Abstract
When stimuli compete for sensory processing and response selection, coherent goal-guided behavior requires cognitive control so that task-relevant “targets” rather than irrelevant distractors are selected. It has been shown that reduced cognitive control under high working memory load increases distractor competition for selection. It remains unknown, though, whether cognitive control by working memory has an effect on the earliest levels of sensory processing in primary visual cortex. The present study addressed this question by having subjects perform a selective attention task involving classification of meaningful target objects while also ignoring congruent and incongruent distractor images. The level of cognitive control over distractor competition was varied through a concurrent working memory task of either low (1 digit) or high (6 digits) load. Functional magnetic resonance imaging revealed greater distractor competition effects not only on behavior but also on the sensory correlates in primary visual cortex (areas V1–V2) in conditions of high (vs. low) working memory load. In addition, high working memory load resulted in increased congruency-related functional connectivity between anterior cingulate cortex and V1. These results are the first to establish the neural correlates of distractor competition effects in primary visual cortex and the critical role of working memory in their cognitive control.
Keywords: attention, cognitive control, fMRI, response competition, working memory
Introduction
Understanding the neural mechanisms that maintain goal-directed behavior and minimize distraction by competing (but goal irrelevant) stimuli is a major challenge for the study of selective attention and cognitive control. Much of the previous work on this issue has focused on the role of prefrontal (e.g., the anterior cingulate) and parietal cortices in maintaining cognitive control over attentional selection, especially in the face of competition for response selection induced by distracting stimuli (e.g., Carter et al. 1998; Hazeltine et al. 2000; MacDonald et al. 2000; van Veen et al. 2001; Bunge et al. 2002; Fan et al. 2003; Kerns et al. 2004). Little is known, though, about the sensory correlates of processing response-competing distractors in early visual cortex (e.g., in primary cortex areas V1 and V2) or the extent to which any such sensory correlates can be subject to high-level cognitive control by working memory.
In the present work, we address this question using the load theory of attention and cognitive control (Lavie et al. 2004; Lavie 2005) to derive specific predictions. The load theory proposes that distractor processing critically depends on the level and type of information load involved in the task. High perceptual load (e.g., large, complex sets of visual information) that consumes all available capacity in the processing of the task information results in reduced distractor perception and visual cortex responses (for reviews, see, e.g., Lavie 2005, 2010), perhaps due to reduced cortical excitability in regions of the brain that are not involved in processing the task stimuli (Muggelton et al. 2008). These effects are found throughout visual cortex (e.g., Rees et al. 1997; Pinsk et al. 2004; Yi et al. 2004), including area V1 (e.g., Lavie 2005, 2010; Schwartz et al. 2005; Bahrami et al. 2007).
In contrast, studies have shown that distractor processing increases under high working memory load (e.g., when subjects perform 1 task while also actively maintaining information relevant to another task). Load theory asserts that task performance under these conditions results in increased processing of irrelevant distractors because of the reduced ability to actively maintain the stimulus-processing priorities of the main task while working memory is loaded in another task. In support of this claim, both behavioral measures of distractor interference effects (Lavie 2000; Lavie et al. 2004; Lavie and De Fockert 2005; Dalton et al. 2008) and neural responses to irrelevant distractors (e.g., images of faces or scenes) in visual association cortex (e.g., fusiform face area and parahippocampal place area) were found to increase under high working memory load (De Fockert et al. 2001; Rissman et al. 2009).
But, the previous research has not as yet addressed the effects of working memory load on the response to irrelevant distractors in early sensory cortex. A differential response to distractors in visual association cortex in the previous research may reflect higher level semantic processing (e.g., belonging to 1 type of semantic category or another), as this is indeed required for performance of the classification tasks that were used. The effect of working memory load on distractor responses in visual association regions may therefore be confined to semantic processing and may not indicate any effect on earlier sensory perceptual processing.
Thus, while we know that selective attention under high perceptual load can result in a reduced sensory response to distractors in areas such as primary visual cortex (V1), it is currently unclear whether the effects of working memory load (and the resultant reduction in cognitive control) can affect sensory processing of distractors at such an early stage. Furthermore, previous studies have so far only examined how load interacts with the overall neural response to the presence of a distractor regardless of its congruency with the response elicited by a target. Here we hypothesized that ignoring incongruent distractors would be more taxing on cognitive control, causing the visual cortex response to such distractors to be more sensitive to the effects of working memory load (cf. Yi et al. 2004).
Using functional magnetic resonance imaging (fMRI), we assessed early retinotopic visual cortex activity driven by response-related (congruent or incongruent) distractor objects presented during a selective attention task under either low (1 digit) or high (6 digits) verbal (and therefore task unrelated) working memory load. A mask was then presented for 2 s to erase any visual representation trace of the set digits. Following the mask, the subjects performed a selective attention task involving classifying images of objects as either fruits or household objects while rehearsing the digit set. On half of the trials, an irrelevant distractor object was presented either in right or in left visual field periphery. The distractor objects were either the same as the target (congruent) or from the opposite category (incongruent). Retinotopic mapping and functional localizers allowed us to identify the regions of striate and extrastriate cortices (V1, V2, V3/Vp, and V3a/V4v) that responded to the presence (vs. absence) of the distractor stimuli. We then assessed the effects of distractor congruency and working memory load on activity in these areas.
Materials and Methods
Participants
Thirty-one people recruited from the University College London (UCL) Division of Psychology and Language Subject Pool (13 females, ages 18–35) participated in the behavioral experiment. Fifteen of these also participated in the main fMRI experiment. Of those 15 participants, 2 were not analyzed due to excessive motion while in the scanner. Two other fMRI participants had an outlier congruency effect in the low working memory load condition (436 and 349 ms effects, 2.5 and 1.8 standard deviations from the mean, respectively) and were excluded from the final analysis (but including these participants' data did not change the overall pattern of either the behavioral or the imaging results). Eight additional people (4 females, age 20–31) participated in a control fMRI experiment. All participants provided informed consent in accordance with the UCL ethics board.
Stimuli and Task
Figure 1 shows the layout of task stimuli and the trial procedure. Stimuli was presented on a white background; all text were black. All pictures were presented in gray scale. The pictures were of an apple, pineapple, banana, and strawberry for the fruit category and a couch, electric fan, wooden chair, and a desk for the household objects category. Target pictures were centered 2.3 degrees of visual angle either above or below fixation and were contained within a 2.3 × 2.3 degree square. Distractor pictures were centered 3.4 degrees to the left of right of fixation and were contained within a 3.4 × 3.4 degree square. A distractor picture was present on 50% of trials and was equally likely to be either congruent (same picture) or incongruent (picture from the opposite category) with the target picture. Target and distractor positions and their combinations were equally likely in each congruency condition.
Figure 1.
Sequence for a high load, incongruent distractor trial. Memory set is presented for 1 s followed by visual mask for 2 s. Objects for visual discrimination task presented for 200 ms; arrows show possible positions for target and distractor items. Objects followed by 1.8-s response period and then a 0- to 2-s temporal jitter where only a fixation cross is shown. Probe for memory task is shown for 3 s.
Trials began with a fixation cross presented in the center of the display for 500 ms. This was followed by a single digit presented at fixation (low load) or 6 digits presented in a central row (high load) for 1000 ms. To erase any visual memory of the digits, a visual mask was then presented for 2000 ms, consisting of a row of 17 hash marks taking the same positions as the digit memory set. This prevented visual processing of the memory set from continuing during the selective attention task. At the offset of the mask, the target picture (and a distractor picture on 50% of trials) was presented for 200 ms. A blank interval lasting for 1800 ms followed, providing a total time window of 2 s for the task response. The attention task response was followed with a variable interval of 0, 1, or 2 s in which the fixation cross was presented, followed by a memory probe digit. The memory probe was displayed for 3000 ms, during which time participants made their memory task response.
Participants were instructed to hold the digits in memory while performing the attention task, making speeded classification responses indicating whether the target image was of a fruit or a household object and ignoring the distractor images. Upon the appearance of the memory probe, the participants were instructed to indicate whether or not the probe digit was one of the to-be-remembered digits presented at the start of the trial. Responses were made using the computer keyboard for the behavioral participants and magnetic resonance–compatible response boxes for the imaging participants.
The memory probe digit was a match on 50% of trials selected at random. Digits for the memory set were selected randomly on each trial with the following constraints: In the low-load condition, the memory set digit was not repeated on consecutive trials. In the high-load condition, no more than 3 consecutive digits could be in ascending or descending order. The probe digit was selected randomly from the memory set (match trials) or from the unused digits (non-match trials); the probe digit was never repeated on consecutive trials. Each digit occurred equally often in the memory set and was equally often in the memory probe.
The main experiment was divided into 4 blocks for 48 trials. Each block began and ended with 12 s of fixation and lasted 8 min in total, which was the length of a single scan session for the main experiment. At the end of each block, participants were given feedback on their accuracy for both tasks. All participants completed 4 blocks of 48 trials. Each block was either high or low load, alternated in an ABAB fashion (the level of load of the first block was counterbalanced across participants). Experimental blocks were preceded with 2 blocks of 16 practice trials each of either low load or high load.
FMRI Procedure
FMRI sessions included retinotopic mapping and localizer scans. Two of each such scan were conducted for each participant following the main experimental scans. The retinotopic mapping scans consisted of flickering checkerboard wedges presented alternately at the horizontal and vertical meridians of the display for 18 s in each position (Slotnick and Yantis 2003; Qiu et al. 2006). Participants were instructed to maintain fixation throughout the scan. Each retinotopic mapping scan lasted 288 s. Functional localizer scans were used to identify the regions of retinotopic cortex that were most responsive to the presence of the distractor pictures. The functional localizer scans consisted of flashing white and black disks presented on a gray background. The disks alternated between the left and the right distractor locations, switching every 24 s. Twelve seconds of fixation were placed at the beginning and end of the scans. Participants were instructed to fixate a cross at the center of the display and respond with a button press whenever it changed into an X; this occurred once during each 24-s stimulation period. Each localizer scan lasted 312 s.
For the control experiment, the stimuli were identical to those used in the selective attention task in the main experiment (with the exception that there were no “distractor-absent” trials). Trials consisted only of the target and distractor image pairs appearing for 200 ms. Participants completed 2 blocks of trials in the scanner. Each block consisted of 80 trials, separated by a variable inter-trial interval (ITI) of 3–7 s, with 12 s of fixation appearing at the start and end of each block. Target location and identity, distractor location and congruency, and ITI were all counterbalanced within each block. Participants were instructed to maintain fixation and monitor for a “jiggle” in either of the 2 pictures. This jiggle consisted of the image shifting 1 pixel to the left of its standard location ∼67 ms after its appearance and then shifting 1 pixel to the right of its standard location ∼67 ms later. Jiggle events were evenly distributed between target and distractor items and occurred every 7–9 trials (once every 40 s on average). Each scan session also consisted of 2 blocks of meridian mapping, 2 blocks of functional localizers, and a structural scan as in the main experiment.
Stimuli were generated using custom Matlab software (MathWorks) using the Psychophysics Toolbox extensions (Brainard 1997; Pelli 1997). During scans, stimuli were projected onto a screen mounted at the opening of the scanner bore using an LCD projector (display resolution: 1280 × 1024; size of the visible region of display: 23 × 14 degrees); participants viewed the display through a mirror mounted on the head coil.
Imaging Data Collection and Analysis
Imaging data were collected at the Birkbeck-UCL Center for NeuroImaging (London) using a 1.5-T Siemens Avanto Scanner with a 12-channel head coil. Anatomical images were acquired using a magnetization-prepared rapid gradient-echo T1-weighted sequence optimized for gray–white matter contrast, yielding images with a 1-mm isovoxel resolution (time repetition [TR] = 2730 ms, time echo [TE] = 3.57 ms, flip angle = 8°). Whole-brain echoplanar functional images were acquired in 35 transverse slices (TR = 3000 ms, TE = 50 ms, matrix = 64 × 64, field of view = 192 mm, slice thickness = 3 mm, no gap, ascending interleaved order).
Data were processed and analyzed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Functional images collected from the scanner were slice time corrected, aligned with a representative functional volume and motion corrected using a rigid body spatial transformation, morphed into the standard Montreal Neurological Institutes space, and spatially smoothed using a 4-mm full-width at half-maximum Gaussian kernel. For the main task, separate events representing congruent and incongruent trials for distractors in the left and right position and no distractor trials, all under low and high load, were modeled using a general linear model (GLM). These events were modeled as impulses of activity at the onset of the picture classification stimuli and were convolved with the canonical hemodynamic response function (HRF) included in SPM5. For the control task, the data were analyzed by grouping each trial into 1 of 4 event types based on distractor location (left of right) and congruency and modeling these event types with a GLM as previously described. For the retinotopic and localizer scans, boxcars representing the duration of each block were convolved with the HRF and similarly modeled using a GLM. Results of the analyses were overlaid on the structural images collected for each subject.
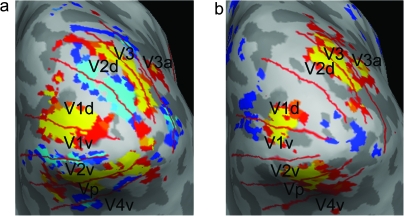
Data from the retinotopic mapping scans were used to define the borders between areas V1, V2, V3/Vp, and V3a/V4v in each hemisphere (Engel et al. 1994; Sereno et al. 1995) (see Fig. 1). Data from the localizer scans were then used to define the segments of each of these areas that responded most strongly to stimuli in the location of the distractors. To define regions of interest (ROIs) in early visual cortex, the functional data were projected onto inflated representations of each participant's cortex. Cortical reconstruction and volumetric segmentation were performed with the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). These ROIs were imported into SPM5, and the beta weights corresponding to stimuli appearing in the contralateral visual field for each of the previously described events were extracted from each ROI, allowing for analysis of the response to the different distractor types under the different load conditions. Table 1 shows the mean location and volume for each of the imported ROIs, averaged across all subjects showing for which that region could be identified.
Table 1.
Mean size and location of and number of subjects showing each retinotopic ROI
| n | Vol (mL) | x | y | z | |
| Left hemisphere | |||||
| V1d | 11 | 0.29 (0.06) | −10 (1.1) | −95 (0.8) | 0.9 (1.1) |
| V2d | 10 | 0.21 (0.04) | −17 (1) | −98 (0.6) | 9.4 (1.2) |
| V3 | 10 | 0.32 (0.05) | −22 (1) | −94 (1) | 13 (1.4) |
| V3a | 9 | 0.35 (0.09) | −29 (2.4) | −89 (0.8) | 10 (1.5) |
| V1v | 11 | 0.24 (0.04) | −7 (1.3) | −91 (1.2) | −5 (0.8) |
| V2v | 11 | 0.38 (0.05) | −11 (1.1) | −87 (1) | −10 (1) |
| Vp | 11 | 0.35 (0.04) | −19 (1.4) | −84 (0.8) | −10 (1) |
| V4v | 11 | 0.26 (0.06) | −27 (1.6) | −80 (1) | −9 (1) |
| Right hemisphere | |||||
| V1d | 11 | 0.26 (0.04) | 11 (2.7) | −93 (0.6) | 1.3 (1.9) |
| V2d | 11 | 0.18 (0.03) | 20 (1.4) | −95 (0.9) | 11 (1.6) |
| V3 | 11 | 0.30 (0.03) | 26 (0.7) | −93 (1.1) | 14 (2.1) |
| V3a | 10 | 0.38 (0.07) | 31 (1.8) | −86 (1.3) | 12 (1.7) |
| V1v | 11 | 0.19 (0.03) | 9.9 (0.8) | −90 (0.5) | −4 (1.5) |
| V2v | 11 | 0.39 (0.05) | 14 (1.1) | −86 (0.7) | −10 (1.3) |
| Vp | 10 | 0.38 (0.05) | 21 (1.5) | −79 (1) | −10 (1) |
| V4v | 10 | 0.19 (0.04) | 28 (1.9) | −78 (1.3) | −13 (1) |
SEMs are listed in parentheses.
To examine which cortical regions showed a greater blood oxygen level–dependent (BOLD) response to the various trial types in the main experiment, full-brain statistical parametric maps were generated using an uncorrected voxel-level threshold of P < 0.001 and a cluster size threshold of 6 voxels resulting in a corrected threshold of P < 0.05. This threshold was calculated using the function CorrClusTh.m, developed for use with SPM (see http://www.sph.umich.edu/∼nichols/JohnsGems5.html).
In addition to the full-brain and retinotopic analyses, an analysis of “effective connectivity” (Friston et al. 1997; Gitelman et al. 2003; Stephan et al. 2003) was conducted using the psychophysiological interaction (PPI) approach. In this approach, 2 seed regions were defined for each subject consisting of left and right V1 (encompassing both dorsal and ventral portions) as defined using the meridian mapping and functional localizer data. The PPI function in SPM5 was used to extract the first eigenvariate of the BOLD time course for the voxels, which was then convolved with a function representing the interaction between distractor congruency and working memory load. T maps were then produced representing the level of effective coupling within each voxel. These maps were submitted to a group-level analysis, which was used to identify regions of effective connectivity with the seed regions. Significant regions were again identified using an uncorrected voxel-level threshold of P < 0.001 and minimum cluster size threshold of 6 voxels, resulting in a cluster-level threshold of P < 0.05.
Results
Retinotopic mapping and functional localizers allowed us to identify the regions of striate and extrastriate cortices (V1, V2, V3/Vp, and V3a/V4v) that showed the greatest response to the presence (vs. absence) of a stimulus in the distractor location. This was confirmed by the increased BOLD response to distractor present (compared with distractor absent) trials (P < 0.01 for all regions); furthermore, the BOLD response was larger to distractors present in the contralateral visual field than those in the ipsilateral visual field (P < 0.008 for all regions), which is consistent with contralateral mapping of visual space in retinotopic cortex. Further analyses of the distractor congruency conditions were therefore conducted on the contralateral distractor conditions.
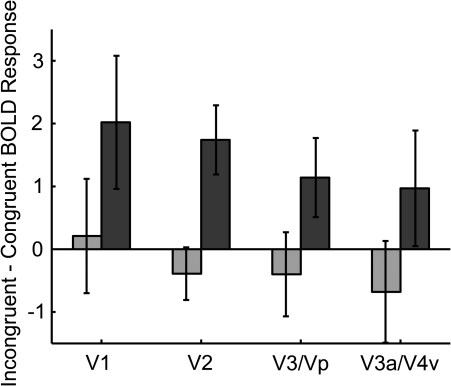
Importantly, these analyses showed that the activity in each of the retinotopic regions that responded to the distractor was strongly modulated as a function of both the distractor congruency conditions and working memory load. As can be seen in Figure 2, the distractor congruency effect (incongruent − congruent) was significantly greater under high load than low load in V1 (t(26) = 2.45, P < 0.034) and V2 (t(26) = 3.34, P < 0.007). This pattern was also present numerically in V3/Vp and V3a/V4 but reached only marginal significance in V3/Vp (t(26) = 2.02, P = 0.071) and was not significant in V3a/V4v (t(26) = 1.24, P > 0.2). These findings are the first to establish a differential response in primary visual cortex areas V1 and V2 to distractor stimuli in accordance with their congruency with the target response and the level of working memory load. Increased working memory load resulted in enhanced distractor effects in primary visual cortex, the opposite effect to that previously seen for perceptual load (e.g., Schwartz et al. 2005), as we predicted. This pattern of results, along with the pattern in the behavioral data, makes it unlikely that one can account for the findings in terms of effects of perceptual load as related to the size of the memory set displays. Such an alternative account is also unlikely given the presentation of a mask for 2 s immediately following the memory set displays which should have removed any visual difference by the time the attention displays were presented.
Figure 2.
Retinotopic mapping and functional localizer data from an exemplar subject overlaid on inflated cortical surface. (a) Data from meridian mapping; warm colors represent BOLD response to horizontal meridian; and cool colors represent BOLD response to vertical meridian. (b) Data from functional localizer for distractor locations showing the contrast left location > right location.
There was no indication of a baseline shift due to load as on distractor-absent trials, the BOLD response within distractor processing ROIs was not significantly different in the high-load condition compared with the low-load condition for any region (all P values > 0.15).
The enhancement of distractor congruency effects on primary cortex by working memory load clearly cannot be attributed to any stimulus difference: the distractors were identical under the low and high working memory load conditions. In addition, a control experiment ruled out the possibility that the neural response to a congruent distractor stimulus (i.e., one that was identical to the target item) would be inherently lower than that to an incongruent distractor in the absence of cognitive modulation. In the control experiment, participants no longer attempted to ignore the distractor while attending to targets. Instead, they simply viewed the same congruent and incongruent stimulus pairs that were previously presented as target and distractor while monitoring for an infrequent jiggle in either of the 2 pictures. This provided a strong test for any possible low-level visual interactions between the target and the distractor images.
The results showed no difference in response to visually congruent or incongruent stimulus pairs in any of the regions; in fact, any numerical trends were in the opposite direction, that is, for a smaller response to visually incongruent compared with congruent stimuli in all regions (V1: I − C = −0.61, standard error of the mean [SEM] = 0.74; V2: I − C = −0.16, SEM = 0.50; V3/Vp: I − C = −0.30, SEM = 0.48; V3a/V4v: I − C = −0.83, SEM = 0.38; all P values > 0.44, expect for V3a/V4v, P < 0.07). Thus, the congruency effects on striate and extrastriate cortices do not reflect low-level visual interactions. Instead, our results suggest that the congruency effects in visual cortex are due to higher level competition between the target and the distractor for attentional selection that requires the availability of working memory to be resolved.
The full-brain contrast of congruency effects (incongruent > congruent trials) revealed increased BOLD signal in several frontal and parietal regions as shown in Table 2. A contrast for the interaction of congruency and load revealed a significant cluster in medial superior parietal lobule (x = −9, y = −54, z = 60; t = 5.39; volume = 0.19 mL) that showed a greater response to incongruent (vs. congruent) distractors under high compared with low working memory load (see Fig. 4). Thus, parietal regions implicated in distractor response competition effects (see also Hazeltine et al. 2000; Milham et al. 2001; Fan et al. 2005) showed greater competition-related activity under conditions of high working memory load. Neither the reverse contrast for congruency effects (congruent > incongruent) nor the reverse interaction (incongruent − congruent greater in low vs. high load) revealed any significant clusters.
Table 2.
Regions showing greater BOLD signal in the incongruent compared with congruent distractor conditions
| Cluster | Brodmann area | Coordinates (x, y, z) | Volume (mL) | T |
| R lingual gyrus | 19 | 27, −60, −9 | 0.162 | 8.77 |
| R middle occipital gyrus | 19 | 39, −78, 15 | 0.162 | 4.89 |
| R superior occipital gyrus | 19 | 33, −81, 33 | 0.270 | 5.31 |
| R precuneus | 7 | 6, −78, 42 | 0.243 | 9.40 |
| R superior parietal lobule | 7 | 3, −63, 60 | 0.378 | 6.43 |
| R middle frontal gyrus | 9 | 51, 18, 27 | 0.189 | 5.39 |
| L intraparietal sulcus | 18/31 | −30, −63, 33 | 0.999 | 7.37 |
| L precuneus | 7 | −6, −72, 45 | 0.621 | 5.73 |
R, right; L, left.
Figure 4.
Top panel: portion of superior parietal lobule (SPL) that showed a significant BOLD response to the load × congruency interaction. Bottom panel: difference in mean BOLD response (measured in beta values) as a function of distractor congruency under low load (light) and high load (dark) in SPL. Error bars represent SEM.
Figure 3.
Difference in mean BOLD response (measured in beta values) as a function of distractor congruency under low load (light) and high load (dark) in regions of early visual cortex. Error bars represent SEM.
In addition to the full-brain contrasts, analysis of effective connectivity using a PPI approach with left V1 as the seed region revealed that the interaction of congruency and load was associated with increased functional connectivity of left V1 and left anterior cingulate cortex (ACC; t = 10.51; x = −12, y = 30, z = 27; volume: 0.162 mL). This finding further reinforces our conclusions that distracter congruency effects on V1 activity are subject to top-down control. In light of the known role for ACC in conflict monitoring in various response competition tasks (Barch et al. 2001; van Veen and Carter 2002; Carter and van Veen 2007), our finding suggests that the connectivity of V1 and ACC found with the greater distractor congruency effects (under higher working memory load) may serve to mediate the registration of a greater conflict-monitoring signal.
The pattern of results observed in the imaging data was also mirrored in the behavioral data. As can be seen in Table 3, working memory task performance was slower (t (26) = 10.57, P < 0.001) and less accurate (t (26) = 4.78, P < 0.001) under high load compared with low load, confirming that our load manipulation was effective. In the attention task, the incongruent (compared with congruent) distractor condition produced slower responses (t (26) = 4.88, P < 0.001), and more importantly, the distractor congruency effects on response times were significantly larger under high load than low load (t (26) = 1.97, P = 0.03, 1 tailed). There were no significant differences in the error rates for any of the conditions of the attention task (all P values > 0.19).
Table 3.
Behavioral performance for all task conditions averaged across subjects
| Low load |
High load |
|||
| RT (ms) | Error (%) | RT (ms) | Error (%) | |
| WM task | 857 (33) | 5.0 (0.9) | 1104 (36) | 9.2 (1.2) |
| Congruent flanker | 803 (33) | 2.9 (0.7) | 806 (31) | 3.4 (1.1) |
| Incongruent flanker | 890 (36) | 4.3 (1.2) | 923 (38) | 4.9 (1.5) |
SEMs are listed in parentheses. RT, response time; WM, Working Memory.
Discussion
The present findings are the first to establish sensory correlates of distractor competition effects in primary visual cortex. We have shown that reduced cognitive control under high working memory load results in extensive modulation of the distractor competition effects not only on behavior and on conflict resolution areas in frontoparietal cortex but also on the sensory correlates found in primary visual cortex areas V1 and V2.
The previous research into the neural mechanisms of control over selection in the presence of response-competing distractors was typically conducted under conditions that allowed for optimal cognitive control, as the cognitive control mechanisms addressed were not presented with high information load (Carter et al. 1998; Hazeltine et al. 2000; MacDonald et al. 2000; Bunge et al. 2001; Milham et al. 2001; van Veen et al. 2001; Bunge et al. 2002; Fan et al. 2003, 2005; Kerns et al. 2004). In such situations, the distractors' impact may be rather low and the competition between the target and distractors may be limited to response selection and higher level semantic classification as per the task instructions, with little effect on sensory visual cortex. In contrast, when attentional control is limited under conditions of high working memory load, an irrelevant distractor has larger interference effects (as shown by the behavioral reaction time measures) and the competition between the target and the distractor extends to the level of sensory stimulus processing as early as primary visual cortex.
The findings that, under high working memory load, primary visual cortex responded differentially to incongruent compared with congruent distractors and that these effects were only found when the distractors' congruency was response related (as indicated in the control experiment) merit further explanation. The functional connectivity analysis provides insight into this issue. This analysis indicated a higher connectivity between V1 and ACC as a result of the interaction between working memory load and distractor congruency. ACC is known to monitor for potential conflict with the current target selection (Barch et al. 2001; van Veen and Carter 2002; Carter and van Veen 2007). The changes in connectivity between ACC and V1 based on working memory load and distractor congruency suggest an account for the congruency effects in V1 in terms of the monitoring feedback from ACC. Such feedback (most likely through parietal cortex, as indicated by the full-brain contrast data) would be useful in situations where the behavioral irrelevance of distractor stimuli is less clear due to a load on working memory. Thus, when an irrelevant distractor was present under high working memory load, it would have elicited greater congruency-monitoring signals from ACC which in turn fed back to V1, resulting in a larger differential primary cortex signal related to the distractor congruency.
These effects provide strong evidence for a novel prediction derived from the load theory of attention (Lavie et al. 2004; Lavie 2005) that working memory is critical for the sensory resolution of distractor-induced response congruency effects. Load theory argues that working memory is involved in selective attention not only through biasing attention toward information held in memory but also through cognitive control by minimizing irrelevant distraction via active maintenance of stimulus-processing priorities. When working memory is loaded with material unrelated to the task at hand (e.g., verbally maintaining a set of digits while performing a visual classification task), it is no longer available to control visual selective attention (e.g., ignoring pictures of distractor objects). This results in greater effects of competing distractors, extending from behavioral interference effects to frontoparietal response competition network and even to sensory activity in primary visual cortex.
The role of working memory in visual selective processing has been considered in other frameworks; one influential framework is the biased competition model (Desimone and Duncan 1995), in which working memory is needed in order to bias competitive interactions in favor of goal-relevant stimuli. Most evidence for this role has thus far been confined to content-specific modulations of visual activity, such as facilitation of activity related to information held, and suppression of information not held, in working memory (Bunge et al. 2001; Postle 2005; D'Esposito 2007; Soto et al. 2008). In contrast, the effects of working memory load with task-unrelated information shown here clearly demonstrate a more general, cognitive control role of working memory in visual selection. An “executive” cognitive control role for working memory was envisaged in neuropsychological models accounting for the substantial deficits in achieving goal-directed control following lesions of frontal cortex (Shallice and Burgess 1991; Baddeley and Della Sala 1996). Clearly, to explain the ubiquitous nature of these deficits, one must consider the more general (noncontent specific) cognitive role for working memory in goal-directed control of visual attention demonstrated here.
Determining that working memory load can affect sensory distractor processing as early as primary sensory cortex is critical to the understanding of attention and cognitive control. It informs us about the reach of cognitive control and also serves to advance a resolution for the most enduring controversy in attention research concerning the question of whether the effects of selective attention can extend to early sensory processing (for a recent review, see Lavie, forthcoming). Much insight into this question had been gained from neuroscientific demonstrations that both perceptual load (as discussed earlier) and spatial orienting (Motter 1993; Gandhi et al. 1999; Martínez et al. 1999; Liu et al. 2005; Serences and Yantis 2006) can affect the response to distractors in primary visual cortex, but the question of whether distractor effects on primary sensory cortex activity can also be subject to higher level cognitive control by working memory was previously open. The present study provides clear evidence for such far-reaching effects of cognitive control.
Funding
Wellcome Trust (WT080568MA to N.L.).
Acknowledgments
We thank Marty Sereno for advice with design and data analysis and Jonathan Roiser for help with the PPI analysis. Conflict of Interest: None declared.
References
- Baddeley A, Della Sala S. Working memory and executive control. Philos Trans R Soc Lond B Biol Sci. 1996;351:1397–1404. doi: 10.1098/rstb.1996.0123. [DOI] [PubMed] [Google Scholar]
- Bahrami B, Lavie N, Rees G. Attentional load modulates responses of human primary visual cortex to invisible stimuli. Curr Biol. 2007;17:509–513. doi: 10.1016/j.cub.2007.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulated cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Dalton P, Lavie N, Spence C. The role of working memory in tactile selective attention. Q J Exp Psychol. 2008;62:635–644. doi: 10.1080/17470210802483503. [DOI] [PubMed] [Google Scholar]
- De Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, Shadlen MN. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18:42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimagaing. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiological interaction in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Poldrack E, Gabrieli JDE. Neural activation during response competition. J Cogn Neurosci. 2000;12(Suppl 2):118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulated conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lavie N. Selective attention and cognitive control: dissociating attentional functions through different types of load. In: Monsell S, Driver J, editors. Attention and performance XVIII. Cambridge (MA): MIT Press; 2000. pp. 175–194. [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N. Attention, distraction and cognitive control under load. Curr Dir Psychol Sci. Forthcoming 2010 [Google Scholar]
- Lavie N. From the mother lode to load. In: Robertson L, Wolfe G, editors. From perception to consciousness: searching for Anne Treisman. Oxford University Press; Forthcoming. [Google Scholar]
- Lavie N, De Fockert JW. The role of working memory in attentional capture. Psychon Bull Rev. 2005;12:669–674. doi: 10.3758/bf03196756. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, De Fockert JW, Viding E. Load theory of selective attention and cognitive control. J Exp Psychol Gen. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Liu T, Pestilli F, Carrasco M. Transient attention enhances perceptual performance and FMRI response in human visual cortex. Neuron. 2005;45:469–477. doi: 10.1016/j.neuron.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulated cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Martínez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2 and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Muggleton N, Lamb R, Walsh V, Lavie N. Perceptual load modulates visual cortex excitability to magnetic stimulation. J Neurophysiol. 2008;100:516–519. doi: 10.1152/jn.01287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Pinsk MA, Doniger GM, Kastner S. Push-pull mechanism of selective attention in human extrastriate cortex. J Neurophysiol. 2004;92:622–629. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- Postle BR. Delay-period activity in the prefrontal cortex: one function is sensory gating. J Cogn Neurosci. 2005;17:1679–1690. doi: 10.1162/089892905774589208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Rosenau BJ, Greenberg AS, Hurdal MK, Barta P, Yantis S, Miller MI. Estimating linear cortical magnification in human primary visual cortex via dynamic programming. Neuroimage. 2006;31:125–138. doi: 10.1016/j.neuroimage.2005.11.049. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. The effect of non-visual working memory load on top-down modulation of visual processing. Neuropsychologia. 2009;47:1637–1646. doi: 10.1016/j.neuropsychologia.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Frith C, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cereb Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. Higher—order cognitive impairments and frontal-lobe lesions in man. In: Levin H, Benton AJ, editors. Frontal lobe function and dysfunction. New York: Oxford University Press; 1991. pp. 125–138. [Google Scholar]
- Slotnick S, Yantis S. Efficient acquisition of human retinotopic maps. Hum Brain Mapp. 2003;18:22–29. doi: 10.1002/hbm.10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Hodsoll J, Rotshtein P, Humphreys GW. Automatic guidance of attention from working memory. Trends Cogn Sci. 2008;12:342–348. doi: 10.1016/j.tics.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, Fink GR. Lateralized cognitive processes and lateralized task control in the human brain. Science. 2003;301:384–386. doi: 10.1126/science.1086025. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14:1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Yi DJ, Woodman GF, Widders D, Marois R, Chun MM. Neural fate of ignored stimuli: dissociable effects of perceptual and working memory load. Nat Neurosci. 2004;7:992–996. doi: 10.1038/nn1294. [DOI] [PubMed] [Google Scholar]