Abstract
Sex differences in age- and puberty-related maturation of human brain structure have been observed in typically developing age-matched boys and girls. Because girls mature 1–2 years earlier than boys, the present study aimed at assessing sex differences in brain structure by studying 80 adolescent boys and girls matched on sexual maturity, rather than age. We evaluated pubertal influences on medial temporal lobe (MTL), thalamic, caudate, and cortical gray matter volumes utilizing structural magnetic resonance imaging and 2 measures of pubertal status: physical sexual maturity and circulating testosterone. As predicted, significant interactions between sex and the effect of puberty were observed in regions with high sex steroid hormone receptor densities; sex differences in the right hippocampus, bilateral amygdala, and cortical gray matter were greater in more sexually mature adolescents. Within sex, we found larger volumes in MTL structures in more sexually mature boys, whereas smaller volumes were observed in more sexually mature girls. Our results demonstrate puberty-related maturation of the hippocampus, amygdala, and cortical gray matter that is not confounded by age, and is different for girls and boys, which may contribute to differences in social and cognitive development during adolescence, and lasting sexual dimorphisms in the adult brain.
Introduction
The beginning of adolescence is a time of dramatic physical, emotional, and social change (Yurgelun-Todd 2007) and is also associated with the onset of psychopathology such as depression, suicide, and substance abuse (Dahl and Gunnar 2009). The study of typically developing children and adolescents adds a critical component to evolving understandings of developmental disorders, including how risks during adolescence can tip the balance toward pathological outcomes in youth and adulthood. Models of typical brain development would be improved by evaluation of interactions between hormonal and physical sexual maturity and the still-maturing neural systems responsible for social interactions, affective states, and cognitive control.
Previous structural magnetic resonance imaging (sMRI) studies have shown that the volume of structures within the medial temporal lobe (MTL) (specifically, the amygdala and hippocampus) increases (Giedd et al. 1996; Sowell and Jernigan 1998; Yurgelun-Todd et al. 2003) and that gray matter in the dorsal frontal and parietal lobes thins between childhood and adulthood (Giedd et al. 1999; Sowell et al. 2003; Sowell, Thompson, Leonard, et al. 2004; Shaw et al. 2008). These structures are known to play important roles in executive and affective functioning. For example, the amygdala is intimately involved in the interplay between emotion and cognition, and is critical in the modulation of emotional events (for review, see McGaugh 2004) and consolidation of memory (Da Cunha et al. 1999). Interactions between the prefrontal cortex and the amygdala allow attachment of emotional valences to facial expressions (Hariri et al. 2002). Maturation of connections between these areas may underlie the dramatic changes in social and emotional domains in early adolescence (Dahl 2004; Ernst et al. 2005; Steinberg 2005; Steinberg et al. 2006).
The cellular etiology of human brain maturational change is not well understood given the paucity of postmortem material in the childhood to adolescent age range. Recent MRI studies with large samples of children and adolescents studied longitudinally have shown that gray matter maturation has an inverted u-shaped size-by-age trajectory that varies by region in onset, duration, and shape (Lenroot et al. 2007). The rise in gray matter volume that occurs in most regions during childhood has been attributed to a second wave of synaptogenesis and may even be related to late neurogenesis observed in animal models (Giedd et al. 1999). Cortical thinning observed with MRI has been attributed to synaptic pruning and myelination, cellular changes known to continue through adolescence in humans (reviewed in Sowell, Thompson, and Toga 2004). It has been postulated that MTL volumetric increases are related to the rise of gonadal hormones at puberty (Giedd et al. 1996; Sowell and Jernigan 1998) because animal studies have shown high densities of steroid hormone receptors in these regions (for review, see Sarkey et al. 2008) and steroid hormones exert trophic effects on the amygdala and hippocampus (Galea et al. 2006; Zhang et al. 2008).
Much that is known about typical adolescent brain development in humans comes from studies of both male and female participants across wide age ranges, analyzed as a heterogeneous group. Sexual dimorphisms have been found in the mammalian amygdala and, to a lesser extent, the hippocampus. The posterodorsal nucleus of the medial amygdala in male rats has a higher synaptic density (Nishizuka and Arai 1981a, 1981b, 1982) and larger average soma sizes than in females (Mizukami et al. 1983). A large number of androgen receptors are located in the amygdala, and these hormone receptors are thought to play a critical role in the development and maintenance of structural sex differences into adulthood (for review, see Cooke 2006). Functional MRI (fMRI) studies show that the amygdala activates differentially in males and females. For example, one fMRI study found a sex difference in the correlation between circulating testosterone (TES) levels and activity within the amygdala, where activity in response to angry faces was associated with TES levels of adult men but not women (Stanton et al. 2009). Another study found that facial expressions displaying disgust activated the amygdala in adult women but not men (Aleman and Swart 2008). The hippocampal mediation of fear conditioning in rats and humans is sexually dimorphic as well (Gray 1971; Archer 1975; Gupta et al. 2001), driven by the higher estrogen (EST) levels in women, which attenuate these responses by suppressing long-term potentiation in the hippocampus (Gupta et al. 2001). A combination of sex differences in prenatal, early childhood, and peripubertal maturation in adult gonadal steroid hormone levels likely subtends sexual dimorphisms in brain structure and function found in adult brains (for review, see Cooke and Woolley 2005; Cooke 2006).
Sex differences in both the size and the relationship between measures of puberty and MTL maturation have been found in typically developing human adolescents (Neufang et al. 2008; Peper et al. 2009), but interpretation of sex differences in populations of age-matched male and female subjects is complex. Girls, on average, mature sexually 1–2 years earlier than boys (Marshall 1986) and, consequently, the male populations in previous studies (Neufang et al. 2008; Peper et al. 2009) were likely in earlier stages of sexual maturity than their female counterparts. Thus, it is not clear whether age-based matching of boys and girls, who are likely in different stages of puberty, mediated sex differences found in previous studies of puberty-related brain maturation.
To clarify these issues, in the present report we used volumetric measurements of brain structure and 2 measures of puberty in typically developing adolescents who were matched on pubertal status, quantified using Tanner’s stages (TS) from a physical exam (Marshall and Tanner 1968), rather than age, to test whether sex differences in the volumes of sexually dimorphic regions like the MTL, thalamus, and basal ganglia are driven by sex differences in puberty-related maturation. We hypothesized that those differences related to pubertal influences would be more pronounced in later than in earlier stages of puberty. We specifically hypothesized that sex differences in amygdala and hippocampal volume would be more pronounced in later stages of puberty (and in adolescents with higher than with lower circulating TES levels) and that changes in volume of these structures during adolescence would be related to pubertal influences.
To determine the extent to which increasing amygdala and hippocampal volume during adolescence is related to the progression of pubertal status, independent of other factors concomitant with increasing age, we controlled for age effects by first using a sample with little variability in age (boys range 11.7–14.0 years and girls range 10.8–13.5 years) and then by statistically controlling for age using multiple regression analysis. Given that we expected sex differences in the trajectory of gray matter change during adolescence, we examined effects of puberty and age in boys and girls separately as well.
To differentiate global effects of puberty from local effects within the MTL, we measured relationships between pubertal status and global cortical gray matter in each hemisphere and hypothesized that increased pubertal status would be associated with global cortical gray matter decreases and that both hippocampal and amygdala gray matter volume increases would be associated with increased pubertal status, independent of age or whole-brain volume (WBV).
Materials and Methods
Data Collection and Analysis
R.E.D., E.E.F., and colleagues at the University of Pittsburgh collected all data, including measures of sexual maturity, blood samples, and MRI. J.E.B., E.R.S., and colleagues conducted analysis of sMRI data at the University of California, Los Angeles. C.M.W. and colleagues assayed hormone levels in blood samples at Emory University. A subset of the same subjects described in this report have been evaluated to assess relationships between pubertal maturation and brain activation (Forbes et al. 2009; Holm et al. 2009).
Subjects
One hundred thirty healthy adolescents from Pittsburgh, PA, and the surrounding community were recruited through advertisements, flyers, and demographically targeted phone lists. Adolescents were free of lifetime psychiatric disorders, did not have braces, and had no history of head injury, serious medical illness, or psychotropic medication. All participants provided informed consent according to the guidelines of the University of Pittsburgh Institutional Review Board. Three participants withdrew, and 5 participants were excluded because information regarding pubertal status was not obtained and 6 due to inadequate image quality. An additional 8 participants were excluded because they did not have a physical exam, and 28 participants were excluded because we were unable to assay circulating TES levels. We focus on TES in our report of hormonal effects on brain maturation because TES was collected in both boys and girls. Table 1 provides demographic descriptions of the 80 participants with complete data sets.
Table 1.
Demographic characteristics of 80 normally developing adolescents, by gender
| Demographics |
Mean | SD | |
| Sex | Description | ||
| Girls | Age (years) | 11.97 | 0.66 |
| Age range (years) | 10.75–13.48 | — | |
| n | 48 | — | |
| No. pre/early puberty | 19 | — | |
| Mean TS | 3.17 | 1.17 | |
| Mean [TES] | 0.29 | 0.11 | |
| Handedness (no. right) | 45 | — | |
| Boys | Age (years) | 12.88 | 0.67 |
| Age range (years) | 11.73–13.98 | — | |
| n | 32 | — | |
| No. pre/early puberty | 9 | — | |
| Mean TS | 2.91 | 0.93 | |
| Mean [TES] | 1.64 | 1.48 | |
| Handedness (no. right) | 30 | — | |
Note: Experiment 1—demographics from 80 participants included in tests of the effects of sexual maturity, using TS and circulating TES on brain structure. Demographics are tabulated for girls and boys as data from each gender were analyzed separately in some statistical tests. SD, standard deviation.
Sexual Maturity
Adolescents underwent a physical examination by a research-trained nurse practitioner to determine stage of sexual maturation with the criteria specified by Marshall and Tanner (1968). While the literature states that the criteria for TS are reliable (Slora et al. 2009), we were concerned about the resolution of TS to clearly differentiate 5 stages. Thus, we divided our participants into groups based on their TS. Consistent with our earlier studies (e.g., Forbes et al. 2004), adolescents with a TS of 1 or 2 were classified as being in a pre/early stage of sexual maturity and those with a TS of 3 or higher were classified as being in a mid/late stage of sexual maturity. Group descriptions are detailed in Table 1.
Circulating TES Levels
Blood samples were collected and analyzed for TES level in both boys and girls. Samples were obtained at the same time for all subjects (between 8:20 and 8:35 AM), using minimally invasive finger-stick procedure developed by Worthman and Stallings (1997). This method provides several advantages over salivary assays for gonadal steroids, and the correspondence of bloodspot-derived level and plasma level is high. Hormone assays were a modification of a commercially available serum/plasma radioimmunoassay kit (TES: DSL/Beckman Coulter). Sensitivity measured as the minimum detectable dose (MDD) and inter-assay coefficients of variation (CV) for low, medium, and high BioRad external controls for TES were MDD = .04 ng/mL; CV = 7.2% (low), 11.4% (medium), and 4.3%.
Structural Image Acquisition
All subjects were scanned in a Siemens Allegra head-only 3-Tesla magnet with a 3D T1-weighted protocol (Siemens). Scan parameters were as follows: repetition time, 1540 ms; echo time, 3.04 ms, flip angle, 8°; field of view, 256 × 256; image voxel size, 1 × 1 × 1 mm; acquisition time, 4.48 m.
Image Processing
Preprocessing and definition of cortical and subcortical gray matter regions on structural images were conducted in the UCLA Laboratory of Neuro Imaging Pipeline Processing Environment (Rex et al. 2003, 2004; Dinov et al. 2009) and using FreeSurfer’s automated segmentation software (FreeSurfer 4.0.3, http://surfer.nmr.mgh.harvard.edu), as described in the works of Fischl and Dale (Dale et al. 1999; Fischl et al. 1999, 2002). During preprocessing, magnetization prepared rapid gradient echo acquisitions for each participant were motion corrected and brain extracted, and gray/white matter boundaries were automatically delineated. A surface of connected white matter voxels was refined to create submillimeter voxel resolution in the gray/white matter boundary (Dale et al. 1999; Fischl et al. 1999). The gray/white matter boundary was then deformed outward to estimate the pial surface with the following constraints; the surface needed to be smooth and maintain the natural topology of the brain.
Procedures for the automatic quantification of gray matter volumes for a variety of brain structures are detailed by Fischl et al. (2002) and include the hypothesis-driven regions investigated here (amygdala, hippocampus, and total cortical volume). We also studied the thalamus and caudate because sex differences in these structures have been observed during adolescence (Neufang et al. 2008; Peper et al. 2009). Briefly, a neuroanatomical label was assigned to each voxel in an individual’s sMRI based on probabilistic information estimated from a manually labeled training set. This manually labeled training set is a result of validated methods from the Center of Morphometric Analysis (http://www.cma.mgh.harvard.edu). To disambiguate the overlap in intensities between different anatomical structures, FreeSurfer utilized spatial information. Two transformations were performed. First, an optimal linear transformation was carried out by maximizing the likelihood of the native image given a manually labeled atlas. Second, a nonlinear transformation was executed on the output of the prior registration step. Finally, a Bayesian parcellation was conducted by using prior spatial information (Fischl et al. 2004; Desikan et al. 2006). At the end of this processing stream, 3 probabilities were calculated for each voxel: 1) the probability of the voxel belonging to each of the label classes, based on its location; 2) the neighborhood function, used to determine the likelihood that the voxel belongs to a class, based on the classification of neighboring voxels; and 3) the result of the probability distribution function for each voxel based on its intensity.
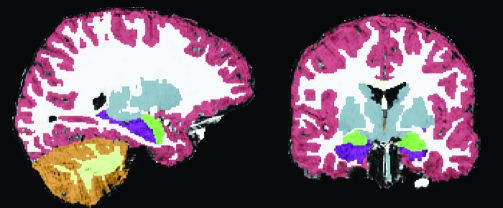
An example of a segmented brain from one of the subjects studied here is shown in Figure 1. As with manual methods, the validity of regional delineations by FreeSurfer varies by region, with some regions, like the amygdala and hippocampus, being less valid than others (Fischl et al. 2002). However, being completely automated, FreeSurfer volume estimates are highly reliable, and comparison of manual and automated hippocampal and amygdala volume estimates revealed no discernible statistical difference (Fischl et al. 2002). Nonetheless, in the current study, each brain image was visually inspected for validity of all regions, and hippocampal and amygdala delineations were edited, when necessary, by a highly experienced neuroanatomy expert (J.E.B.). The 2 structures were differentiated using a band of cerebrospinal fluid, which divides the 2 when visible, and when they directly abutted one another, using the white matter tract on the superior aspect of the hippocampus as landmark. Intra-rater variability and reliability were assessed for edited delineations of the 4 subregions (left and right hippocampus and amygdala) in 10 subjects edited twice. We used the nonparametric sign test to assess the paired differences between the delineations at 2 time points across subjects and regions of interests using the Statistics Online Computational Resource (SOCR; www.socr.ucla.edu) (Dinov 2006; Che et al. 2009). The sign test statistics (P = 0.286) provide statistical evidence that the results of the 2 delineations were statistically indistinguishable. We also employed the intraclass correlation coefficient (ICC) (Koch 1982) using the IRR package (McGraw and Wong 1996) of the R Statistical Computing environment (Everitt 2005) to assess the concordance/consistency of the delineations. The 95% confidence interval estimated for ICC was as follows 0.993 < ICC < 0.998, showing that volumes resultant from the 2 sessions are nearly perfectly correlated.
Figure 1.
Example segmented brain. On the left is a sagittal view and on the right is a coronal view demonstrating tissue segmentation of a single participant’s brain. Segmentation includes cortical gray (red) and white matter (white) segmentation, and subcortical structures, the majority of which are colored in gray. Subcortical regions of interest are highlighted in bright colors (the hippocampus colored in purple and the amygdala in green).
Statistical Analyses
We used a one-tailed 2-independent samples t-test to test whether boys and girls in later stages of puberty were significantly older than those in earlier stages and whether participating boys had larger WBVs than participating girls.
Sex differences in regional volume (left and right amygdala, hippocampus, caudate, thalamus, and cortical gray matter) were evaluated using a 2-tailed 2–independent samples t-test. The ability of sex to predict the volumes of the above regions independent of age was investigated using simultaneous multiple regression analysis using the following equation: volume = constant + sex + age.
The ability of sex to predict the volumes of regions significant in the 2 prior analyses, independent of WBV, was investigated using simultaneous multiple regression analysis with the following equation: volume = constant + sex + age + WBV.
To test the hypothesis that sex differences in regional volume would be greater in individuals in later stages of puberty (independent of age), we conducted simultaneous multiple regression analyses to test for interactions between sex and pubertal status (using either TS, which was binarized based on definitions of pre/early and mid/late puberty described above [Forbes et al. 2004], or circulating TES) in predicting regional volumes using the following equations: volume = constant + TS + sex + TS × sex and volume = constant + TES + sex + TES × sex.
We assessed the relationship between pubertal status and regional volume using the following statistical methods, which were common to analyses using both measures of pubertal status (TS and circulating TES levels). Males and females were analyzed separately because we did not want to confound pubertal effects with sex by puberty interactions on brain structure (Dewing et al. 2003; Arnold 2004; Lenroot et al. 2007). To ensure that increases in volume measures associated with pubertal status were not attributable to increased overall brain size, we used multiple regression analysis, adjusting for WBV.
The effects of physical sexual maturation (difference between pre/early and mid/late puberty groups) on regional volume were evaluated using the Wilcoxon–Mann–Whitney 2–independent samples nonparametric rank-sum test (Che et al. 2009), thresholded at a nonparametric 0.95 confidence interval. This nonparametric test allowed us to quantify the differences between the underlying distributions of the pre/early and mid/late groups, without making assumptions about the distributions of the data (e.g., normality was not required). While boys sampled had a significantly smaller proportion of pre/early : mid/late puberty participants than girls sampled (P = 0.038, calculated using the SOCR Fisher’s exact test applet [http://socr.ucla.edu/htmls/SOCR_Analyses.html]; Che et al. 2009), this sampling difference did not affect either estimates of population means or variances used in the nonparametric statistical tests applied here (Hellmann and Fowler 1999).
Plasma concentration of TES was a continuous, not a binary, variable. The effects of circulating hormones on the same regions assessed in the above experiment within each sex were evaluated using Pearson’s correlation analysis with a false-positive rate α = 0.05.
We also conducted simultaneous multiple regression analyses to predict regional gray matter volumes using either sexual maturity group and age or circulating TES concentration and age using the following equations: volume = constant + TS + age and volume = constant + TES + age. This allowed us to assess the relative contributions of puberty and age-related factors to changes in brain structure.
In regions found to be significantly positively associated with TS or TES, independent of age, WBV was used to normalize regional volume (volume/WBV). A 2–independent samples t-test was performed on each normalized region found to be significant in both of the previous analyses.
In these experiments, we conduct a relatively small number of hypothesis-driven statistical tests to keep experiment-wise error low, and thus, results presented were not corrected for multiple comparisons. Conditions of collinearity were met for all multiple regression analyses described above using the following criteria: the correlations between any 2 simultaneous predictors must be less than 0.800 [CORR(x,y) < 0.800) (O'brien 2007).
Results
Relationships between Sex, Age, Sexual Maturity, and Circulating TES Levels
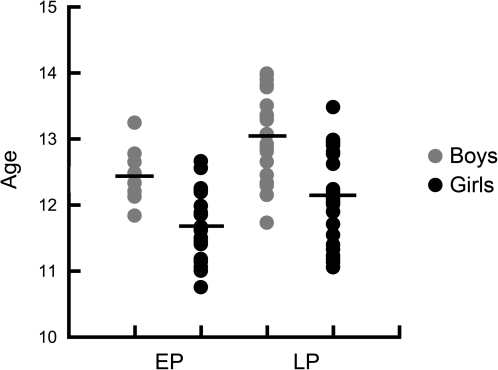
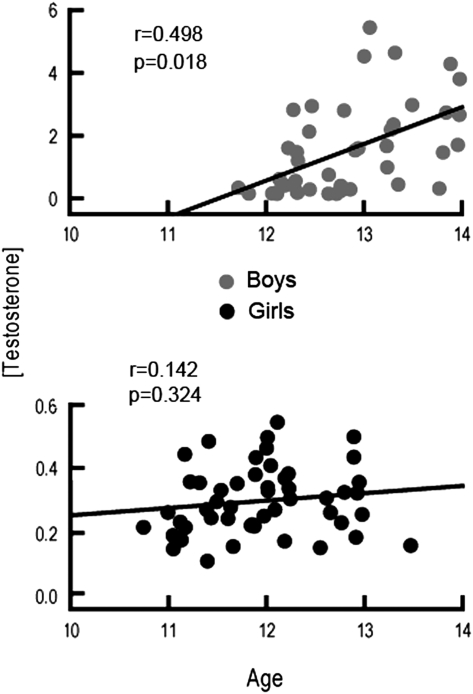
Even within the restricted age range studied, older boys and girls tended to be in later pubertal stages, assessed with TS, than younger boys (P = 0.004) and girls (P = 0.007) (see Table 1). Similarly, TES level was positively correlated with age in boys, suggesting that older boys (P = 0.004) had higher TES levels than younger boys. TES was not significantly associated with age in girls. Table 2 shows the correlations between age and both TS and TES, as well as the correlation between TS and TES. Figure 2 shows sexual maturity group membership for participating boys and girls as a function of age. Figure 3 shows TES levels for each individual as well as the distribution of TES measures across the age range for both boys and girls.
Table 2.
Correlations between TES, TS, age, and WBV
| [TES] |
TS |
|||
| Boys | Girls | Boys | Girls | |
| TS | 0.437* | 0.553* | — | — |
| Age | 0.486* | 0.143 | 0.439* | 0.336* |
| WBV | 0.058 | −0.228 | −0.060 | −0.241 |
Note: TS was measured using Tanner breast/genital exam and was converted to a 2-level variable: pre/early puberty (TS = 1–2) and mid/late puberty (TS = 3–5). Correlation measured using Pearson’s r.
Significance threshold was P < 0.05.
Figure 2.
Physical sexual maturity as a function of age in boys and girls. Scatter plot showing the relationship betweens between sexual maturity (x-axis) and age (y-axis) in boys and girls. While the y-axis is in years, age is actually plotted in months, to best demonstrate the age of individual adolescents as well as the narrowness of the age range studied. Sexual maturity was assessed using TS based on physical exam. Pre/early puberty (EP) represented TS 1 or 2, while mid/late puberty (LP) represented TS 3–5. The mean for each sex/sexual maturity group is represented using a horizontal bar through the corresponding scatter plot. While there was a correlation between sexual maturity and age for both boys and girls, there was no significant interaction between sex, age, and sexual maturity. Therefore, age was not a confounding factor when testing sex by sexual maturity interactions on brain volumes in this sample.
Figure 3.
Circulating TES levels as a function of age. Scatter plots showing the relationships between circulating TES (y-axis) and age (x-axis) in boys and girls. As with Figure 2, age is plotted in months. Circulating TES level was an order of magnitude greater in boys than in girls (reflected in the range on the y-axis). The correlation coefficient (r) is also displayed. TES was significantly correlated with age in boys, but not in girls.
Sex Differences
Sex Differences in Regional Volume
On average, boys had larger brains than girls (P < 0.0001). As predicted, there was a significant main effect of sex such that girls had smaller volumes than boys in all regions assessed (P < 0.001), with the exception of the caudate, which was not significantly different between the 2 sexes. However, no significant effects of sex on regional volume could be dissociated from both age and WBV differences between groups.
Sex Differences in the Effects of Puberty (TS and Circulating TES) on Gray Matter Volumes
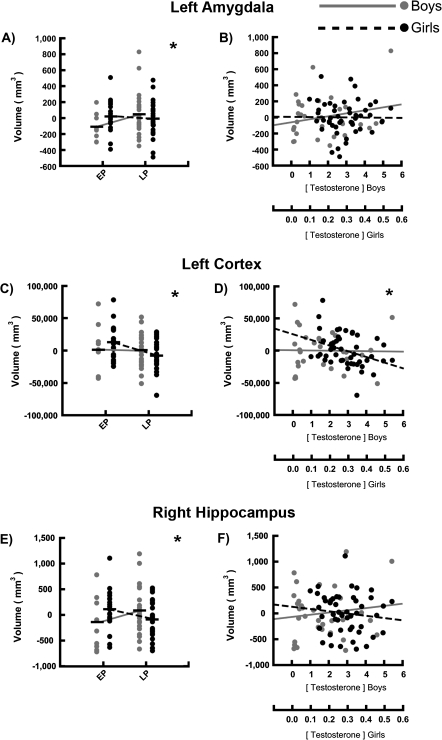
To assess whether sex differences were driven by pubertal status, we conducted simultaneous multiple regression analyses to test for sex by pubertal status interactions in predicting regional volumes. As predicted, we found a significant interaction between sex and the ability of physical sexual maturity to predict the volumes of the right hippocampus (β = −0.540, P = 0.013) and bilateral amygdala (right: β = −0.572, P = 0.010; left: β = −0.470, P = 0.035) (see Table 3 and Fig. 4). In each of these regions, the effect of sex was larger in mid/late puberty (TS = 3, 4, or 5) than in pre/early puberty (TS = 1 or 2). In these regions, smaller volumes were observed in girls at later than earlier pubertal stages, with trends for boys to increase in volume with advancing pubertal status (see Table 4). We were unable to detect any interactions between sex and the effects of pubertal status on the thalamus or caudate using either TS or TES as predictors. In the bilateral thalamus, boys had larger volumes than girls in both pre/early and mid/late puberty; this effect was not different in pre/early than mid/late puberty.
Table 3.
Interactions between sex and the effect of pubertal status on developing regional volume
| Region | Sex |
Pubertal status |
Interaction |
|||
| β | P | β | P | β | P | |
| Experiment 1: pubertal status determined by TS | ||||||
| R hippocampus | −0.033 | 0.855 | 0.338 | 0.049* | −0.540 | 0.013*,† |
| L hippocampus | −0.236 | 0.199 | 0.177 | 0.308 | −0.277 | 0.206 |
| R amygdala | 0.049 | 0.791 | 0.261 | 0.134 | −0.572 | 0.010*,† |
| L amygdala | −0.030 | 0.869 | 0.347 | 0.049*,† | −0.470 | 0.035*,† |
| R thalamus | −0.419 | 0.022* | −0.026 | 0.880 | −0.084 | 0.694 |
| L thalamus | −0.407 | 0.027* | −0.045 | 0.791 | −0.080 | 0.713 |
| R caudate | 0.179 | 0.372 | 0.267 | 0.162 | −0.376 | 0.118 |
| L caudate | 0.241 | 0.230 | 0.212 | 0.264 | −0.407 | 0.091 |
| R cortex | −0.260 | 0.134 | 0.003 | 0.984 | −0.340 | 0.102 |
| L cortex | −0.263 | 0.127 | −0.022 | 0.891 | −0.336 | 0.105 |
| Experiment 2: pubertal status determined by TES | ||||||
| R hippocampus | −0.142 | 0.537 | 0.172 | 0.180 | −0.187 | 0.378 |
| L hippocampus | −0.295 | 0.202 | −0.016 | 0.899 | −0.163 | 0.443 |
| R amygdala | 0.057 | 0.809 | 0.068 | 0.606 | −0.404 | 0.066 |
| L amygdala | −0.164 | 0.483 | 0.206 | 0.117 | −0.088 | 0.683 |
| R thalamus | −0.164 | 0.454 | 0.243 | 0.049* | −0.186 | 0.358 |
| L thalamus | −0.220 | 0.326 | 0.193 | 0.125 | −0.136 | 0.511 |
| R caudate | 0.220 | 0.379 | 0.200 | 0.153 | −0.215 | 0.351 |
| L caudate | 0.286 | 0.255 | 0.179 | 0.202 | −0.249 | 0.284 |
| R cortex | −0.061 | 0.781 | −0.020 | 0.873 | −0.479 | 0.020*,† |
| L cortex | −0.041 | 0.853 | −0.022 | 0.859 | −0.502 | 0.015*,† |
Note: Statistical results, including estimates of main and interaction effect sizes from multiple regression analysis. Sex: ability of sex to predict volumes of regions of interest. Pubertal status: ability of pubertal status to predict volumes of regions of interest. Results from 2 measures of pubertal status are tabulated. Experiment 1: sexual maturity, measured with TS, using Tanner breast/genital exam and converted to a 2-level variable—pre/early puberty (TS = 1–2) and mid/late puberty (TS = 3–5). Experiment 2: circulating TES, with respect to regional volumes. Interaction: Interaction statistic demonstrating the ability of sex to predict the effect of pubertal status on regional volume. β, standardized regression coefficients; L, left; R, right.
Significance threshold was P < 0.05.
†Significance threshold was P < 0.05 after correcting for age effects. Important values bolded to improve visability.
Figure 4.
Interactions between sex and the effect of pubertal status on development of select regions of interest. Sex differences in the effects of puberty on regional volume were assessed and scatter plots demonstrate sex by pubertal status (x axis) on gray matter volume (y axis) interactions in the left amygdala, left cortex, and right hippocampus. Two measures of pubertal status are plotted—(left) sexual maturity, measured with TS using TS (Tanner breast/genital exam) and converted to a 2-level variable: pre/early puberty (TS = 1–2) (EP) and mid/late puberty (TS = 3–5) (LP); and (right) circulating TES, with respect to regional volumes. The mean is represented using a horizontal black bar through the corresponding scatter plot for binarized measures of TS for each group (A, C, and E). A line connecting mean regional volumes of adolescents in EP and LP is drawn separately in boys and girls to demonstrate the interactions found. Circulating TES was plotted against regional volume in boys and girls (B, D, and F). Significant interactions between sex and the effect of sexual maturity on regional volume were found (A, C, and E). Significant interactions between sex and the effect of circulating TES on regional volume were found (D). No significant interactions between sex and the effect of sexual maturity on regional volume were found in B or F. *Significance threshold was P < 0.05 for sex by pubertal status effect on volume interaction.
Table 4.
Regional volumes predicted by 2 measures of pubertal status
| Region | Experiment 1 |
Experiment 2 |
||||||
| Pre/early puberty |
Mid/late puberty |
Size difference (grouped by Tanner’s scale) |
Size prediction by TES |
|||||
| Mean volume (mm3) | SD | Mean volume (mm3) | SD | z | P | r | P | |
| Girls | ||||||||
| R hippocampus | 4535 | 398 | 4341 | 362 | −1.57 | 0.058 | −0.126 | 0.394 |
| L hippocampus | 4205 | 279 | 4126 | 388 | −0.66 | 0.253 | −0.116 | 0.431 |
| R amygdala | 1952 | 239 | 1785 | 196 | −2.51 | 0.006 | −0.289 | 0.046 |
| L amygdala | 1683 | 196 | 1622 | 217 | −1.09 | 0.139 | −0.043 | 0.770 |
| R thalamus | 7246 | 504 | 7083 | 497 | −0.85 | 0.393 | −0.191 | 0.198 |
| L thalamus | 7169 | 475 | 6985 | 533 | −1.18 | 0.238 | −0.136 | 0.360 |
| R caudate | 4152 | 522 | 4032 | 459 | −1.15 | 0.251 | −0.144 | 0.334 |
| L caudate | 4080 | 424 | 3871 | 478 | −1.71 | 0.088 | −0.171 | 0.252 |
| R cortex | 33 1024 | 27 402 | 30 9516 | 22 834 | −2.60 | 0.005 | −0.355 | 0.014* |
| L cortex | 33 4051 | 26 388 | 31 1022 | 22 092 | −2.88 | 0.002 | −0.380 | 0.008* |
| Boys | ||||||||
| R hippocampus | 4566 | 506 | 4896 | 467 | 1.61 | 0.053 | 0.212 | 0.245 |
| L hippocampus | 4389 | 391 | 4530 | 338 | 0.96 | 0.168 | −0.023 | 0.901 |
| R amygdala | 1926 | 250 | 2069 | 283 | 1.55 | 0.060 | 0.082 | 0.654 |
| L amygdala | 1698 | 170 | 1874 | 270 | 1.82 | 0.034* | 0.253 | 0.162 |
| R thalamus | 7854 | 901 | 7816 | 778 | −0.02 | 0.983 | 0.273 | 0.138 |
| L thalamus | 7752 | 884 | 7685 | 763 | −0.06 | 0.950 | 0.222 | 0.230 |
| R caudate | 3956 | 634 | 4256 | 608 | −1.07 | 0.285 | 0.252 | 0.171 |
| L caudate | 3824 | 657 | 4055 | 586 | −0.69 | 0.489 | 0.214 | 0.248 |
| R cortex | 34 7306 | 34 509 | 34 7521 | 26 762 | 0.13 | 0.448 | −0.027 | 0.884 |
| L cortex | 35 0627 | 39 467 | 34 9184 | 25 847 | −0.09 | 0.465 | −0.030 | 0.875 |
Note: Experiment 1—volume differences between pre/early and mid/late pubertal boys and girls using a nonparametric 2-samples test for mean differences (column 1). Groups were divided by physical sexual maturity: pre/early puberty = TS 1–2, mid/late puberty = TS 3–5. Statistics calculated using a one-tailed 2–independent samples Wilcoxon rank-sum test. Regional volumes for each group are tabulated. Experiment 2: volume predicted by circulating TES, using Pearson’s correlation [CORR(volume, TES)] in pubertal boys and girls. Data are tabulated separately for girls and boys. Note that effects of both sexual maturity (TS) and circulating TES on the right hippocampus and amygdala in girls were in the unpredicted direction and therefore not statistically significant in this one-tailed analysis. L, left; R, right; SD, standard deviation; r, Pearson’s correlation coefficient.
Significance threshold was P < 0.05, using a 2-tailed test for the volumes of the thalamus and caudate and a one-tailed test for MTL and cortical volumes: MTL volume increases and cortical volume decreases were predicted. Important values bolded to improve visability.
We also found a significant interaction between sex and how well circulating TES predicted the volume of cortical gray matter bilaterally (right: β = −0.479, P = 0.020; left: β = −0.502, P = 0.015) (see Table 3), where the sex differences in volume are greater among those at higher TES levels than those at lower TES levels. In these regions, volume reductions were observed in girls at earlier than at later pubertal stages, whereas no differences were observed in the boys. Results of these statistical analyses are summarized in Table 3.
Results for males and females (mean, standard deviation, and statistical test of difference in means) from all a priori zregional volumes in each physical sexual maturity group (pre/early and mid/late based on TS) are shown in Table 4. Correlation results showing the predictability of regional volume by circulating TES are also included in Table 4. Effect sizes (standardized coefficients) and statistical tests of significance (P value) from multiple regression analyses using each measure of pubertal status (TS and circulating TES) and age as predictors of interest are shown in Table 5.
Table 5.
Volumetric results of simultaneous multiple regression analysis using measures of pubertal status and age to predict cortical volumes
| Region | Experiment 1, volume = TS + age |
Experiment 2, volume = TES + age |
||||||
| Size prediction by TS |
Size prediction by age |
Size prediction by [TES] |
Size prediction by age |
|||||
| β | P | β | P | β | P | β | P | |
| Girls | ||||||||
| R hippocampus | −0.270 | 0.087 | 0.058 | 0.709 | −0.123 | 0.416 | −0.015 | 0.921 |
| L hippocampus | −0.170 | 0.281 | 0.168 | 0.287 | −0.140 | 0.353 | 0.133 | 0.376 |
| R amygdala | −0.363 | 0.018† | −0.002 | 0.990 | −0.275 | 0.064 | −0.080 | 0.581 |
| L amygdala | −0.090 | 0.568 | −0.152 | 0.335 | −0.011 | −0.942 | −0.182 | 0.229 |
| R thalamus | −0.228 | 0.147 | 0.193 | 0.219 | −0.157 | 0.307 | 0.080 | 0.603 |
| L thalamus | −0.235 | 0.136 | 0.167 | 0.285 | −0.111 | 0.474 | 0.064 | 0.676 |
| R caudate | –0.080 | 0.613 | −0.121 | 0.444 | −0.077 | 0.608 | −0.224 | 0.139 |
| L caudate | −0.195 | 0.215 | −0.079 | 0.612 | −0.108 | 0.470 | −0.205 | 0.174 |
| R cortex | −0.379 | 0.013† | −0.062 | 0.676 | −0.330 | 0.027† | −0.107 | 0.460 |
| L cortex | −0.415 | 0.006† | −0.061 | 0.674 | −0.356 | 0.016† | −0.105 | 0.464 |
| Boys | ||||||||
| R hippocampus | 0.284 | 0.154 | 0.052 | 0.792 | 0.170 | 0.423 | 0.085 | 0.687 |
| L hippocampus | 0.213 | 0.295 | −0.073 | 0.717 | −0.040 | −0.852 | 0.035 | 0.872 |
| R amygdala | 0.299 | 0.138 | −0.157 | 0.430 | 0.131 | 0.541 | −0.098 | 0.647 |
| L amygdala | 0.340 | 0.089 | −0.061 | 0.754 | 0.284 | 0.180 | −0.062 | 0.768 |
| R thalamus | −0.049 | 0.811 | 0.066 | 0.749 | 0.358 | 0.102 | −0.139 | 0.517 |
| L thalamus | −0.100 | 0.623 | 0.149 | 0.467 | 0.268 | 0.226 | −0.078 | 0.721 |
| R caudate | 0.221 | 0.275 | −0.001 | 0.996 | 0.261 | 0.238 | −0.065 | 0.767 |
| L caudate | 0.166 | 0.415 | 0.019 | 0.923 | 0.274 | 0.217 | −0.134 | 0.541 |
| R cortex | 0.009 | 0.967 | −0.012 | 0.955 | −0.008 | 0.973 | −0.036 | 0.874 |
| L cortex | −0.015 | 0.943 | −0.016 | 0.939 | 0.012 | 0.958 | −0.076 | 0.738 |
Note: Statistical results and estimates of effect sizes from multiple regression analysis. Experiment 1: ability of physical sexual maturity (pre/early and mid/late adolescence, assessed using Tanner breast/genital score) to predict volumes of regions of interest with age regressed, and ability of age to predict these volumes with Tanner breast/genital score regressed. Experiment 2: as experiment 1 but analysis using [TES], rather than Tanner breast/genital score. Data are tabulated separately for girls and boys. L, left; R, right; SD, standard deviation; β, standardized regression coefficients.
Significance threshold was P < 0.05 after correcting for age effects and using a 2-tailed test statistic. Important values bolded to improve visability.
There were sex differences in age (P < 0.0001) and WBV (P < 0.0001) that did not change with advancing puberty (i.e., the difference between boys and girls at early puberty was the same as differences between boys and girls in late puberty). However, to be certain that neither age nor brain volume differences affected our interaction statistic, we reran our analyses using both age and brain volume as predictors. We found that all interactions significant in the prior analysis remained significant when WBV was included in the regression equation.
Boys
Regional Gray Matter Volumes and Physical Sexual Maturity in Boys
As predicted, male participants in mid/late puberty, assessed using TS, had larger volumes in MTL structures than those in earlier stages (see Fig. 4 and Table 4). Specifically, boys in mid/late puberty had significantly larger amygdala (z = 1.82, P = 0.034) and a trend toward larger right amygdala (z = 1.55, P = 0.060) and right hippocampal (z = 1.61, P = 0.053) volumes than boys in pre/early puberty. Sexual maturity was not predictive of total hemispheric cortical gray matter in boys, suggesting some regional specificity to the impact of sexual maturity on MTL brain structures.
Because boys in earlier stages of puberty, as measured using TS, are younger than boys in later stages of puberty (P < 0.01), even over this narrow age range, and because previous studies have found significant correlations between age and MTL volume increases during adolescence (Giedd et al. 1996; Sowell and Jernigan 1998; Yurgelun-Todd et al. 2003) multiple regression analyses were conducted using both physical sexual maturity and age as predictors. We were unable to dissociate effects of sexual maturity on amygdala volume from concomitant age effects in boys.
Regional Gray Matter Volumes and Circulating TES in Boys
We were unable to detect effects of circulating TES on regional volumes when male participants were studied as a separate population.
Girls
Regional Gray Matter Volumes and Physical Sexual Maturity in Girls
As predicted, female participants in mid/late puberty, assessed using TS, had significantly smaller right and left cortical gray matter volumes than girls in earlier stages of puberty (z = −2.60, P = 0.005; z = −2.88, P = 0.002, respectively). Advanced sexual maturity also had an unpredicted association with volumes in MTL structures. Specifically, girls in mid/late puberty had a smaller right amygdala (z = 2.51, P = 0.006) than girls in pre/early puberty (see Fig. 4 and Table 4), though not significantly so, as these were results from a one-tailed test and in the unpredicted direction.
Girls in earlier stages of puberty, as measured using TS, are younger than girls in later stages of puberty (P = 0.003), even over this narrow age range. Therefore, multiple regression analyses were conducted using both physical sexual maturity and age as predictors. Advanced physical sexual maturity significantly predicted declining volume bilaterally in the amygdala (right: β = −0.363, P = 0.018; left: β = −0.090, P = 0.568) and bilaterally in the cortex (right: β = −0.379, P = 0.013; left: β = −0.415, P = 0.006), independent from and better than age. Age was not a significant predictor of regional or cortical gray matter volume for any region in the multiple regression analyses (see Table 5).
Regional Gray Matter Volumes and Circulating TES in Girls
Consistent with our findings using TS, we found an inverse correlation between circulating TES and volume of the right amygdala (r = −0.289, P = 0.046) (see Table 4).
Consistent with both our hypotheses and our findings using measures of physical sexual maturity, female participants with higher circulating TES levels had smaller bilateral cortical gray matter volumes (right: r = −0.355, P = 0.014; left: r = −0.380, P = 0.008) than female participants with lower circulating TES levels.
When we performed multiple regression analysis, including circulating TES and age as predictors, increased circulating TES significantly predicted loss of cortical gray matter in both hemispheres (right: β = −0.330, P = 0.027; left: β = −0.356, P = 0.016) (see Table 5), but neither circulating TES nor age contributed significant unique variance to MTL volume. As with physical sexual maturity, circulating TES typically predicted regional volume qualitatively better than age; puberty had a larger estimated beta than age in most tests.
Discussion
As hypothesized, we found interactions between sex and the effect of pubertal status on the development of the hippocampus, amygdala, and cortical gray matter. In these structures, we show that the effects of sex are not significant in pre/early puberty but are significant in mid/late pubertal children. Significant interactions of sex with physical sexual maturity for the right hippocampus were driven by nonsignificant volume increases with increased sexual maturity in boys, and significant volume decreases with increased sexual maturity in girls, independent of age. Significant sex by puberty interactions in the cerebral cortex were driven by volume reductions in girls at more advanced pubertal stages, but no effect of sexual maturity on cortical volumes in boys was seen. All these effects were significant even when age was used as an additional predictor, suggesting that sexual dimorphisms in MTL and cortical gray matter are driven more by pubertal influences, and less so by other factors co-occurring with increased age during adolescence.
In boys, sexual maturity, measured using TS, predicted volume increases in the MTL, significantly so in the left amygdala. However, we were unable to dissociate these from concomitant age effects in our population.
In girls, larger TS measurements were associated with a more mature pattern of cortical gray matter. Girls in earlier stages of puberty had larger gray matter volumes in the right and left cortex than girls of the same age in later stages of puberty. However, effects of sexual maturity on MTL volume were in an unpredicted direction. MTL volume was smaller in more than in less sexually mature girls. When we used multiple regression analysis, modeling effects of both advancing pubertal status and age, we found that girls in earlier stages of puberty had significantly larger gray matter volumes in the right amygdala than girls in later stages, independent of age. Consistent with our findings using TS, adolescent girls with higher levels of TES had smaller bilateral cortical gray matter than adolescent girls of the same age with lower levels. Furthermore, circulating TES predicted right MTL volume, but as with TS results, it was in an unpredicted direction. Girls with higher levels had smaller right amygdala and hippocampal volumes than girls with lower levels of circulating TES, though these effects did not remain significant when age was used as an additional predictor.
Mean volume increases in MTL volume in boys but statistically significant decreases in girls with the progression of puberty may be related to sex differences in the number of new cells added to the amygdala during puberty. In rats, it has been found that more new cells are added to the amygdala in males than in females during puberty and that reducing endogenous levels of pubertal hormones via gonadectomy reduces the number of new cells created (Ahmed et al. 2008). Another potential cellular mechanism of the observed sex differences is that boys and girls differ in the soma sizes of neurons with gonadal steroid hormone receptors within the amygdala. This sexual dimorphism has been observed in the amygdala of rodents and has also been found to depend on TES levels (Romeo and Sisk 2001).
We found sex differences in the right and left thalamus, similar to previous reports (Neufang et al. 2008; Peper et al. 2009). However, we found no evidence that pubertal influences are significantly associated with sex differences in this region. We did find that circulating TES significantly predicted thalamic volume when boys and girls were pooled. However, TES did not significantly predict thalamic volume in either boys or girls alone or independent of concomitant age effects. Previous human brain imaging studies have also been unable to link sex differences in basal ganglia or thalamic volumes (Neufang et al. 2008; Peper et al. 2009) to pubertal influences (Neufang et al. 2008; Peper et al. 2009), further supporting the hypothesis that any true sexual dimorphisms in these structures are likely unrelated to puberty-driven brain maturation.
The functional relevance of these structural findings in understanding puberty-related changes in adolescent boys and girls could be numerous. Interactions between the amygdala, critical for processing emotional information, and thinning cortical regions, important for controlling impulses and evaluation of risk (i.e., the frontal lobes), should be considered in interpretation of sex differences in puberty-related brain development (for review, see McGaugh 2004). Thinner cortex in frontal and parietal cortices has been linked to more mature brain activation patterns in children and adolescents (Lu et al. 2007). If thinner cortex is associated with improved executive control over the emotional processing centers of the amygdala, then one might expect differences in emotional processing between boys and girls, depending on pubertal status and the state of brain regions involved. Indeed, the incidence of depression and anxiety symptoms are much higher in girls than in boys starting in late childhood (Bailey et al. 2007; Van Oort et al. 2009), and perhaps earlier development of frontal lobes and frontolimbic connections in the right than in left hemisphere explains why clinically relevant anxiety and depression occurs in girls during adolescence. Higher left-sided and lower right-sided connectivity between the amygdala and frontal lobes, more right-lateralized frontal lobe activity, and lower rates of left-sided prefrontal cortical activity measured using fMRI and electroencephalography have all been associated with anxiety and depression in adults (Davidson et al. 1999; Davidson and Irwin 1999). Previous studies have shown that right frontal cortical thinning occurs prior to left frontal cortical thinning in children 5–11 years of age (Sowell et al. 2001). Perhaps there is a greater lag between right and left hemisphere frontolimbic system maturation in girls than in boys, a hypothesis that warrants further study.
While risk and reward processing and decision-making skills develop in both boys and girls during adolescence, boys tend to be less risk averse than girls during adolescence (Van Leijenhorst et al. 2008). Our findings of trophic changes in medial temporal limbic regions without the presumed increased efficiency and cognitive control that accompany cortical thinning in the frontal lobes may explain why boys are more likely to take risks than girls, whose cortical development occurs so much earlier. Conversely, the relatively early cortical maturation in girls, while possibly related to increased anxiety and depression symptoms, may also reduce their propensity to take unnecessary risks.
Our findings both agree and differ from previous reports, and may help shed light on discrepancies in the literature regarding MTL maturation. We show that MTL volumes rise in boys, and decline in girls, when evaluated as a function of sexual maturity. Both may be due to pubertal hormones causing an increase in MTL size when adolescents are young and a decrease in size when adolescents are older. Perhaps when girls are younger than age 10, gonadal steroid hormones initiate cellular mechanisms, like cellular proliferation or synaptogenesis, which could cause significant volume increases. But after age 10 or 11, the same hormones could facilitate cellular mechanisms like synaptic pruning, causing a decrease in size of the MTL. The results presented here, and those of prior studies (Neufang et al. 2008), further support the notion that the impact of rising pubertal hormones on brain structure may vary depending on the sex of the individual and the state of brain maturation at the time the hormones are introduced. Also, as we state above, the offsets between maturation of cortical and limbic structures, or right and left hemispheres, may differ by gender, even in puberty-matched populations. The age range of male and female participants, which varies across studies, undoubtedly affects conclusions that can be drawn by comparing findings. Future longitudinal studies will be ideal in parsing hormonal or other age-related influences on changes in the trajectories of gray matter volumes.
Finally, note that this study is framed in a larger context of identifying puberty-specific, as opposed to other age-related, effects on adolescent brain maturation. Puberty-related changes in specific neural systems and structures are a critical component for building more complete accounts of both typical development and pathology. For example, rates of depression in females increase sharply during adolescence, with evidence that pubertal hormones are stronger predictors than age (Angold et al. 1999). As noted by several researchers (Dahl 2004, 2008; Nelson et al. 2005; Blakemore and Choudhury 2006; Dahl and Gunnar 2009; see Steinberg and Cauffman 2006), the clinical and social policy implications of understanding adolescent brain development require a deeper understanding of the interactions among the various neural systems that must integrate cognitive, affective, and social information processing, not just the individual systems for each of these functions. Future research designed to specifically disentangle pubertal effects on brain development, including longitudinal studies, can advance these goals and better inform clinical and social policy impacting the health of youth.
Funding
National Institute of Drug Abuse (grants R01 DA017831 to E.R.S. and R01 DA018910 to R.E.D.); National Institute of Child Development Health and Human Development (grant RO1 HD053893-01 to E.R.S); National Institute of Health Roadmap for Medical Research, National Institutes of Health (grant U54 RR021813 entitled Center for Computational Biology and NIH/NCRR 5 P41 RR013642 to A.W.T.).
Acknowledgments
We thank Eric Kan for managing the data; Cornelius Hojatkashani and the rest of the UCLA Laboratory of Neuro Imaging Pipeline Team, Rico Magsipoc and Jonathan Pierce, for improving and maintaining our computing resources; and Jessie Chen for her assistance with editing figures. Conflict of Interest: None declared.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Swart M. Sex differences in neural activation to facial expressions denoting contempt and disgust. Plos ONE. 2008;3:e3622. doi: 10.1371/journal.pone.0003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Archer J. Rodent sex differences in emotional and related behavior. Behav Biol. 1975;14:451–479. doi: 10.1016/s0091-6773(75)90636-7. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Bailey MK, Zauszniewski JA, Heinzer MM, Hemstrom-Krainess AM. Patterns of depressive symptoms in children. J Child Adolesc Psychiatr Nurs. 2007;20:86–95. doi: 10.1111/j.1744-6171.2007.00090.x. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Che A, Cui J, Dinov I. SOCR Analyses: implementation and demonstration of a new graphical statistics educational toolkit. Statistics Online Computational Resource. J Stat Softw. 2009;30(3):1–19. doi: 10.18637/jss.v030.i03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Roozendaal B, Vazdarjanova A, McGaugh JL. Microinfusions of flumazenil into the basolateral but not the central nucleus of the amygdala enhance memory consolidation in rats. Neurobiol Learn Mem. 1999;72:1–7. doi: 10.1006/nlme.1999.3912. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent development and the regulation of behavior and emotion: introduction to part VIII. Ann N Y Acad Sci. 2004;1021:294–295. doi: 10.1196/annals.1308.034. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol. 1999;9:228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- Dinov I. SOCR: Statistics Online Computational Resource: socr.ucla.edu. Stat Comput Graphics. 2006;17:11–15. doi: 10.18637/jss.v016.i11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinov ID, Van Horn JD, Lozev KM, Magsipoc R, Petrosyan P, Liu Z, Mackenzie-Graham A, Eggert P, Parker DS, Toga AW. Efficient, distributed and interactive neuroimaging data analysis using the LONI Pipeline. Front Neuroinformat. 2009;3:22. doi: 10.3389/neuro.11.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2005;35:1–14. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B. An R and S-PLUS companion to multivariate analysis. London: Springer; 2005. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. 2009 doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Williamson DE, Ryan ND, Dahl RE. Positive and negative affect in depression: influence of sex and puberty. Ann N Y Acad Sci. 2004;1021:341–347. doi: 10.1196/annals.1308.042. [DOI] [PubMed] [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gray JA. Medial septal lesions, hippocampal theta rhythm and the control of vibrissal movement in the freely moving rat. Electroencephalogr Clin Neurophysiol. 1971;30:189–197. doi: 10.1016/0013-4694(71)90053-8. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Hellmann JJ, Fowler GW. Bias, precision, and accuracy of four measures of species richness. Ecol Appl. 1999;9:824–834. [Google Scholar]
- Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Pubertal maturation, reward-related brain function, and sleep in adolescents. J Adolesc Health. 2009;45(4):326–334. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch GG. Intraclass correlation coefficient. In: Kotz S, Johnson NL, editors. Encyclopedia of statistical sciences. New York: John Wiley; 1982. pp. 213–217. [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Toga A, Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Marshall WA. Puberty. In: Tanner J, Falkner F, editors. Human growth: a compendium treatise. New York: Plenum Press; 1986. pp. 171–209. [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- Mizukami S, Nishizuka M, Arai Y. Sexual difference in nuclear volume and its ontogeny in the rat amygdala. Exp Neurol. 1983;79:569–575. doi: 10.1016/0014-4886(83)90235-2. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR, Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nishizuka M, Arai Y. Organizational action of estrogen on synaptic pattern in the amygdala: implications for sexual differentiation of the brain. Brain Res. 1981a;213:422–426. doi: 10.1016/0006-8993(81)90247-x. [DOI] [PubMed] [Google Scholar]
- Nishizuka M, Arai Y. Sexual dimorphism in synaptic organization in the amygdala and its dependence on neonatal hormone environment. Brain Res. 1981b;212:31–38. doi: 10.1016/0006-8993(81)90029-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka M, Arai Y. Synapse formation in response to estrogen in the medial amygdala developing in the eye. Proc Natl Acad Sci U S A. 1982;79:7024–7026. doi: 10.1073/pnas.79.22.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41:673–690. [Google Scholar]
- Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, Delemarre-Van de Waal HA, Boomsma DI, Kahn RS, Hulshoff Pol HE. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI Pipeline processing environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Rex DE, Shattuck DW, Woods RP, Narr KL, Luders E, Rehm K, Stoltzner SE, Rottenberg DA, Toga AW. A meta-algorithm for brain extraction in MRI. Neuroimage. 2004;23:625–637. doi: 10.1016/j.neuroimage.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- Sarkey S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D, DonCarlos LL. Classical androgen receptors in non-classical sites in the brain. Horm Behav. 2008;53:753–764. doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slora EJ, Bocian AB, Herman-Giddens ME, Harris DL, Pedlow SE, Dowshen SA, Wasserman RC. Assessing inter-rater reliability (IRR) of Tanner staging and orchidometer use with boys: a study from PROS. J Pediatr Endocrinol Metab. 2009;22:291–299. doi: 10.1515/jpem.2009.22.4.291. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL. Further MRI evidence of late brain maturation: limbic volume increases and changing asymmetries during childhood and adolescence. Dev Neuropsychol. 1998;14:599–617. [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Wirth MM, Waugh CE, Schultheiss OC. Endogenous testosterone levels are associated with amygdala and ventromedial prefrontal cortex responses to anger faces in men but not women. Biol Psychol. 2009;81:118–122. doi: 10.1016/j.biopsycho.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Blatt-Eisengart I, Cauffman E. Patterns of competence and adjustment among adolescents from authoritative, authoritarian, indulgent, and neglectful homes: replication in a sample of serious juvenile offender. J Res Adolesc. 2006;16:47–58. doi: 10.1111/j.1532-7795.2006.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer DJ, Masten AS, Pine DS. The study of developmental psychopathology in adolescence: integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol 2: Developmental neuroscience. 2nd ed. Hoboken (NJ): John Wiley; 2006. p. xvii, 876. [Google Scholar]
- Van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the cake gambling task: age and gender analyses of probability estimation and reward evaluation. Dev Neuropsychol. 2008;33:179–196. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- Van Oort FV, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC. The developmental course of anxiety symptoms during adolescence: the TRAILS study. J Child Psychol Psychiatry. 2009;50(10):1209–1217. doi: 10.1111/j.1469-7610.2009.02092.x. [DOI] [PubMed] [Google Scholar]
- Worthman CM, Stallings JF. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am J Phys Anthropol. 1997;104:1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD, Cintron CB. Cognitive correlates of medial temporal lobe development across adolescence: a magnetic resonance imaging study. Percept Mot Skills. 2003;96:3–17. doi: 10.2466/pms.2003.96.1.3. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]