Abstract
Autism spectrum disorders (ASDs) are typically characterized by impaired social interaction and communication, narrow interests, and repetitive behaviors. The heterogeneity in the severity of these characteristics across individuals with ASD has led some researchers to suggest that these disorders form a continuum which extends into the general, or “typical,” population, and there is growing evidence that the extent to which typical adults display autistic traits, as measured using the autism-spectrum quotient (AQ), predicts performance on behavioral tasks that are impaired in ASD. Here, we show that variation in autism spectrum traits is related to cortical structure and function within the typical population. Voxel-based morphometry showed that increased AQ scores were associated with decreased white matter volume in the posterior superior temporal sulcus (pSTS), a region important in processing socially relevant stimuli and associated with structural and functional impairments in ASD. In addition, AQ was correlated with the extent of cortical deactivation of an adjacent area of pSTS during a Stroop task relative to rest, reflecting variation in resting state function. The results provide evidence that autism spectrum characteristics are reflected in neural structure and function across the typical (non-ASD) population.
Keywords: autism, fMRI, resting state, voxel-based morphometry
Introduction
Autism spectrum disorders (ASDs) are characterized by impaired social interaction and communication, restricted interests, and repetitive behaviors. The severity of these characteristics are posited to lie on a continuum that extends into the general population (Frith 1991; Baron-Cohen 1995). To determine the extent to which adults of at least average intelligence display characteristics associated with ASD, Baron-Cohen, Wheelwright, Skinner, et al. (2001) developed a self-administered questionnaire, the autism-spectrum quotient (AQ). Individual differences in AQ have been shown to predict performance in both typical and ASD populations on tasks that are impaired in ASD, including self-focused attention (Lombardo et al. 2007), local versus global processing (Grinter et al. 2009), inferring others’ mental state from the eyes (Baron-Cohen, Wheelwright, Hill, et al. 2001), and attentional cueing from gaze (Bayliss and Tipper 2005). An important question is whether variation in autism spectrum traits are associated with changes in structure and patterns of activation in typical participants’ brains.
A recent review by Zilbovicius et al. (2006) emphasized that ASD is associated with changes in gray matter (GM) and white matter (WM) structure along the length of the superior temporal sulcus (STS) and superior temporal gyrus (Barnea-Goraly et al. 2004; Boddaert et al. 2004; McAlonan et al. 2005). Gray matter changes have typically been localized to the upper and lower banks of more posterior STS (pSTS) and posterior temporal regions more generally while WM changes extend along its length and into pSTS and adjacent temporoparietal junction (TPJ) (for details of location of previously reported structural and functional changes, see Supplementary Fig. S1). These latter regions also play a key role in social processing and along with other regions, notably the medial prefrontal cortex (mPFC), have repeatedly shown abnormal responses to socially meaningful tasks in individuals with ASD (Happe et al. 1996; Castelli et al. 2002; Pelphrey et al. 2005). Changes in the WM structure of mPFC in ASD have also been reported (Barnea-Goraly et al. 2004), suggesting underlying structural changes in this network of “social” regions.
To address whether AQ scores are related to structural variation in local gray or WM volume of these areas in typical participants’ brains, we performed a voxel-based morphometry (VBM) analysis. We were also interested in whether AQ affected changes in the blood oxygen level–dependent (BOLD) response and whether the areas affected corresponded to those that showed structural changes. It was important to ensure that any AQ-related changes in BOLD signal were not secondary to altered performance or strategy, as might be expected for certain perceptual or social cognitive tasks that are impaired in individuals with ASD or in typical participants with high AQ scores. We therefore compared the change in BOLD response while performing a Stroop task, not typically affected by ASD (Kennedy et al. 2006), relative to rest to address “deactivation” of the default-mode or resting state network. This network comprises a number of regions that are more active at rest but reduce in activation, or “deactivate,” during attention-demanding tasks and comprises mPFC, posterior cingulate cortex, precuneus, and posterior lateral cortices, including pSTS/TPJ and angular gyrus (Gusnard and Raichle 2001; Buckner et al. 2008). A number of studies have suggested that activation in these regions at rest may reflect internal self-reflective thought processes, and the extent of deactivation during a task may therefore indicate the degree to which these processes are suppressed during an attention-demanding task (Shulman et al. 1997; Gusnard and Raichle 2001; Mazoyer et al. 2001). These same resting state regions show remarkable overlap with areas implicated in social functions displaying abnormal activation in ASD (Buckner et al. 2008). More recent research has specifically addressed rest activation in individuals with ASD and shown abnormal function in the resting state network (Cherkassky et al. 2006; Kennedy et al. 2006; Kennedy and Courchesne 2008; Monk et al. 2009), and specifically within regions of the STS, particularly pSTS and adjacent angular gyrus. Thus, we were interested in whether the extent of deactivation in typical controls during the Stroop task was related to individual differences in AQ scores.
Materials and Methods
Participants
Ninety-five right-handed healthy adults participated in the VBM study. Participants were recruited from the MRC Cognition & Brain Sciences Unit’s volunteer panel, which comprises members of the local Cambridge community, including a large proportion from Cambridge University, none had a history of psychiatric illness and/or physical illness. Two were excluded from statistical analyses because of excessive head movement (>2 mm), and a further 2 participants who also took part in the functional magnetic resonance imaging (fMRI) study were removed from the VBM (but not from fMRI) analysis because their structural scans had very poor image intensity distributions. This left 91 participants’ data for analysis (mean age = 25 ± 5 years, range: 18–42; 53 females). Where time permitted, a subset of these volunteers (31) underwent 2 structural scans, which were then averaged to improve image quality for segmentation purposes. All participants completed the AQ questionnaire (mean score: 16 ± 7, range: 2–33). This 50-item questionnaire is a validated measure of the degree of autism spectrum characteristics found within the typical population, as well as in individuals with high-functioning autism and Asperger Syndrome (Baron-Cohen, Wheelwright, Skinner, et al. 2001). Examples of items include: “I would rather go to the library than to a party,” “I find it difficult to work out people’s intentions,” and “I notice patterns in things all the time.” A higher AQ score indicates a greater extent of autism spectrum characteristics. One of the 91 volunteers scored 33, just above the 32-point cutoff determined by Baron-Cohen, Wheelwright, Skinner, et al. (2001). However, the AQ is not a diagnostic measure, and none had with a clinical diagnosis of an ASD. Written informed consent was obtained from all participants, and the study was approved by the local research ethics committee.
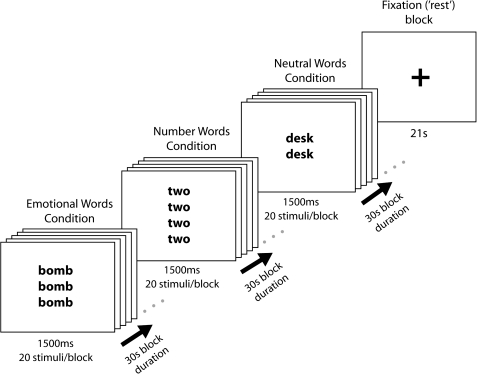
A randomly selected subset of 19 volunteers (12 females; mean age = 24 ± 4 years) also completed the fMRI Stroop task (mean AQ score = 18 ± 7, range: 5–30); the remaining participants completed unrelated fMRI experiments. Materials and design for the fMRI study are summarized in Figure 1. The Stroop task used a similar design to Kennedy et al. (2006) that consisted of 3 task conditions—number words, negatively valenced emotional words, and neutral words. Between 2 and 4, identical words appeared on the screen at any one time. Participants were instructed to count the number of times the word was displayed and to respond as quickly and as accurately as possible by button press. Number words were always incongruent with respect to the number of times the word appeared on the screen (e.g., “2” written 4 times). Ten negatively valenced emotional words (e.g., “bomb”) and 10 neutral words were chosen from the Affective Norms for English Words list (Bradley and Lang 1999). Stimuli were presented in a blocked design in one of 2 pseudorandom orders using E-prime (Psychology Software Tools). Each stimulus was presented for 1.5 s, with each block containing 20 presentations from the same word category. Each set of task conditions (number, emotional, and neutral words) was repeated 4 times with three 21-s fixation rest blocks between repeats of task blocks. For fixation blocks, participants were instructed to stare passively at the low-contrast fixation cross presented at the center of the screen.
Figure 1.
Materials and design for the fMRI Stroop task.
Reaction times, measured from stimulus onset to participant response, and percent correct responses were recorded for all participants. To verify that participants were reading the words in the scanner, they were given a surprise word recognition memory test immediately after scanning which comprised a list of 20 emotional and 20 neutral words. Half of the words had been presented in the scanner and the other half were novel words whose arousal and valence were matched with those shown in the scanner. Participants had to indicate which words they remembered seeing in the scanner. Hits, misses, and false positives were converted into a percent correct score.
Magnetic Resonance Imaging Acquisition and Analysis
All data were acquired on a Siemens 3-T Tim Trio scanner, and all analyses were performed in SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). A high-resolution structural magnetization prepared rapid gradient echo scan (voxel size = 1 × 1 × 1 mm, repetition time = 2250 ms, echo time = 2.99 ms, inversion time = 900 ms, flip angle = 9o, total scan time = 4 min 16 s) was acquired in all participants. VBM analysis was performed in SPM5, which enables automated spatial normalization, tissue classification, and radio-frequency bias correction to be combined within the segmentation step. Default values for segmentation and normalization within SPM5 were used. Following normalization and segmentation into GM and WM, a modulation step was incorporated to take into account volume changes caused by spatial normalization which can cause certain brain regions to shrink or expand. This was done by multiplying the voxel values in the segmented images by the Jacobian determinants from the spatial normalization step. The segmented, normalized, and modulated images were smoothed using a Gaussian kernel of 10-mm full-width at half-maximum (FWHM) and entered into a regression model with AQ. An absolute threshold mask was set at 0.1 to ensure that the voxels included in the analysis had a higher probability of being WM or GM, respectively. Global WM and global GM volumes were calculated from segmentations in native space. Global WM volume was included as a covariate of no interest in the VBM analysis of WM volume differences, to account for any gross differences in total WM volume across participants. Similarly, global GM volume was included as a covariate of no interest when changes in GM volume were being modeled. As the AQ is a behavioral measure tapping social–communicative skills and behavior associated with ASD, we expected to find effects primarily in regions associated with social cognition, in particular the mentalizing network comprising mPFC, TPJ, and STS, which is impaired in ASD. We corrected for multiple comparisons by family-wise error using a small-volume correction with a 20-mm sphere centered on the average of the pSTS/TPJ and mPFC peak activations from 10 mentalizing studies listed in Frith U and Frith CD (2003), such that for the left and right hemispheres, the sphere was centered at x = ±55, y = −55, z = 11 for pSTS/TPJ and x = −4, y = 45, z = 34 for mPFC. To take into account more ventral aspects of mPFC/anterior cingulate cortex (ACC) often identified in theory-of-mind (ToM) and the resting state, we included an additional region of interest (ROI) centered on the average of peak coordinates in ventral mPFC/ACC identified in a subset of the same 10 studies: x = −1, y = 47, z = 4. In addition, any other regions surviving P < 0.001 (uncorrected) and a cluster extent threshold of 10 voxels are reported in Table 1.
Table 1.
Functional deactivation as a function of AQ (P < 0.001 uncorrected, unless otherwise indicated; cluster extent threshold 10 voxels)
| Brain region | Hemisphere | Cluster size | T | x | y | z |
| All versus fixation | ||||||
| STS | L | 83 | 5.29* | −42 | −42 | 4 |
| R | 204 | 6.83* | 40 | −40 | 12 | |
| Posterior insula | L | 12 | 3.60 | −34 | −4 | 24 |
| Brainstem | L | 22 | 3.48 | −8 | −26 | −28 |
| Midcingulate | L | 18 | 3.38 | −8 | 16 | 40 |
| Number versus fixation | ||||||
| STS | L | 95 | 4.70* | −42 | −42 | 4 |
| R | 54 | 5.48* | 40 | −40 | 12 | |
| Emotional versus fixation | ||||||
| STS | L | 250 | 5.89* | −42 | −40 | 4 |
| R | 209 | 8.99* | 40 | −40 | 12 | |
| Supplemental motor area | L | 31 | 4.51 | −12 | 2 | 58 |
| R | 33 | 5.26 | 18 | 14 | 62 | |
| Hippocampus | R | 92 | 5.22 | 38 | −34 | −10 |
| Subparietal sulcus | L | 124 | 5.15 | −2 | −74 | 40 |
| R | 14 | 3.81 | 34 | 2 | 30 | |
| Mid-cingulate | L | 27 | 3.40 | −6 | 12 | 38 |
| R | 22 | 3.24 | 6 | 10 | 30 | |
| Brainstem | L | 26 | 3.38 | −8 | −22 | −28 |
| Neutral versus fixation | ||||||
| STS | L | 100 | 4.67* | −44 | −48 | 8 |
| Brainstem | L | 85 | 4.51 | −8 | −26 | −28 |
| Visual cortex | R | 32 | 4.07 | 12 | −90 | 0 |
| Frontal operculum | L | 24 | 3.58 | −30 | 30 | −6 |
| Dorsal anterior cingulate | L | 11 | 3.56 | −16 | 18 | 46 |
| Pars triangularis | L | 15 | 3.34 | −32 | 12 | 28 |
P < 0.05 small-volume correction with a 20-mm sphere centered at x = ±55, y = −55, z = 11.
For the fMRI study, participants underwent gradient-recalled echo-planar imaging (EPI) consisting of 40 axial oblique slices (repetition time = 2.4 s, echo time = 30 ms, 3 × 3 × 3-mm voxel size). The first 3 volumes were discarded to allow for equilibration effects. The EPI images were sinc interpolated in time to correct for slice time differences and realigned to the first scan by rigid body transformations to correct for head movements. EPI and structural scans were coregistered and normalized to the T1 standard template in Montreal Neurological Institute space (Montreal Neurological Institute–International Consortium for Brain Mapping) using linear and nonlinear transformations and smoothed with a Gaussian kernel of 10-mm FWHM. Condition effects were estimated using boxcar regressors convolved with the canonical hemodynamic response function in the general linear model. Data were high-pass filtered (cutoff 128 s) to remove low-frequency signal drift. Spatial realignment parameters were included as regressors of no interest in the model to account for any residual movement-related variance. Statistical parametric maps were generated for each individual by estimating activation contrasts between conditions. Data were analyzed at the group level by conducting a random-effects analysis. A multiple regression model was used to estimate the correlation between AQ scores and all voxels in the brain for a given response contrast. Small-volume correction for multiple comparisons was applied using the same ROIs described in the VBM analysis.
Results
VBM
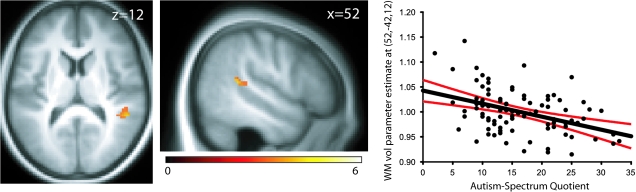
We performed a VBM analysis on magnetic resonance imaging scans of 91 healthy adults, who completed the AQ questionnaire. The results showed a significant reduction in WM volume with increased AQ scores in just one region: right pSTS (cluster size = 64 voxels, T = 4.32, x = 52, y = −42, z = 12, P < 0.02 small-volume corrected [SVC]) (Fig. 2). Age and gender were not significantly associated with AQ (P = 0.25 and P = 0.17, respectively) and repeating the analysis including these variables as covariates-of-no-interest produced highly similar results (cluster size = 43; T = 4.14; x = 52, y = −42, z = 12; P < 0.02 svc). No other regional WM changes were observed as a function of AQ. Changes in GM volume with AQ were not found in our a priori ROI; however, increasing AQ was associated with increased GM in left superior frontal sulcus (cluster size = 34; T = 3.73; x = −30, y = 14, z = 62; P < 0.001 uncorrected).
Figure 2.
The pSTS region showing significantly reduced WM volume with increased AQ displayed on the average structural scan for all participants. Scatterplot shows the effect size of WM volume reduction in right STS at the peak voxel as a function of AQ (T = 4.32) with 95% confidence intervals. Data are displayed at P < 0.001 uncorrected. Correlation plot is for illustrative purposes only.
Functional Imaging: Stroop Task
A subset of 19 participants also underwent fMRI while performing a counting Stroop task to determine whether AQ scores were related to activation of the resting state network. In view of the VBM results, we were particularly interested in the relationship between AQ and deactivation in the pSTS.
Consistent with the observation of Kennedy et al. (2006) that behavioral performance on the 3 conditions of the Stroop task was relatively unaffected by ASD, analysis of covariance showed no significant correlation between participants’ accuracy on the task and AQ (F2,34 = 0.26, P = 0.78), and a nonsignificant trend between reaction times and AQ across conditions (F2,34 = 2.8, P = 0.08). Postscanning recognition memory for the neutral and emotional words used in the task conditions was good (80%), indicating that subjects attended throughout. In addition, there was no significant relationship between AQ and participants’ memory for words in the postscan assessment (F1,17 = 0.3, P = 0.59). Hence, any changes in brain activity are unlikely to reflect AQ-related differences in task performance.
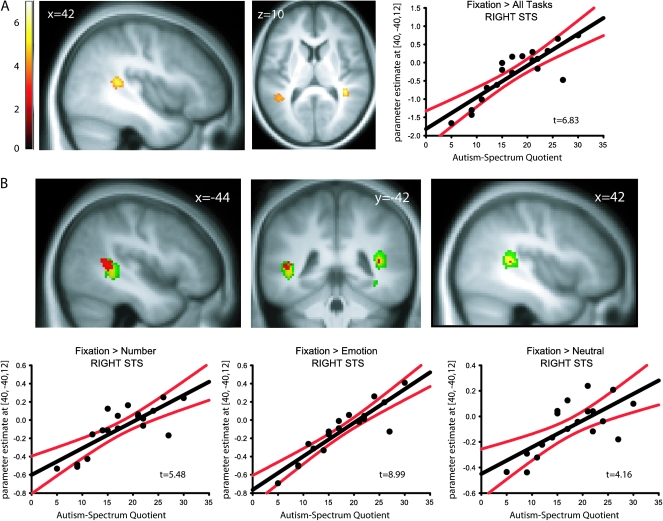
Irrespective of AQ, separate comparisons of each task condition with fixation produced significant deactivation in resting state regions (see Supplementary Fig. S2). Of more interest, AQ scores predicted deactivation in bilateral pSTS for all task conditions compared with fixation, such that individuals with higher AQ scores displayed greater deactivation of pSTS (Table 1; Fig. 3a). Furthermore, when each task condition (number words, emotional words, and neutral words) was compared with fixation independently, we found a highly similar relationship with AQ in pSTS for each, indicating that the effect was robust across each task condition (Table 1 and Fig. 3b).
Figure 3.
(A) Regions whose deactivation significantly correlated with AQ for all task conditions compared with fixation. Effect sizes of right pSTS peak voxel fixation response are plotted as a function of AQ (T = 6.83) with 95% confidence intervals. (B) Regions whose deactivation significantly correlated with AQ for the number words condition compared with fixation (yellow), emotional words compared with fixation (green), and neutral words compared with fixation (red). Effect sizes of right pSTS fixation response as a function of AQ for fixation versus number words (T = 5.48), fixation versus emotional words (T = 8.99), and fixation versus neutral words (T = 4.16) are plotted with 95% confidence intervals. Data are displayed at P < 0.001 uncorrected. Correlation plots are for illustrative purposes only.
Taking the functional and structural results together, it is striking that the location of the WM reduction with AQ identified by VBM is in close proximity to the functional STS deactivation observed in the Stroop task. To rule out the possibility that the reduction of WM in pSTS was driven by the subset of participants who participated in both the VBM and fMRI components, we performed an additional VBM analysis excluding those participants and found identical results (cluster size = 70; T = 4.59; x = 52, y = −42, z = 12; P < 0.007 svc), indicating that the functional and structural changes in pSTS as a function of AQ can be found in independent samples.
Given that the VBM and fMRI Stroop tasks identified changes in proximal regions of the pSTS, we conducted a further post hoc correlation analysis to test for any relationship between the extracted BOLD response and WM volume at pSTS for participants who took part in both fMRI and VBM experiments. This revealed no significant relationship. However, since we had an independent sample consisting of the individuals who took part in the VBM study only, we used the correlation coefficient of WM volume with AQ (r = −0.46) from this independent sample as an estimate of effect size. A power analysis (80% power) revealed the minimum required sample size to detect a correlation with WM volume is 31 participants. Consequently, a significant correlation between WM and BOLD response may be found in a larger sample.
The scale of Baron-Cohen, Wheelwright, Skinner, et al. (2001) was originally devised to assess autism spectrum traits across 5 different areas of behavior. More recent work by Hoekstra et al. (2008) showed that a number of these subscales are intercorrelated and that factor analysis identified just 2 distinct scales tapping “social interaction” and “attention to detail.” Hoekstra et al. (2008) also used a slightly different method of scoring the AQ questionnaire. We therefore performed additional analyses examining the total AQ scores according to Hoekstra et al. (2008), as well as the 2 proposed subscales. For all 3, we identified AQ-related changes in WM and deactivation in the pSTS for the VBM and Stroop tasks, respectively (VBM Hoekstra total: T = 3.44, x = 50, y = −42, z = 14; VBM Hoekstra social: T = 3.26, x = 40, y = −42, z = 18; VBM Hoekstra detail: T = 3.22, x = 52, y = −42, z = 12; Stroop Hoekstra total: T = 4.19, x = −42, y = −42, z = 2; Stroop Hoekstra social: T = 3.93, x = −42, y = −42, z = 2; Stroop Hoekstra detail: T = 3.90, x = 38, y = −46, z = 18; all P ≤ 0.002 uncorrected). Furthermore, in the VBM analysis, the social interaction subscale, which includes items relating to ToM, such as “When I’m reading a story, I find it difficult to work out the characters’ intentions,” identified a negative correlation with WM volume in ACC/mPFC (T = 3.08, x = −12, y = 40, z = −2; P < 0.002 uncorrected), an area strongly implicated in ToM (Frith CD and Frith U 1999; Gallagher and Frith 2003).
Discussion
We present converging evidence from structural and functional neuroimaging data that variation in autism spectrum characteristics in the typical (non-ASD) population is correlated with both WM volume and BOLD response in pSTS. Individuals with ASD show considerable variation in the severity and extent to which they exhibit characteristics of the disorder. This heterogeneity has led some researchers to posit that ASD lies on a continuum of social–communication difficulties (Frith 1991; Baron-Cohen 1995) that extends into the “typical” population. Consistent with this proposal, behavioral studies in typical participants show an effect of autism spectrum traits on tasks that are impaired in ASD (Baron-Cohen, Wheelwright, Hill, et al. 2001; Lombardo et al. 2007; Grinter et al. 2009), including attentional cueing from eye gaze, which is strongly associated with pSTS (Bayliss and Tipper 2005). Indeed, in as yet unpublished work, we have found that AQ predicts increased cortical response to changes in gaze direction in typical adults in a remarkably similar region of pSTS to that identified here. While all these studies have used AQ as a measure of autism spectrum traits, work using a related self-report quantitative measure, the Social Responsiveness Scale (SRS), also provides evidence of a continuum in social communicative function across a large sample of typical adolescents (Constantino and Todd 2003). Indeed, a recent functional connectivity study found evidence of a negative relationship between SRS scores in typical adults and cingulo-insular connectivity (Di Martino et al. 2009). Our current results provide a new addition to the study of autism spectrum traits within the typical population by demonstrating their relationship with the WM structure and regional BOLD response of an area implicated in social cognition and ASD.
Previous structural brain imaging studies have emphasized abnormalities in the STS region in ASD, revealing local reductions in GM volume (Boddaert et al. 2004; McAlonan et al. 2005) along the upper and lower banks of pSTS and reduced fractional anistotropy of WM, reflecting altered connectivity or general integrity of WM tracts, along the length of the STS extending into pSTS/TPJ region (Barnea-Goraly et al. 2004). While we did not observe any significant changes in GM volume in these regions, the reduction in WM volume with greater self-reported autistic traits is consistent with the findings of Barnea-Goraly et al. (2004) in ASD participants and suggests that structural changes in WM in pSTS extend from the typical to ASD population and, in the typical population at least, are coupled with behavioral characteristics associated with ASD.
The location of our observed changes in WM volume and BOLD response is also particularly striking given the role of the pSTS and adjacent TPJ in both the resting state network and higher order social function. Several imaging studies have examined brain function at rest in ASD using various techniques and consistently reported differences along the STS, although the direction of the effect and the location of the changes show some variability from one study to the next. Ohnishi et al. (2000) and Zilbovicius et al. (2000) found reduced perfusion at rest in individuals with ASD along the length of the STS, including regions of pSTS, and Kennedy et al. (2006) similarly found reduced task-independent deactivation in ASD in more anterior STS. A more recent study has reported increased functional connectivity at rest of mid-STS with other resting state regions (Monk et al. 2009). In the posterior aspects of the STS region more traditionally associated with the resting state network, reduced resting state connectivity of the angular gyrus, which lies adjacent to the posterior ascending limb of the STS, with other components of the network has also been reported in ASD (Kennedy and Courchesne 2008).
The pSTS/TPJ region also plays an important role in higher order social function and has been shown to display reduced task-related activation in individuals with ASD in response to social stimuli such as eye gaze and “Heider–Simmel” animations of 2D shapes moving in a socially meaningful manner (Castelli et al. 2002; Pelphrey et al. 2005). Our findings of increased fixation activation or increased task-independent deactivation, with increased autism spectrum traits lie in a remarkably similar region of pSTS to those identified in task-related activation studies, and adjacent to the angular gyrus identified in the resting state study of Kennedy and Courchesne (2008).
While these studies have typically reported reduced activation in the ASD group which is thought to reflect abnormalities in ToM, perception of social interactions, and, particularly in the case of task-independent or resting state studies, internally directed thought processes more generally, we have found increased rest activation with greater autistic traits in the typical population. Similarly, some fMRI studies have investigated the relationship between cortical response and ASD symptom severity in clinical populations with ASD, which is typically measured using the Autism Diagnostic Interview (Lord et al. 1994). These studies have found that symptom severity in the ASD participants is negatively related to the magnitude of the change in activation (Pelphrey et al. 2005; Kennedy et al. 2006). Our results appear at odds with these findings, however, it is important to note that our sample consisted of neurotypical controls only, whereas these studies looked for correlative measures within the clinical ASD population only. A key next step in understanding the relationship between autism spectrum traits and neural function will be to examine the effect of AQ across the whole autism spectrum, from typical to ASD individuals. In fact, there is some evidence at the behavioral level to suggest such seemingly opposing relationships between autistic traits in the typical and ASD populations are not without basis. Lombardo et al. (2007) showed an inverted-U relationship between AQ and a behavioral measure of self-focused attention associated with mentalizing ability in ASD—typical controls displayed a positive relationship between AQ and self-focused attention, whereas the relationship became negative at the higher end of the AQ scale in individuals with ASD. In addition, there is evidence that WM volume and BOLD response may not be positively correlated. Multiple sclerosis is characterized by deterioration of WM, but this is associated with increased BOLD response in the early stages of the disease and decreased BOLD response in later stages of the disease (Rocca et al. 2005). While we are not dealing with a progressive clinical population, it is possible that such an adaptive relationship between BOLD response and WM volume may exist at the neurophysiological level, such that reductions in WM volume in the normal population are associated with relatively increased BOLD response. For the present, however, our study provides convergent evidence from 2 distinct neuroimaging techniques that variation in AQ in the typical population is linked to altered neurophysiology of the pSTS.
As discussed earlier, Baron-Cohen, Wheelwright, Skinner, et al. (2001) originally devised the AQ to assess autism spectrum characteristics across 5 different areas. By contrast, more recent work by Hoekstra et al. (2008) using a factor analysis of AQ data identified just 2 distinct subscales relating to social interaction and attention to detail. Additional analyses of our VBM and fMRI data showed that both of these subscales and the total scale of Hoekstra et al. (2008) also predicted changes in structure and function of pSTS. Interestingly, however, the social interaction subscale, which relates more to ToM and difficulties in social interaction, was unique in identifying a cluster of reduced WM volume in ACC/mPFC, an area with an established role in ToM. Although the cluster did not survive small-volume correction, it is of interest that Barnea-Goraly et al. (2004) also found that participants with ASD showed changes in WM structure in very similar regions of mPFC and pSTS/TPJ to those identified here. On the basis of the current results, we speculate that pSTS has a role in the broader aspects of Autism, whereas ACC/mPFC has a greater specificity to autistic traits related to ToM and social function.
Following other studies (Greicius et al. 2004; Kennedy et al. 2006), we have interpreted altered patterns of deactivation in a cognitive (e.g., Stroop) task relative to rest as reflecting differences in the neural response during the fixation (“rest”) condition. As with any contrast comparing 2 conditions, however, it is not possible to be certain which condition is driving the effect. However, this seems a reasonable interpretation given that the pSTS/TPJ forms part of the network that is deactivated during demanding cognitive tasks (Gusnard and Raichle 2001); the same area is not classically associated with Stroop tasks (Nee et al. 2007); and performance on the Stroop task was not correlated with AQ. However, even if the activation was driven by the Stroop conditions per se, our study still demonstrates that AQ is related to abnormal patterns of BOLD response in a highly proximal region of pSTS to the area that shows structural changes as function of AQ.
Finally, a limitation of the current study is that we are not able to draw conclusions about the relationship between the regional changes in BOLD response we observed and the changes in WM volume. Although WM volume and BOLD response each showed a significant relationship with AQ in highly proximal regions of pSTS, the relationship between WM and BOLD response was not an apriori focus of the current study and did not reach statistical significance. However, a post hoc analysis showed that we did not have sufficient power to directly test this relationship independent of AQ. Future studies should address this issue in order to determine whether the changes in BOLD response are a direct neurophysiological consequence of decreased WM volume.
In conclusion, our study presents evidence that variation in autism spectrum traits in the healthy typical population is correlated with both the structure and function of pSTS, an area implicated in ASD and social cognition more generally. Individuals with higher AQ displayed a greater reduction in pSTS WM volume and greater changes in BOLD response in this area than individuals with lower AQ. These results support the theory that aspects of the autism phenotype extend into the typical population and affect the structure and function of regions implicated in higher order social and emotional processing (Frith CD and Frith U 1999). Our findings also have important implications for research on ASD with reference to the selection of a “control” group, particularly when addressing pSTS function and may explain the variability in neuroimaging studies comparing ASD and control populations. In other words, significant differences in brain function between ASD and control groups could depend on whether the majority of controls fall at the higher or lower end of the typical autism spectrum range. The elusive search for the neurological basis of ASD (Amaral et al. 2008) may therefore be constrained as much by heterogeneity of typical controls as the heterogeneity of ASD. Here, we have shown that at least some aspects of this heterogeneity in the typical population reflect a relationship between the extent of autism spectrum traits and variability in the neuroanatomy of the pSTS. Thus, a correlational approach might provide a complementary methodological approach to the neural basis of autism spectrum traits in addition to the standard group-based comparisons employed to date.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
UK Medical Research Council (U.1055.02.001.00001.01 to A.J.C.); Academy of Finland (#119088 to L.N.).
Acknowledgments
We thank Simon Strangeways for help with figures and the radiographers at the Cognition and Brain Sciences Unit. Conflict of Interest: None declared.
References
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: an essay on autism and theory of mind. Boston: MIT Press/Bradford Books; 1995. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bayliss AP, Tipper SP. Gaze and arrow cueing of attention reveals individual differences along the autism spectrum as a function of target context. Br J Psychol. 2005;96:95–114. doi: 10.1348/000712604X15626. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Technical report C-1. The Center for Research in Psychophysiology, University of Florida; 1999. Affective norms for English words (ANEW): stimuli, instruction manual and affective ratings. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds–a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U. Autism and Asperger's syndrome. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of 'theory of mind'. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinter EJ, Maybery MT, Van Beek PL, Pellicano E, Badcock JC, Badcock DR. Global visual processing and self-rated autistic-like traits. J Autism Dev Disord. 2009;39:1278–1290. doi: 10.1007/s10803-009-0740-5. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Happe F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, Dolan R, Frackowiak R, Frith C. ‘Theory of mind' in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Cath DC, Boomsma DI. Factor structure, reliability and criterion validity of the Autism-Spectrum Quotient (AQ): a study in Dutch population and patient groups. J Autism Dev Disord. 2008;38:1555–1566. doi: 10.1007/s10803-008-0538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103:8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-referential cognition and empathy in autism. PLoS One. 2007;2:e883. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Yip L, Murphy DG, Chua SE. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123(Pt 9):1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Colombo B, Falini A, Ghezzi A, Martinelli V, Scotti G, Comi G, Filippi M. Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol. 2005;4:618–626. doi: 10.1016/S1474-4422(05)70171-X. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks. 2. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Boddaert N, Belin P, Poline JB, Remy P, Mangin JF, Thivard L, Barthelemy C, Samson Y. Temporal lobe dysfunction in childhood autism: a PET study. Positron emission tomography. Am J Psychiatry. 2000;157:1988–1993. doi: 10.1176/appi.ajp.157.12.1988. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends Neurosci. 2006;29:359–366. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.