsia and it will be intriguing to pursue this and similar genes as potential biomarkers of preeclampsia in early gestation. A potentially related finding is that the expression of WFDC1 is robustly increased in severe preeclampsia. WFDC1 is a putative tumor suppressor that is also known to inhibit the proliferation of senescent cells(32). WFDC1 encodes a member of the WAP-type four disulfide core domain families and the encoded protein shares 81% amino acid identity with the rat ps20 protein, which was originally identified as a secreted growth inhibitor (25, 26). The fact that WFDC1 is secreted, and also altered in severe preeclampsia at the protein level (Figure 5), is interesting because its elevated expression may be detectable at the protein level in the serum of preeclampsia patients and may therefore represent a novel marker for the diagnostic and prognostic management of this disease.
It is also significant that our data display a gene expression profile that is potentially indicative of altered immunoreactivity in preeclampsia patients. For example, we found that IL15 expression was decreased in severe preeclampsia. IL15 is known to be a potent stimulator of CD8 memory T cells(33) and NK cells(34). Interestingly it has been suggested that NK cells play a central role in the pathology of PE(35). Also notable is IL4, which induces differentiation of naive helper T cells (Th0 cells) to Th2 cells, since it has been shown that reduced Th2 response may be a component of PE(35). Similarly, VSIG4 is a strong negative regulator of murine and human T cell proliferation(20) and our discovery that its expression is increased in severe preeclampsia is significant given the above observation that PBMCs from these individuals also display an antiproliferative phenotype at the level of mRNA expression. We also found that the receptor for IL10 (IL10RA), an anti-inflammatory cytokine, was reduced in PBMCs from severe preeclampsia, which is significant because IL10 has previously been shown to be reduced in PBMCs from preeclampsia patients(36). Consistent with this we also observed a reduction in expression of HMOX1, which encodes hemoxygenase. Carbon monoxide (which is produced by the activity of HMOX1) is known to have anti-inflammatory properties(37).
Among the upregulated anti-apoptotic genes in severe preeclampsia cases was BIRC5, the expression of which is in general limited to fetal tissues and tumors(38). Interestingly, we found that E2F1 and CDCA8 were also elevated in these samples. Notably, CDCA8 has been shown to be a functional partner of BIRC5, whose promoter is positively regulated via E2F1 signaling(39). This finding is suggestive of a potential regulatory network involving genes identified in this study and again underlies the potential importance of E2F1 signaling pathways in severe preeclampsia.
The observation that the expressions of a number of genes encoding pro-angiogenic factors are reduced is intriguing. Platelet-derived endothelial cell growth factor (ECGF1) is a potent angiogenic factor that is expressed in leukocytes(40). In our data, ECGF1 is reduced in PBMCs from patients with preeclampsia, a finding that is consistent with previous results showing its expression is reduced in the placentas of preeclampsia patients(41). Similarly, the expression of neuropilin-1 (NRP1), a membrane-bound co-receptor that is associated with a tyrosine kinase receptor for both vascular endothelial growth factor (VEGF) and semaphorin family members, was also reduced amongst severe preeclampsia cases. NRP1 plays a central role in angiogenesis, potentially acting as a co-factor for VEGF-165 and thereby enhancing the angiogenic stimulus. Furthermore, SASH1 over expression, which was identified in severe preeclampsia cases in our study, has been associated with hypertension in a model of VEGF-R blockade(42). Finally, the expression of the TBXA2R gene, which encodes the thromboxane receptor, was reduced in severe preeclampsia cases. This protein is important in the context of both the regulation of platelet aggregation and vascular tone(43, 44). Not surprisingly its expression is associated with arterial hypertension. Its activity has also been linked to the impairment of angiogenesis(45) and this is significant given the above findings relating to ECGF1 and NRP1.
Our long-term goal is to identify maternal biomarkers for preeclampsia. Because the maternal samples used were obtained at term, results from the current study do not directly address this objective. These data do however serve as a useful proof of concept that maternal gene expression profiles are altered in severe preeclampsia cases. Our data suggest that the gene expression changes observed and reported here may be end stage changes rather than early changes that initiate the disease process. Significantly, the mechanisms underlying the rapid acceleration of the disease process associated with severe preeclampsia are poorly understood as are the fundamental causes of preeclampsia. Therefore, understanding the biological phenotypes that are associated with the severity of preeclampsia is also of vital importance and the current data provide valuable insight in this regard.
It should be noted that differences observed in DNA microarray data were not fully confirmed by individual gene analysis as determined by real-time PCR. Although the concordance rate between the two methods is generally high, the discrepancies are likely due to the potential for probe cross-hybridization on the microarray and the potential for the multiple probes on the microarray to record the presence of splice variants that are not detected by QRTPCR. Additionally, discrepancies in the protein expression and transcription data may be due to a variety of factors including biological phenomena relating to post-transcriptional modifications in which differences in mRNA levels are not reflected directly at the protein expression level. Other explanations may relate to the chosen analytical methods or possibly the effect of intracellular protein compartmentalization. For example, when we analyzed E2F1 we used total protein extracts and found no difference in protein expression despite the fact that DNA microarray differences were confirmed by QRTPCR. It is possible that the use of a nuclear extract for this analysis may have confirmed the expression difference at the level of protein expression. Another important factor to be considered when interpreting our data may be the effect of inter-individual variation in expression levels caused by, for example, differences in immune tolerance during gestation.
In summary, we have performed an exploratory genome wide analysis of mRNA transcription in PBMCs obtained from preeclampsia cases and normal pregnant controls. Agglomerative hierarchical clustering of the resulting data lead to the identification of a molecular phenotype that is associated with severe preeclampsia. Analysis of differentially expressed transcripts that distinguish these cases from normal pregnant samples in combination with the use of computational analysis revealed a number of biological pathways that appear to be coordinately dysregulated in severe preeclampsia. These data provide a foundation for future studies of preeclampsia pathobiology in the context of the maternal circulation. Furthermore, this study suggests that the development of non-invasive methods directed towards the maternal PBMC transcriptome and/or proteome might be a productive future research focus.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (RO3-055219 to ARK) and The Magee-Womens Research Institute and Foundation (to DGP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009 Jun;33(3):130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Luft FC. Soluble endoglin (sEng) joins the soluble fms-like tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant. 2006 Nov;21(11):3052–4. doi: 10.1093/ndt/gfl439. [DOI] [PubMed] [Google Scholar]
- 3.ten Dijke P, Goumans MJ, Pardali E. Endoglin in angiogenesis and vascular diseases. Angiogenesis. 2008;11(1):79–89. doi: 10.1007/s10456-008-9101-9. [DOI] [PubMed] [Google Scholar]
- 4.Kita N, Mitsushita J. A possible placental factor for preeclampsia: sFlt-1. Curr Med Chem. 2008;15(7):711–5. doi: 10.2174/092986708783885309. [DOI] [PubMed] [Google Scholar]
- 5.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 6.Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009 Jan;30(1):15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response--a review. Placenta. 2003 Apr;24(Suppl A):S21–7. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 8.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999 Dec;222(3):222–35. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 9.Redman CW, Sargent IL. Preeclampsia and the systemic inflammatory response. Semin Nephrol. 2004 Nov;24(6):565–70. doi: 10.1016/s0270-9295(04)00127-5. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki S, Sekizawa A, Purwosunu Y, Farina A, Wibowo N, Okai T. Placenta-derived, cellular messenger RNA expression in the maternal blood of preeclamptic women. Obstet Gynecol. 2007 Nov;110(5):1130–6. doi: 10.1097/01.AOG.0000286761.11436.67. [DOI] [PubMed] [Google Scholar]
- 11.Sun CJ, Zhang L, Zhang WY. Gene expression profiling of maternal blood in early onset severe preeclampsia: identification of novel biomarkers. J Perinat Med. 2009;37(6):609–16. doi: 10.1515/JPM.2009.103. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003 Mar;41(3):437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 13.Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol. 1984 Feb;91(2):128–32. doi: 10.1111/j.1471-0528.1984.tb05895.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002 Feb 15;30(4):e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 16.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003 Feb 12;19(3):368–75. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 17.Systems I. Ingenuity Pathways Analysis. 2008. Ingenuity Pathways Analysis. [Google Scholar]
- 18.Rajakumar A, Doty K, Daftary A, Markovic N, Conrad KP. Expression of von Hippel Lindau (pVHL) protein in placentae from normal pregnant women and women with preeclampsia. Placenta. 2006 Apr–May;27(4–5):411–21. doi: 10.1016/j.placenta.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Wagner LK. Diagnosis and management of preeclampsia. Am Fam Physician. 2004 Dec 15;70(12):2317–24. [PubMed] [Google Scholar]
- 20.Vogt L, Schmitz N, Kurrer MO, Bauer M, Hinton HI, Behnke S, et al. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J Clin Invest. 2006 Oct;116(10):2817–26. doi: 10.1172/JCI25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004 Oct 8;279(41):43117–25. doi: 10.1074/jbc.M403538200. [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss KA, Ashton AW, Schwartz EL. Thymidine phosphorylase and 2-deoxyribose stimulate human endothelial cell migration by specific activation of the integrins alpha 5 beta 1 and alpha V beta 3. J Biol Chem. 2003 May 23;278(21):19272–9. doi: 10.1074/jbc.M212670200. [DOI] [PubMed] [Google Scholar]
- 23.Kikuno N, Yoshino T, Urakami S, Shigeno K, Kishi H, Hata K, et al. The role of thymidine phosphorylase (TP) mRNA expression in angiogenesis of prostate cancer. Anticancer Res. 2003 Mar–Apr;23(2B):1305–12. [PubMed] [Google Scholar]
- 24.Cheng L, Jia H, Lohr M, Bagherzadeh A, Holmes DI, Selwood D, et al. Anti-chemorepulsive effects of vascular endothelial growth factor and placental growth factor-2 in dorsal root ganglion neurons are mediated via neuropilin-1 and cyclooxygenase-derived prostanoid production. J Biol Chem. 2004 Jul 16;279(29):30654–61. doi: 10.1074/jbc.M402488200. [DOI] [PubMed] [Google Scholar]
- 25.Karumanchi SA, Lindheimer MD. Advances in the understanding of eclampsia. Curr Hypertens Rep. 2008 Aug;10(4):305–12. doi: 10.1007/s11906-008-0057-3. [DOI] [PubMed] [Google Scholar]
- 26.Blumenstein M, McMaster MT, Black MA, Wu S, Prakash R, Cooney J, et al. A proteomic approach identifies early pregnancy biomarkers for preeclampsia: novel linkages between a predisposition to preeclampsia and cardiovascular disease. Proteomics. 2009 Jun;9(11):2929–45. doi: 10.1002/pmic.200800625. [DOI] [PubMed] [Google Scholar]
- 27.Varkonyi T, Nagy B, Fule T, Tarca AL, Karaszi K, Schonleber J, et al. Microarray Profiling Reveals That Placental Transcriptomes of Early-onset HELLP Syndrome and Preeclampsia Are Similar. Placenta. Jun 11; doi: 10.1016/j.placenta.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitras V, Paulssen RH, Gronaas H, Leirvik J, Hanssen TA, Vartun A, et al. Differential placental gene expression in severe preeclampsia. Placenta. 2009 May;30(5):424–33. doi: 10.1016/j.placenta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Polager S, Ginsberg D. E2F - at the crossroads of life and death. Trends Cell Biol. 2008 Nov;18(11):528–35. doi: 10.1016/j.tcb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Blanchard JM. Cyclin A2 transcriptional regulation: modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem Pharmacol. 2000 Oct 15;60(8):1179–84. doi: 10.1016/s0006-2952(00)00384-1. [DOI] [PubMed] [Google Scholar]
- 31.Joaquin M, Watson RJ. Cell cycle regulation by the B-Myb transcription factor. Cell Mol Life Sci. 2003 Nov;60(11):2389–401. doi: 10.1007/s00018-003-3037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madar S, Brosh R, Buganim Y, Ezra O, Goldstein I, Solomon H, et al. Modulated expression of WFDC1 during carcinogenesis and cellular senescence. Carcinogenesis. 2009 Jan;30(1):20–7. doi: 10.1093/carcin/bgn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng NP, Liu K, Catalfamo M, Li Y, Henkart PA. IL-15 is a growth factor and an activator of CD8 memory T cells. Ann N Y Acad Sci. 2002 Dec;975:46–56. doi: 10.1111/j.1749-6632.2002.tb05940.x. [DOI] [PubMed] [Google Scholar]
- 34.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994 Oct 1;180(4):1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sargent IL, Borzychowski AM, Redman CW. NK cells and pre-eclampsia. J Reprod Immunol. 2007 Dec;76(1–2):40–4. doi: 10.1016/j.jri.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Hennessy A, Orange S, Willis N, Painter DM, Child A, Horvath JS. Transforming growth factor-beta 1 does not relate to hypertension in pre-eclampsia. Clin Exp Pharmacol Physiol. 2002 Nov;29(11):968–71. doi: 10.1046/j.1440-1681.2002.03763.x. [DOI] [PubMed] [Google Scholar]
- 37.Pae HO, Lee YC, Chung HT. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov. 2008;2(3):159–65. doi: 10.2174/187221308786241929. [DOI] [PubMed] [Google Scholar]
- 38.Iskandar ZA, Al-Joudi FS. Expression of survivin in fetal and adult normal tissues of rat. Malays J Pathol. 2006 Dec;28(2):101–5. [PubMed] [Google Scholar]
- 39.Jiang Y, Saavedra HI, Holloway MP, Leone G, Altura RA. Aberrant regulation of survivin by the RB/E2F family of proteins. J Biol Chem. 2004 Sep 24;279(39):40511–20. doi: 10.1074/jbc.M404496200. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura A, Kuwazuru Y, Furukawa T, Yoshida H, Yamada K, Akiyama S. Purification and tissue distribution of human thymidine phosphorylase; high expression in lymphocytes, reticulocytes and tumors. Biochim Biophys Acta. 1990 Apr 23;1034(1):107–13. doi: 10.1016/0304-4165(90)90160-x. [DOI] [PubMed] [Google Scholar]
- 41.Jarvenpaa J, Vuoristo JT, Savolainen ER, Ukkola O, Vaskivuo T, Ryynanen M. Altered expression of angiogenesis-related placental genes in pre-eclampsia associated with intrauterine growth restriction. Gynecol Endocrinol. 2007 Jun;23(6):351–5. doi: 10.1080/09513590701350291. [DOI] [PubMed] [Google Scholar]
- 42.Sakao S, Taraseviciene-Stewart L, Cool CD, Tada Y, Kasahara Y, Kurosu K, et al. VEGF-R blockade causes endothelial cell apoptosis, expansion of surviving CD34+ precursor cells and transdifferentiation to smooth muscle-like and neuronal-like cells. FASEB J. 2007 Nov;21(13):3640–52. doi: 10.1096/fj.07-8432com. [DOI] [PubMed] [Google Scholar]
- 43.Russell JA. Substance P elicits thromboxane-induced contraction of canine pulmonary veins. Pulm Pharmacol. 1988;1(3):153–9. doi: 10.1016/s0952-0600(88)80013-9. [DOI] [PubMed] [Google Scholar]
- 44.Needleman P, Minkes M, Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–5. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- 45.Benndorf RA, Schwedhelm E, Gnann A, Taheri R, Kom G, Didie M, et al. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: a potential link between oxidative stress and impaired angiogenesis. Circ Res. 2008 Oct 24;103(9):1037–46. doi: 10.1161/CIRCRESAHA.108.184036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.