Abstract
The functional significance of diverse neuropeptide coexpression and convergence onto common second messenger pathways remains unclear. To address this question, we characterized responses to corticotropin-releasing factor (CRF), pituitary adenylate cyclase–activating peptide (PACAP), and vasoactive intestinal peptide (VIP) in rat neocortical slices using optical recordings of cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA) sensors, patch-clamp, and single-cell reverse transcription–polymerase chain reaction. Responses of pyramidal neurons to the 3 neuropeptides markedly differed in time-course and amplitude. Effects of these neuropeptides on the PKA-sensitive slow afterhyperpolarization current were consistent with those observed with cAMP/PKA sensors. CRF-1 receptors, primarily expressed in pyramidal cells, reportedly mediate the neocortical effects of CRF. PACAP and VIP activated distinct PAC1 and VPAC1 receptors, respectively. Indeed, a selective VPAC1 antagonist prevented VIP responses but had a minor effect on PACAP responses, which were mimicked by a specific PAC1 agonist. While PAC1 and VPAC1 were coexpressed in pyramidal cells, PAC1 expression was also frequently detected in interneurons, suggesting that PACAP has widespread effects on the neuronal network. Our results suggest that VIP and CRF, originating from interneurons, and PACAP, expressed mainly by pyramidal cells, finely tune the excitability and gene expression in the neocortical network via distinct cAMP/PKA-mediated effects.
Keywords: cerebral cortex, fluorescence resonance energy transfer, G-protein–coupled receptor, genetically encoded sensor, neuropeptides
Introduction
Neuropeptides are expressed throughout the mammalian brain and exert neuromodulatory effects by acting at G-protein–coupled receptors. It is well documented that diverse neuropeptides share common second messenger pathways, sometimes bind to the same receptors and often coexist in the same neurons (Zupanc 1996; Merighi 2002). However, the role of this apparent redundancy in the modulation of brain networks is poorly understood.
The neuropeptides corticotropin-releasing factor (CRF), pituitary adenylate cyclase–activating peptide (PACAP), and vasoactive intestinal peptide (VIP), which all activate the cyclic cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) second messenger pathway, are expressed in the neocortex. The neocortical network comprises glutamatergic pyramidal cells and γ-aminobutyric acid (GABA)ergic interneurons (Peters and Jones 1984; Thomson and Deuchars 1994), which exhibit cell-type specific expression patterns of neuropeptides (DeFelipe 1993; Kawaguchi and Kubota 1997; Cauli et al. 2000; Karagiannis et al. 2009). CRF and VIP are specifically found in interneurons, where their expression partially overlaps (Taki et al. 2000; Gallopin et al. 2006). CRF acts at CRF-1 receptors to enhance the excitability of pyramidal cells (Gallopin et al. 2006). While VIP also enhances pyramidal neuron excitability (Pawelzik et al. 1992), the neocortical distribution of its receptors has not been characterized. VIP binds to PAC1 and VPAC1 receptors (Laburthe et al. 2002; Vaudry et al. 2009), which are both present in the neocortex (Usdin et al. 1994; Tatsuno et al. 2001; Jolivel et al. 2009), as well as to VPAC2 receptors whose expression is restricted to subcortical structures (Usdin et al. 1994; Sheward et al. 1995). These receptors also bind PACAP (Laburthe et al. 2002; Vaudry et al. 2009), which is sparsely expressed in glutamatergic neurons (Koves et al. 1994; Hannibal 2002; Stumm et al. 2007) and whose physiological effects on neocortical neurons have not been reported.
The present study was initially aimed at examining whether VIP and CRF, which are colocalized in interneurons, can exert different effects in the neocortical network. To this end, we performed optical recordings of cAMP/PKA dynamics in pyramidal neurons from rat neocortical slices using genetically encoded sensors based on fluorescence energy transfer (FRET) between 2 variants of the green fluorescent protein (Nikolaev et al. 2004; Zhang et al. 2005; Vincent et al. 2008). PKA activity was also monitored in these neurons using patch-clamp recordings of the slow afterhyperpolarization, which is reduced upon PKA activation (Pedarzani and Storm 1993). This study was completed by single-cell reverse transcription–polymerase chain reaction (RT-PCR) (scPCR; Lambolez et al. 1992) analyses of neuropeptide receptor expression in neocortical neurons. We found that VIP and CRF, and also PACAP, activate cAMP/PKA responses in pyramidal cells. The 3 neuropeptides elicited different cAMP/PKA dynamics by acting at distinct receptors. Our results suggest that distinctive sources, targets, and effects allow these neuropeptides to differentially regulate excitability and gene expression in the cortical network.
Materials and Methods
Subcloning and Virus Production
The PKA sensor AKAR2 (Zhang et al. 2005) and the cAMP sensor Epac1-camps (Nikolaev et al. 2004) were used in this study. The coding sequences of AKAR2, AKAR-NLS (both provided by Jin Zhang; Zhang et al. 2005), AKAR2 Thr391 to Ala mutant (AKAR2mut; Gervasi et al. 2007), and EPAC1-camps (provided by Martin Löhse) were subcloned into the viral vector pSinRep5 (Invitrogen). Sindbis viruses were produced as previously described (Gervasi et al. 2007; Hepp et al. 2007). Recombinant pSinRep5 constructs and helper plasmid pDH26S were transcribed in vitro into capped RNA using the Megascript SP6 kit (Ambion). Baby hamster kidney-21 (ATCC no. CCL-10) cells were electroporated with sensor-containing RNA and helper RNA (2.107 cells, 950 μF, 230 V) and incubated for 24 h at 37 °C in 5% CO2 in Dulbecco's modified Eagle Medium supplemented with 5% fetal calf serum before collecting cell supernatant containing the viruses. The virus titer (106–108 infectious particles/ml) was determined after counting fluorescent baby hamster kidney cells infected using serial dilution of the virus stock.
Brain Slice Preparation
Male Wistar rats (12–23 days old, Janvier, France) were killed by decapitation in accordance with guidelines from the French Ministry of Agriculture and the European Community. The brains were quickly immersed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 20 D-glucose, 5 Na pyruvate, 1 kynurenic acid and saturated with 5% CO2/95% O2. Parasagittal slices (300 μm thick) of somatosensory cortex were cut at an angle of 10° using a Leica VT 1000S Vibratome (Leica). Slices were kept at room temperature (20–25 °C) for 30 min in the same solution before viral infection or electrophysiological recordings.
Brain Slice Viral Infection
Brain slices were placed onto a millicell-CM membrane (Millipore) with culture medium (50% minimum essential medium, 50% Hanks’ balanced salt solution, 6.5 g/L glucose, and 100 U/mL penicillin/100 μg/mL streptomycin; Invitrogen). Infection was performed by adding ≈5 × 105 particles per slice. Slices were incubated overnight at 35 °C in 5% CO2. The next morning, brain slices were equilibrated in ACSF for 1 h and then placed into the recording chamber and perfused continuously at 2 mL/min with ACSF at 32 °C.
FRET Imaging
Recordings were made from visually identified pyramidal neurons in layers II/III and V of rat somatosensory neocortex. Wide-field images were obtained with an Olympus BX50WI or BX51WI upright microscope with a 20× 0.5 NA or 40× 0.8 NA water-immersion objective and either a Micromax digital camera (Roper Scientific) or an ORCA-AG camera (Hamamatsu). Images were acquired using iVision (Biovision). Excitation and dichroic filters were D436/20 and 505dclp. Signals were acquired by alternating the emission filters HQ480/40 for cyan fluorescent protein and D535/40 for yellow fluorescent protein with a filter wheel (Sutter Instruments). All filters were from Chroma Technology (Brattleboro). Images were analyzed using custom routines written in the IGOR Pro environment (Wavemetrics). For AKAR2, the ratio of each pixel was calculated as the emission at 535 nm divided by the emission at 480 nm (F535/F480), whereas this ratio was inversed for Epac1-camps. The pseudocolor images were calculated to simultaneously display the ratio information coded in hue and the fluorescence intensity (averaged F480 and F535). Ratio changes were measured with respect to the baseline. Occasionally, the baseline drifted and the measurement were made with respect to the linear extrapolation of the baseline.
Measurements of Slow AHP Currents
Characterization of slow afterhyperpolarization currents (IsAHP) was performed using patch pipettes (3–5 MΩ) filled with internal solution containing (in mM): 130 potassium methane sulfonate (Fluka), 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) (Fluka), 1 5,5′-dibromo-1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) tetrapotassium (Molecular Probes), 5 MgCl2, 0.1 CaCl2, 4.4 ATP-Na2+ (Sigma), 5 creatine phosphate (Sigma), pH 7.3, 340 mOsm/L. Voltage-clamp recordings were obtained using an Axopatch 200B amplifier and Axograph 4.9 software (Molecular Devices). Recordings were filtered at 1 kHz and digitized at 5 kHz. IsAHP was activated by a train of 20–40 steps of 3-ms duration at a frequency of 80 Hz from a holding potential of −67 to +13 mV, but the amplitude of the sAHP current was less stable than the holding current, which was therefore used in our measurements and called tonic potassium current (tonic IK). A 10-mV hyperpolarizing jump was applied before each train to monitor series and input resistances. Experiments were discarded if the series resistance increased above 10 MΩ. The transient capacitive currents remained constant throughout the experiments.
Characterization of Neurons by Patch-Clamp and scPCR
For scPCR experiments, patch micropipettes (3–5 MΩ) were filled with 8 μL of internal solution containing (in mM): 144 K-gluconate, 3 MgCl2, 0.5 ethyleneglycol-bis(aminoethylether)-tetraacetic acid (EGTA), and 10 HEPES plus 2 mg/mL biocytin (Sigma). The pH was adjusted to 7.2 and osmolarity to 285/295 mOsm. Whole-cell recordings were made as described above, and membrane potentials were not corrected for liquid junction potential. Cells were maintained at a holding potential of –60 mV by continuous current injection and their firing behavior was tested by applying depolarizing current pulses. Signals were filtered at 5 kHz, digitized at 10 kHz, and analyzed off-line with Clampfit 9.2 software (Molecular Devices). At the end of the whole-cell recording, the cell cytoplasm was aspirated into the recording pipette while maintaining a tight seal. The pipette was removed delicately to allow outside-out patch formation. The content of the pipette was expelled into a test tube and reverse transcription (RT) was performed in a final volume of 10 μL as described previously (Lambolez et al. 1992). Next, 2 steps of polymerase chain reaction (PCR) were performed essentially as described (Cauli et al. 1997). The cDNAs present in 10 μL of the RT reaction first were amplified simultaneously using the primer pairs described in Supplementary Table 1 (for each primer pair, the sense and antisense primers were positioned on 2 different exons). Taq polymerase (2.5 U; Qiagen) and 20 pmol of each primer were added to the buffer supplied by the manufacturer (final volume, 100 μL), and 21 cycles (94 °C for 30 s, 60 °C for 30 s, and 72 °C for 35 s) of PCR were run. Second rounds of PCR were performed using 2 μL of the first PCR product as template. In this second round, each cDNA was amplified individually with a second set of primer pairs internal to the first PCR primer pair (nested primers; see Supplementary Table 1). Thirty-five PCR cycles were performed (as described). Then 10 μL of each individual PCR were run on a 2% agarose gel, in parallel with molecular weight markers and stained with ethidium bromide. The RT-PCR protocol was tested on 500 pg of total RNA purified from rat neocortex, and all the transcripts were detected. Sizes of the PCR-generated fragments were as predicted by the messenger RNA (mRNA) sequences (see Supplementary Table 1). A control for mRNA contamination from surrounding tissue was performed by placing a patch pipette into the slice without establishing a seal. Positive pressure was then interrupted and, following the removal of the pipette, its content was processed as described. No PCR product was obtained using this protocol (n = 20).
Drugs
VIP, CRF, PACAP-38, and PACAP6-38 were purchased from Bachem. Astressin was obtained from Tocris. PG97-269 was kindly provided by Dr Patrick Robberecht (Université Libre de Bruxelles, Brussels, Belgium). Maxadilan was a kind gift from Dr Ethan Lerner (Massachusetts General Hospital, Charlestown, MA). All other drugs were from Sigma. Stock solution was prepared and stored as follows: 250 μM VIP, 250 μM CRF, 250 μM PACAP-38, 250 μM PACAP6-38, 250 μM Maxadilan, 250 μM PG97-269, and 250 μM Astressin were prepared in H2O and stored at −80 °C. Solutions of 200 mM 3-isobutyl-1-methyl-xanthine (IBMX) and 25 mM forskolin were prepared in dimethyl sulfoxide and stored at −20 °C. On the day of use, drugs were diluted in ACSF to their working concentration and applied on brain slices through the continuous bath perfusion. VIP and CRF dose–response histograms were obtained using single applications of either peptide per slice.
Statistics
In this report, N represents the number of brain slices tested, while n represents the number of cells on which measurements were performed. Values are expressed as mean ± standard error of the mean. Statistical significance was assessed with Student's t-test. P value <0.05 was considered statistically significant.
Results
VIP and CRF Elicit Different cAMP/PKA Responses in Pyramidal Neurons
We used recombinant Sindbis viruses to express the PKA (AKAR2) and cAMP (Epac1-camps) sensors in neurons of neocortical slices (see Materials and Methods). Expression of the sensors was primarily observed in layers II/III and V pyramidal cells, consistent with previous reports (Lendvai et al. 2000; Furuta et al. 2001; Drobac et al. 2010). Both sensors were seen in the somatic and dendritic cytosol. In contrast with AKAR2, Epac1-camps was excluded from the nucleus (Figs 1 and 4). Optical recordings were performed in layers II/III and V, by measuring the F535/F480 (AKAR2) or F480/F535 (Epac1-camps) fluorescence emission ratio of the sensors.
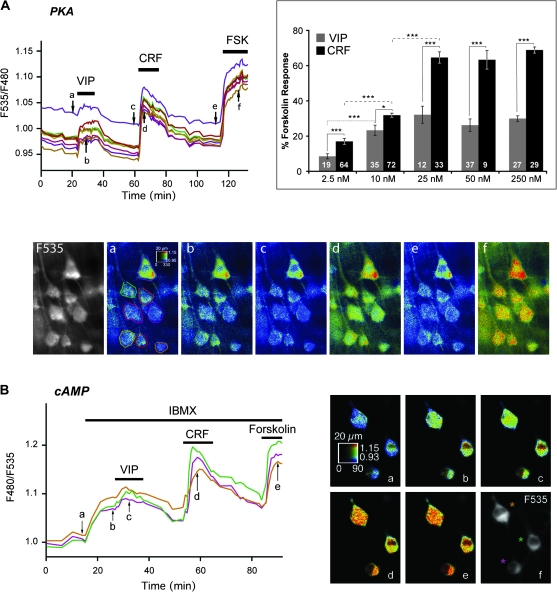
Figure 1.
VIP and CRF elicit different cAMP/PKA responses in pyramidal neurons. (A) PKA activation in pyramidal cells expressing the AKAR2 probe to VIP (250 nM), CRF (250 nM), and forskolin (FSK, 13 μM). Traces show average F535/F480 emission ratios measured at the soma of individual pyramidal neurons delineated on pseudocolor image a. Arrows indicate time points corresponding to pseudocolor images below. Pseudocolor images show the ratio value (coded by the pixel hue) and the intensity of the fluorescence (coded by the pixel intensity), on the scale indicated by the calibration square (20 μm wide). Grayscale image shows the 535 nm fluorescence emission. Inset: VIP and CRF dose–response histograms. Data are expressed in percent of the response to FSK. Numbers of recorded neurons are indicated in each bar (statistical significance: ***P < 0.0001; *P < 0.05). (B) cAMP elevation in pyramidal cells expressing Epac1-camps in response to VIP (250 nM), CRF (250 nM), and FSK (13 μM) in the presence of IBMX (200 μM). Traces show average F480/F535 emission ratios measured at the soma of individual neurons shown on the grayscale image. Arrows indicate time points corresponding to pseudocolor images. Note that cAMP/PKA responses to CRF declined during peptide application.
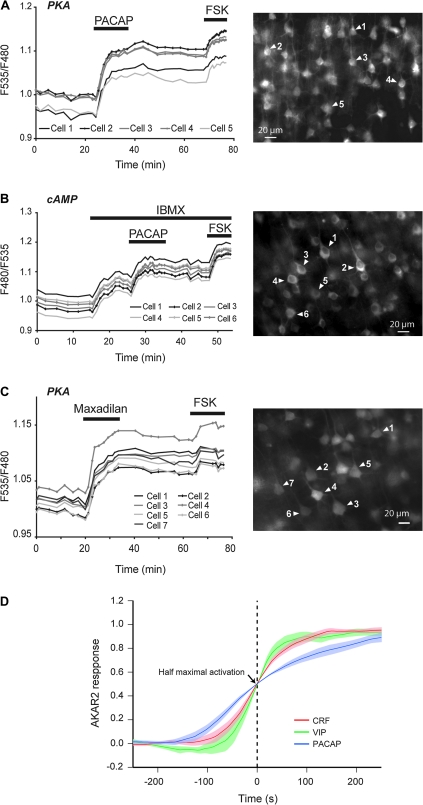
Figure 4.
PACAP elicits persistent cAMP/PKA responses in pyramidal neurons via PAC1 receptors. (A–C) Responses of pyramidal cells expressing AKAR2 (PKA) or EPAC1-camps (cAMP) probes to PACAP (250 nM), to the PAC1 agonist Maxadilan (250 nM), and to FSK (13 μM). Variations of cAMP levels were recorded in the presence IBMX (200 μM). Recorded neurons are indicated by arrowheads on grayscale images showing the 535 nm fluorescence emission (right panels). Note that PACAP and Maxadilan had similar effects and that responses to both agonists persisted after peptide washout. (D) Traces show mean (solid lines) ± standard error of the mean (color shades) onset of AKAR2 responses to 250 nM VIP, CRF, and PACAP. For each experiment, traces recorded for individual neurons were normalized to the maximal response and then averaged. The zero time point was defined as half-maximal activation, and the averaged traces obtained from individual experiments were averaged between different experiments. Note the slow onset of PACAP responses.
Application of 250 nM VIP or CRF produced a reversible increase of the F535/F480 emission ratio of the PKA sensor (Fig. 1A). Responses reached a maximum 4.3 ± 0.7 min (VIP) and 4.1 ± 0.4 min (CRF) after the beginning of peptide application and returned to baseline over 20 min during washout of the peptides. VIP elicited tonic responses that persisted in the presence of the peptide. In contrast, CRF elicited inactivating responses, which declined rapidly during the application of the peptide. Peptidergic responses were quantified by comparison with the response to a maximally effective concentration of the adenylate cyclase (AC) activator forskolin (13 μM; Gervasi et al. 2007). Forskolin elicited a larger increase of the F535/F480 ratio than VIP and CRF, showing that peptidergic responses were within the dynamic range of the sensor. Responses to 250 nM CRF (69.0 ± 1.7% of the forskolin response; N = 4 slices, n = 29 cells) had a significantly larger amplitude than responses to 250 nM VIP (30.0 ± 1.5% of the forskolin response; N = 5 slices, n = 27 cells P < 0.0001). These results are summarized in Table 1. Switching the order of peptide application did not affect the shape and relative amplitude of the responses (not shown, N = 5 slices, n = 22) indicating that the response to either peptide was fully reversible and did not affect the response to the second peptide. VIP and CRF responses were observed in all recorded cells; this suggests that all pyramidal neurons coexpress VIP and CRF receptors, consistent with an earlier report on CRF effects in the neocortex (Gallopin et al. 2006). The same protocol was used on cortical slices transduced with AKAR2mut, a control probe that cannot be phosphorylated by PKA on its consensus site (Zhang et al. 2005, Gervasi et al. 2007). No change of the F535/F480 ratio was observed in AKAR2mut-expressing cells upon VIP, CRF, or forskolin application (not shown, N = 3, n = 7), confirming that the peptide responses were dependent on AKAR2 phosphorylation by PKA. VIP and CRF dose–response histograms (Fig. 1A, inset) show that responses were consistently obtained upon application of 2.5 nM peptide and reached a maximum at 25 nM peptide. CRF responses were significantly larger than VIP responses at each concentration tested (see Fig. 1A). Hence, although both peptides act at nanomolar concentrations, CRF is a more potent activator of PKA in pyramidal neurons than VIP. The rapid decline of the CRF response, consistently observed in the continued presence of 250 nM peptide (Fig. 1A), did not occur when the CRF concentration was 50 nM or below (not shown). Application of 100 nM CRF also elicited tonic responses, except in 1 of 5 slices tested where inactivating responses were observed (not shown). Tonic responses were observed at all VIP concentrations tested.
Table 1.
Summary of VIP, CRF, and PACAP effects on neocortical pyramidal neurons
| Compartment | Measurement method | VIP | CRF | PACAP | ||
| PKA activity | Cytosol | AKAR2 | %FSK time course | 30.0 ± 1.5 tonic | 69.0 ± 1.7 decaying | 75.5 ± 1.3 persistent |
| Nucleus | AKAR2-NLS | %FSK time course | Not detected | 46.3 ± 3.4 transient | 47.2 ± 3.4 persistent | |
| Membrane | Inhibition of membrane current | %FSK onset | 64 ± 5 fast | 91 ± 1 fast | 87 ± 5 slow | |
| cAMP production | Cytosol | EPAC1-camps | %FSK time course | 14.1 ± 1.8 undetermined | 66.1 ± 3.9 decaying | 50.0 ± 1.4 persistent |
Variations of cAMP levels in response to peptidergic stimulation were next examined using the Epac1-camps sensor (Nikolaev et al. 2004). Under normal conditions, application of 250 nM VIP or CRF resulted in barely detectable responses of the cAMP sensor, while forskolin elicited only a small increase of 6.3 ± 0.6% from baseline (N = 3, n = 6; data not shown), consistent with levels of free cAMP remaining in the submicromolar range (Castro et al. 2010). We thus blocked cAMP degradation to reveal variations of cAMP concentration in response to neuropeptides. Application of the phosphodiesterase inhibitor IBMX (200 μM) increased the F480/F535 ratio by 6.6 ± 0.7% (N = 5, n = 41), indicating that under basal conditions, cAMP is continuously produced and degraded (Fig. 1B). In the presence of IBMX, application of 250 nM CRF or VIP elicited rapid and reversible F480/F535 ratio changes (Fig. 1B). Responses to 250 nM CRF exhibited a marked decline in the presence of the peptide similar to that observed with the PKA probe. This decrease of cAMP in presence of IBMX suggests that either IBMX did not block all the phosphodiesterases or/and cAMP is extruded from cells by cyclic nucleotide transporters (Sassi et al. 2008). Responses to CRF and VIP reached 66.1 ± 3.9% and 14.1 ± 1.8% of the forskolin response, respectively (N = 6, n = 34, significantly different with P < 0.0001). These results, summarized in Table 1, suggest that in pyramidal neurons, CRF is a more potent activator of cAMP signaling than VIP, consistent with the respective potency of these peptides in stimulating PKA.
Receptors Involved in cAMP/PKA Responses to VIP and CRF
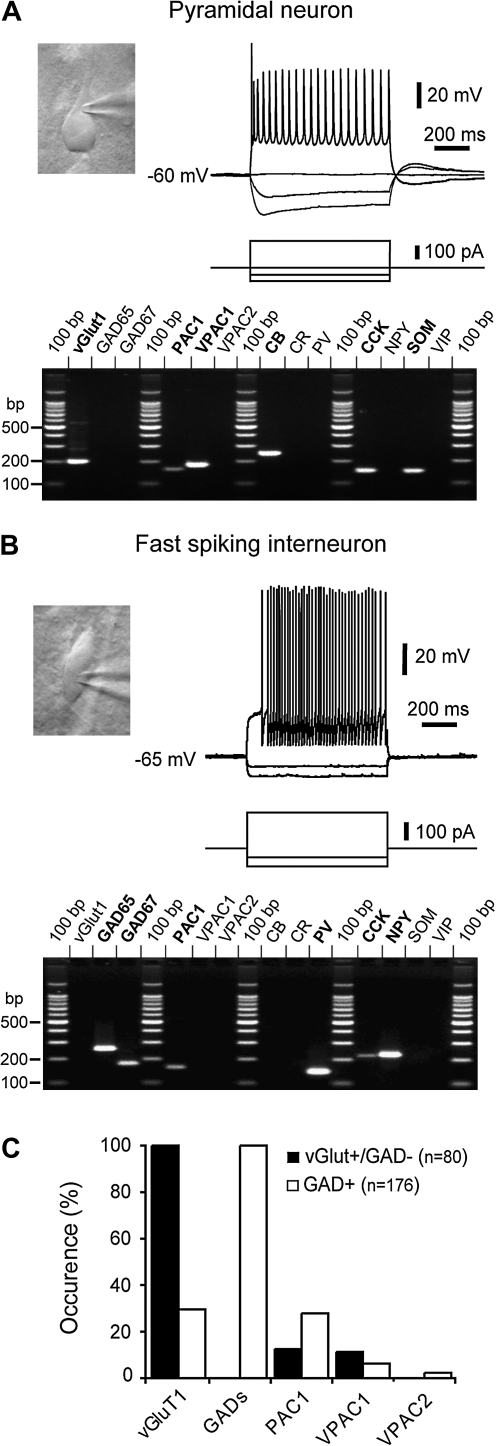
While it is known that the neocortical effects of CRF are mediated by CRF-1 receptors primarily expressed in pyramidal cells (Gallopin et al. 2006), the expression pattern of VIP receptor subtypes in neocortical neurons is largely uncharacterized. The expression of VIP receptors (VPAC1, VPAC2, and PAC1) was investigated in neocortical neurons (n = 256) from layers I–V using scPCR. In addition to VIP receptors, expression of 10 molecular markers commonly used to define subtypes of neocortical neurons was probed by scPCR: the vesicular glutamate transporter (vGluT1); GABA-synthesizing enzymes (glutamic acid decarboxylase, GAD 65 and GAD 67); calcium-binding proteins calretinin, calbindin (CB), and parvalbumin (PV); and neuropeptides VIP, somatostatin (SOM), cholecystokinin (CCK), and neuropeptide Y (NPY). Pyramidal cells (n = 80) and interneurons (n = 176) were selected according to their somatodendritic appearance and action potential firing patterns in response to depolarizing current pulses. Their identity was confirmed by the presence of vGluT1 and the absence of GAD in pyramidal cells (vGluT1+/GAD−) and the presence of GAD in interneurons (GAD+). Expression of the vGluT1 mRNA was detected in 30% of GABAergic interneurons (n = 52 of 176), consistent with its reported expression in several GABAergic cell types throughout the brain (Danik et al. 2005; Gallopin et al. 2006; Karagiannis et al. 2009). An example of a pyramidal neuron expressing VIP receptors is shown in Figure 2A. This neuron exhibited a piriform soma extending a prominent apical dendrite, a reduction of action potential amplitude, and frequency along the discharge typical of pyramidal neurons (Peters and Jones 1984; Connors and Gutnick 1990). In addition to vGluT1, PAC1 and VPAC1 mRNAs were detected in this neuron but not VPAC2. Expression of CB, CCK, and SOM was also detected in this neuron. The vertical fusiform interneuron showed in Figure 2B fired short-duration action potentials with large afterhyperpolarizing potentials at a sustained high frequency, typical of fast-spiking interneurons (Kawaguchi and Kubota 1993; Cauli et al. 1997). This GAD+ neuron expressed PAC1 but did not have detectable levels of VPAC1 or VPAC2 mRNAs. In addition to PV, characteristically expressed in fast-spiking interneurons (Kawaguchi and Kubota 1993; Cauli et al. 1997), CCK and NPY mRNAs were also detected in this Fast Spiking neuron. In our sample of 80 pyramidal neurons, the PAC1 mRNA was detected in 10 cells (13%) and VPAC1 in 9 cells (11%) (Fig. 2C). In GABAergic interneurons, the occurrence of PAC1 (28%, n = 49 of 176) was higher than that of VPAC1, which was detected in only 11 of 176 cells (6%). Expression of PAC1 and VPAC1 was not preferentially associated with any of the molecular markers of interneuron diversity (not shown). The rare occurrence of the VPAC2 mRNA, detected in only 4 GABAergic interneurons and never in pyramidal cells, is consistent with its very low expression level in the neocortex (Usdin et al. 1994; Sheward et al. 1995). These results indicate that PAC1 and/or VPAC1 potentially mediate the effects of VIP on pyramidal cells.
Figure 2.
Expression of VIP receptors in identified neocortical neurons. (A) Electrophysiological and scPCR analysis of a neuron visually selected under infrared video microscopy. This neuron exhibited a piriform soma with a prominent apical dendrite (upper left) and a reduction of action potential amplitude and frequency along the discharge (upper right) typical of pyramidal neurons. Expression of vGlut1, PAC1, VPAC1, CB, CCK, and SOM mRNAs was detected in this neuron upon agarose gel analysis of scPCR products (lower panel) run in parallel with 100 bp DNA ladder as a molecular weight marker. (B) This vertical fusiform interneuron fired short-duration action potentials with large afterhyperpolarizing potentials at a sustained high frequency, typical of fast-spiking interneurons. This neuron expressed GAD65, GAD67, PAC1, PV, CCK, and NPY mRNAs. (C) Expression of VIP receptors in pyramidal neurons (vGluT+/GAD−) and interneurons (GAD+).
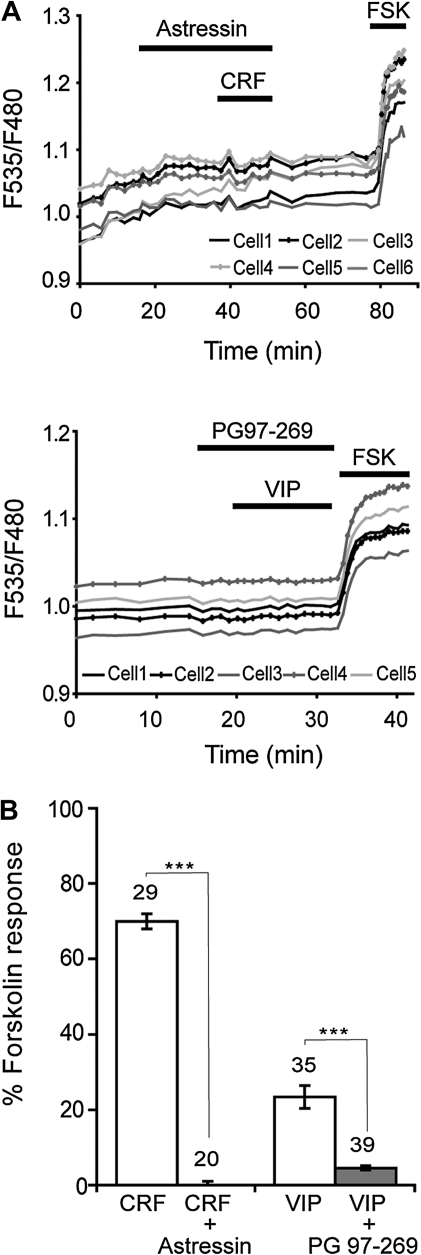
The involvement of CRF-1 and VPAC1 receptors in mediating the cAMP/PKA responses to CRF and VIP was examined using specific antagonists (Fig. 3). The CRF receptor antagonist Astressin (500 nM) prevented the AKAR2 response to 250 nM CRF (N = 5, n= 44), confirming the reported involvement of CRF-1 in neocortical CRF effects (Gallopin et al. 2006). In the presence of PG97-269 (200 nM), a specific VPAC1 antagonist (Gourlet et al. 1997), the AKAR2 response to 10 nM VIP reached only 4.6 ± 0.5% of the forskolin response (N = 4, n = 39), corresponding to an 80% reduction of the response in the absence of the VPAC1 antagonist (see above). These results indicate that responses of pyramidal cells to VIP are primarily mediated by VPAC1 receptors. Nonetheless, the PG97-269-resistant fraction of the VIP response (20%) suggests a possible contribution of PAC1 receptors. It is noteworthy that although VPAC1 mRNAs were only detected in a subset of the pyramidal cells analyzed by scPCR, cAMP/PKA responses to VIP were observed in all recorded cells, suggesting that virtually all neocortical pyramidal neurons express VPAC1.
Figure 3.
Pharmacology of VIP and CRF responses. (A) Upper: responses of individual pyramidal cells expressing AKAR2 to CRF (250 nM) were prevented by the CRF receptor antagonist Astressin (500 nM). Lower: Responses to VIP (10 nM) were prevented by the VPAC1 antagonist PG97-269 (200 nM). Application of forskolin (13 μM) provides control for responsiveness of AKAR2 and allowed for the quantification of peptidergic responses. (B) Responses to CRF (250 nM) were abolished by the CRF receptor antagonist Astressin (500 nM). Responses to VIP (10 nM) were largely reduced by the VPAC1 antagonist PG97-269 (200 nM). Antagonists were applied at least 5 min prior to agonist application. Numbers of tested cells and statistical significance are indicated (***P < 0.0001).
PACAP Elicits a Slow Long-Lasting cAMP/PKA Response via PAC1 Receptors
PACAP is the preferential agonist at PAC1 receptors, to which VIP binds with a lower affinity (Vaudry et al. 2009). We investigated the distribution of PACAP in neocortical neurons by combining in situ hybridization and scPCR. PACAP was sparsely observed in neocortical neurons; primarily in layer V pyramidal cells (see Supplementary Results), in agreement with earlier reports (Koves et al. 1994; Hannibal 2002; Stumm et al. 2007).
Optical recordings with the AKAR2 probe showed that PACAP is a potent activator of PKA in pyramidal neurons (Fig. 4A). Indeed, responses to 10, 50, and 250 nM PACAP reached 24.3 ± 1.6% (N = 3, n = 21), 46.9 ± 1.0% (N = 4, n = 40), and 75.5 ± 1.3% (N = 5, n = 50) of the forskolin response, respectively. The mean amplitude of AKAR2 responses to 250 nM PACAP was similar to that obtained with 250 nM CRF (69.0 ± 1.7%, see above) and was 2.5-fold greater than responses to 250 nM VIP (significantly different with P < 0.0001). While maximal responses to VIP and CRF were reached at a concentration of 25 nM (see Fig. 1), responses to 50 nM PACAP were submaximal, suggesting a lower apparent affinity of PACAP than of VIP and CRF to their cognate receptors in our experimental conditions. At all doses tested, the kinetics of PACAP responses markedly differed from those of VIP and CRF. As seen on averaged and normalized traces in Figure 4D, the onset of PACAP responses was slower than those of VIP and CRF responses. Furthermore, PACAP elicited persistent responses that remained roughly stable for at least 30 min after peptide washout (Fig. 4A). Variations of the cAMP levels in response to PACAP (250 nM) were next investigated in the presence of IBMX (200 μM) using the Epac1-camps probe. Consistent with its effects on PKA activity, PACAP elicited a slow response of the cAMP sensor, which reached 50.0 ± 1.4% of the forskolin effect (N = 4, n = 41) and persisted after peptide washout (Fig. 4B). These results are summarized in Table 1. Application of the specific PAC1 antagonist PACAP6-38 (500 nM) did not detectably reduce the response to 10 nM PACAP (N = 2, n = 20; not shown). Conversely, application of the specific PAC1 agonist maxadilan (250 nM) elicited AKAR2 responses whose amplitude (73.5 ± 1.9%, N = 7; n = 87), onset kinetics (maximum was reached in ∼5 min), and persistence after agonist washout (see example in Fig. 4C) were similar to the PACAP-induced responses. On the other hand, the VPAC1 antagonist PG97-269 only slightly reduced PACAP responses, suggesting only a minor contribution of VPAC1 to the cAMP/PKA response. Indeed, in the presence of PG97-269 (200 nM) responses to 50 nM PACAP reached 41.8 ± 0.9% of the forskolin effect (N = 4, n = 40), corresponding to a 5% reduction of the control response (see above). These results indicate that, in the neocortex, PACAP induces a robust and persistent activation of the cAMP/PKA pathway by acting mainly through PAC1 receptors, consistent with an earlier report (Tatsuno et al. 2001). Responses to PACAP and maxadilan were observed in all recorded cells, suggesting that virtually all neocortical pyramidal neurons express PAC1.
Nuclear PKA Responses to VIP, CRF, and PACAP
Translocation of active PKA to the nucleus activates RNA transcription. We thus recorded neuropeptide effects on PKA activity in the nuclei of pyramidal cells using the AKAR2 sensor tagged with a nuclear localization signal (AKAR2-NLS; Zhang et al. 2005). No response of the AKAR2-NLS probe was observed upon application of 250 nM VIP (N = 6, n = 60; Supplementary Fig. 2). In contrast, CRF (250 nM) and PACAP (250 nM) elicited robust nuclear PKA responses, the mean amplitudes of which were 46.3 ± 3.4% (N = 4, n = 26) and 47.2 ± 3.4% (N = 4; n = 40) of the forskolin response, respectively (Supplementary Fig. 2). Nuclear responses, which reached a maximum in 21.1 ± 4.1 min for CRF and 17.3 ± 1.8 min for PACAP, were significantly slower than the cytosolic responses to these peptides (P < 0.05). CRF responses were reversible, while PACAP responses remained stable for at least 40 min after peptide washout, consistent with our observations with the nontargeted AKAR2 probe. Hence, nuclear PKA dynamics appeared to reflect the differential cytosolic effects of the neuropeptides, but with smaller amplitude and slower time-course, in agreement with previous reports (Zhang et al. 2001; DiPilato et al. 2004; Gervasi et al. 2007). These results are summarized in Table 1.
VIP, CRF, and PACAP Differentially Inhibit a Membrane Potassium Conductance
The apamin-insensitive slow afterhyperpolarization is mediated by a calcium-dependent potassium current (IsAHP) activated by a train of action potentials. IsAHP regulates the excitability of cortical pyramidal neurons by inducing a long-lasting (∼5 s) membrane hyperpolarization that prevents tonic firing (McCormick and Prince 1986; McCormick and Williamson 1989). This transient current is associated with a tonic potassium current (tonic IK), and both currents are inhibited upon PKA activation (Pedarzani and Storm 1995; Lancaster and Batchelor 2000; Goaillard and Vincent 2002; Lancaster et al. 2006; Gervasi et al. 2007; Castro et al. 2010). We thus used IsAHP and tonic IK as physiological reporters of PKA activity to compare the effects of VIP, CRF, and PACAP in neocortical pyramidal cells from acute slices.
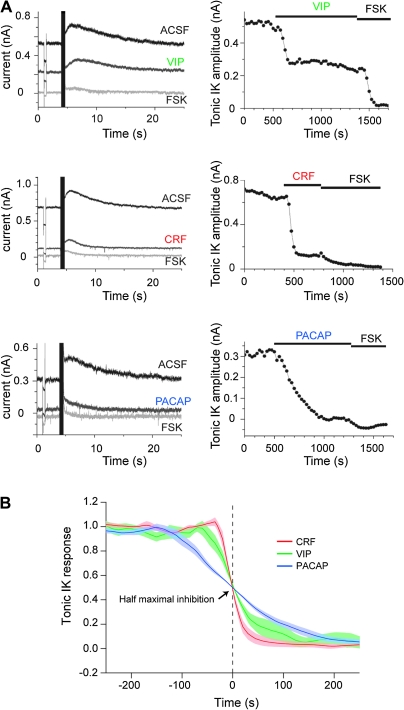
We used 5,5-dibromo-BAPTA as an intracellular calcium buffer, which reportedly increases the magnitude of both IsAHP and tonic IK in hippocampal and cortical pyramidal neurons (Lancaster and Batchelor 2000; Lancaster et al. 2006; Castro et al. 2010). We first verified that substituting 5,5-dibromo-BAPTA for EGTA in the internal solution potentiated IsAHP and tonic IK in neocortical pyramidal cells while maintaining their sensitivity to PKA (Supplementary Fig. 3). Indeed, IsAHP increased from 127 ± 11 pA (n = 24) with 1 mM EGTA to 535 ± 42 pA (n = 36) with 1 mM dibromo-BAPTA, while tonic IK increased from 125 ± 9 pA (n = 24) with EGTA to 635 ± 27 pA (n = 36) with dibromo-BAPTA. IsAHP and tonic IK were simultaneously suppressed by application of forskolin (13 μM) in both EGTA and dibromo-BAPTA conditions (Supplementary Fig. 3), confirming their sensitivity to PKA phosphorylation. The SK channel antagonist Apamin (100 nM) had no effect on these currents when recorded in dibromo-BAPTA conditions (n = 4), in agreement with earlier reports (Lancaster and Adams 1986; Lorenzon and Foehring 1993; Castro et al. 2010). Dibromo-BAPTA was thus used in all subsequent experiments aimed at comparing the effects of neuropeptides on sAHP currents. As shown in Figure 5A, application of VIP, CRF, and PACAP (250 nM) markedly reduced the amplitude of both IsAHP and tonic IK, and addition of 13 μM forskolin following peptide washout virtually abolished the remaining current. These results indicate that all 3 peptides exert excitatory effects on neocortical pyramidal cells by inhibiting the tonic IK and phasic components of the sAHP, as reported in other structures (Haug and Storm 2000). However, the responses to the 3 neuropeptides differed in amplitude and onset kinetics. VIP elicited a smaller reduction of tonic IK amplitude (64 ± 5%, n = 8) than CRF (91 ± 2%, n =5) and PACAP (87 ± 5%, n = 7). Conversely, the onset kinetics of PACAP effects was far slower than those of VIP and CRF effects. Indeed, the time to reach half-maximal inhibition of tonic IK was 165 ± 28 s (n = 5) for PACAP versus 68 ± 4 s (n = 8) and 50 ± 10 s (n = 5) for VIP and CRF, respectively. This is illustrated in Figure 5B showing averaged time-course of tonic IK inhibition by the 3 neuropeptides. Hence, the relative amplitude and onset kinetics of the 3 neuropeptide effects on the sAHP membrane conductance recorded in acute slices were in excellent agreement with their relative effects on cytosolic PKA dynamics recorded in slices transduced with the AKAR2 probe (see Table 1). These results indicate that the different effects of the 3 neuropeptides on cAMP/PKA dynamics in neocortical pyramidal cells effectively result in differential modulation of the membrane excitability of these neurons.
Figure 5.
Neuropeptide-specific inhibition of the IsAHP tonic IK in pyramidal cells. (A) Effects of neuropeptides (250 nM) on the phasic IsAHP activated by a train of action potentials (left panels) and on the holding current (tonic IK, plotted in right panels) were recorded in voltage-clamp mode at a holding potential of −67 mV. Neuropeptides concomitantly reduced the amplitude of IsAHP and tonic IK as compared with control conditions (ACSF). Subsequent forskolin application (FSK, 13 μM) further reduced the amplitude of both IsAHP and tonic IK. VIP elicited a smaller reduction of the slow AHP amplitude than CRF and PACAP. Conversely, the onset of PACAP effects was slower than those of VIP and CRF effects. (B) Traces show mean (solid lines) ± standard error of the mean (color shades) onset of tonic IK inhibition in response to 250 nM of VIP, CRF, or PACAP. Individual traces were normalized to the maximal tonic IK. Zero time point was defined as the point where the inhibition was 50%. Note the slow onset of the PACAP effect.
Discussion
Responses of the cAMP/PKA pathway to VIP, CRF, and PACAP were studied in neocortical slices. VIP elicited a weaker stimulation of cAMP/PKA signaling in pyramidal neurons than CRF and PACAP. Responses to VIP and CRF followed the time-course of peptide application, whereas responses to PACAP had a slow onset and persisted after peptide washout. The effects of VIP, CRF, and PACAP were mediated by distinct receptors, VPAC1, CRF-1, and PAC1, respectively. The relative amplitude and kinetics of the 3 neuropeptide effects on the PKA-sensitive sAHP current in pyramidal neurons were similar to those observed on cytosolic cAMP/PKA dynamics. An increase of nuclear PKA activity was observed in pyramidal cells in response to CRF and PACAP but not to VIP. Our results suggest that VIP and CRF, originating from interneurons, and PACAP, expressed mainly by layer V pyramidal cells, differentially activate cAMP/PKA signaling to modulate the excitability and transcription program of neocortical neurons.
VIP, CRF, and PACAP Act at Distinct Receptors to Elicit Different cAMP/PKA Responses
Converging results using optical recordings of the cAMP and PKA probes and electrophysiological recordings of the PKA-sensitive sAHP indicate that VIP, CRF, and PACAP all activate the cAMP/PKA pathway in neocortical pyramidal neurons, as observed in multiple cell types (Dautzenberg and Hauger 2002; Vaudry et al. 2009). Furthermore, the good agreement between peptidergic responses in acute slices (sAHP) and in virally transduced slices (optical sensors) indicates that receptor-coupling and cAMP/PKA signaling were minimally affected by the expression of the sensors, consistent with our earlier observations (Gervasi et al. 2007, Castro et al. 2010).
Our Astressin results (Fig. 3) confirm that the effects of CRF on neocortical neurons are mediated by the CRF-1 receptor (Gallopin et al. 2006); the other receptor, CRF-2, is virtually absent from the neocortex (Chalmers et al. 1995; Van Pett et al. 2000). VIP and PACAP could potentially act through common receptors, with binding affinities PACAP>>VIP at PAC1 and PACAP = VIP at VPAC1 receptors (Harmar et al. 1998; Vaudry et al. 2009). However, the relative potencies of these peptides depend on alternative splicing and second messenger coupling of these receptors (Ushiyama et al. 2007), for which few selective ligands exist (Laburthe et al. 2007). We found that the VIP and PACAP effects on cAMP/PKA dynamics in neocortical pyramidal cells markedly differ and are primarily mediated by distinct receptors, VPAC1 and PAC1, respectively. Indeed, both onset and decay kinetics of VIP and PACAP responses differed markedly, supporting the involvement of distinct receptors in our experimental conditions. Furthermore, most of the VIP effect was blocked by the specific VPAC1 antagonist PG97-269, while the effect of PACAP was mimicked by the specific PAC1 agonist maxadilan and only slightly reduced by PG97-269. The PAC1 antagonist PACAP6-38 failed to prevent the PACAP response. This discrepancy with our maxadilan results is presumably due to the tissue dependence of PACAP6-38 effects, which can require a higher molar excess than used in the present study to antagonize PACAP responses (e.g., Reglodi et al. 2008; Costa et al. 2009).
The different CRF, PACAP, and VIP responses in neocortical pyramidal neurons suggest that properties of their cognate receptors differ, although other possibilities, such as different integration within local signaling microdomains or subcellular localization, may account for some of our observations. The rapid onset of CRF and VIP responses suggests that CRF-1 and VPAC1 are tightly coupled to AC, perhaps within signaling microdomains (Martin and Cooper 2006; Jarnaess and Tasken 2007), whereas the slow onset of PACAP responses suggests a loose coupling of PAC1 to AC. The decay of CRF and VIP responses upon agonist washout suggests that the activated forms of CRF-1 and VPAC1 are short lived. The rapid decline of CRF responses at supramaximal neuropeptide concentration, at which VIP responses remain tonic, suggests that CRF-1 can undergo desensitization, as earlier reported (Hauger et al. 2000; Reyes et al. 2006). In contrast, the persistence of PACAP responses after peptide washout indicates that the activated form of PAC1 is long lasting. Interestingly, similar persistent cAMP signaling has been recently shown to occur following internalization of hormone G-protein–coupled receptors (Calebiro et al. 2009; Ferrandon et al. 2009). This may indicate that agonist-bound PAC1 continues signaling following internalization in pyramidal neurons, perhaps through activation of AC in the endosomes (Calebiro et al. 2010). Nonetheless, inhibition of phosphatases may also account for the long-lasting effects of PACAP (Ansanay et al. 1995). Finally, while the large amplitude of PACAP responses can be explained by the persistence of activated PAC1, the relative amplitude of CRF and VIP responses suggests that CRF-1 is present at a higher density than VPAC1 at the plasma membrane of pyramidal cells.
We found that CRF and PACAP efficiently induced translocation of active PKA to the nucleus, consistent with their effects on gene expression (Peeters et al. 2004; Eiden et al. 2008). In contrast, no effect of VIP was observed on nuclear PKA activity, suggesting that VPAC1 activation has minimal transcriptional effects in neocortical pyramidal cells. If the amplitude of nuclear responses were linearly related to that of cytosolic responses, VIP would have produced a small but detectable response. This suggests that a PKA activity threshold must be reached in the cytosol in order to propagate the signal into the nucleus. VIP is known to regulate gene expression through the cAMP/PKA pathway in cultured cortical neurons (Vaccarino et al. 1993; Martin et al. 1995). In these latter studies, however, the effective VIP concentrations were ∼100 times those of PACAP, suggesting an involvement of PAC1 in these VIP transcriptional effects.
Peptidergic Control of cAMP/PKA-Mediated Effects on the Neocortical Network
VIP is specifically expressed in a distinct neocortical interneuron type. In contrast CRF has a dual origin: it is coexpressed in VIP-containing interneurons and in SOM-containing interneurons (Gallopin et al. 2006). Conversely, PACAP is expressed in glutamatergic neurons (Koves et al. 1994; Hannibal 2002; Stumm et al. 2007), among which layer V pyramidal cells appear as a major source of this neuropeptide. This suggests that the 3 neuropeptides are differentially released upon activation of the local network. Distinctive release sites (dendrite vs. axon terminal) and diffusion distance (Zupanc 1996; Merighi 2002; Baraban and Tallent 2004) may further differentiate the 3 peptides.
While VPAC1 and PAC1 mRNA expression was only detected in a minority of pyramidal cells by scPCR, our optical and electrophysiological recordings indicated functional expression of these receptors in all recorded cells. This, together with earlier observations on neuropeptide receptors, including CRF-1, in the neocortex (Gallopin et al. 2006; Ferezou et al. 2007) suggests that our scPCR has a relatively low sensitivity compared with our physiological recordings. This presumably relates to the low abundance of neuropeptide receptor mRNAs, as extensively discussed by Gallopin et al. (2006). The present results thus suggest that PACAP released from glutamatergic neurons acts widely on both pyramidal cells and interneurons. In contrast, the effects of CRF are essentially restricted to pyramidal neurons (Gallopin et al. 2006). The lower occurrence of VPAC1 mRNA observed in our sample of interneurons may indicate that VIP preferentially targets pyramidal cells.
The present results indicate that coordinated effects of the 3 neuropeptides may shape cAMP/PKA-mediated responses of the neocortical network. While the tonic VIP response presumably reflects the duration and intensity of VIP release, corelease of CRF may largely enhance the initial phase of the response, before the CRF contribution decreases because of CRF-1 inactivation. In contrast, the contribution of PACAP has a slow onset but is likely to prolong the cAMP/PKA-mediated effects far beyond the clearance of neuropeptides. Interestingly, exogenous CRF and PACAP are more potent activators of neocortical cAMP/PKA signaling than norepinephrin or dopamine, whose effects are intermediate between those of CRF/PACAP and those of VIP (Vincent et al. 2008). However, it is likely that the slow release and diffusion of neuropeptides, the action of peptidases (Zupanc 1996; Baraban and Tallent 2004), and the discrete distribution of their sources limit the amplitude and spatial extent of endogenous VIP, CRF, and PACAP effects. Conversely, each of these neuropeptides exhibits a distinctive pattern of sources, targets, and effects. This may allow the 3 neuropeptides to elicit coordinated, time- and space-defined, effects on neocortical excitability and gene expression that are finely adjusted to local network activity.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
Centre National de la Recherche Scientifique (CNRS), Université Pierre et Marie Curie-P6; Human Frontier Science Program (RGY0070/2007); CNRS (“Nitrex”); Ecole des Neurosciences de Paris (“Network for Viral Transfer”); National Institutes of Health (NS17323 to R.H-W.).
Acknowledgments
We thank Rosine Wehrle, Bhaskar Saha, and Ludovic Tricoire for their valuable assistance. Conflict of Interest: None declared.
References
- Ansanay H, Dumuis A, Sebben M, Bockaert J, Fagni L. cAMP-dependent, long-lasting inhibition of a K+ current in mammalian neurons. Proc Natl Acad Sci U S A. 1995;92:6635–6639. doi: 10.1073/pnas.92.14.6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban S, Tallent M. Interneuron diversity series: neuropeptides-endogenous regulators of neuronal excitability. Trends Neurosci. 2004;27:135–142. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:1–25. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Persani L, Lohse MJ. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci. 2010;31:221–228. doi: 10.1016/j.tips.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Castro LR, Gervasi N, Guiot E, Cavellini L, Nikolaev VO, Paupardin-Tritsch D, Vincent P. Type 4 phosphodiesterase plays different integrating roles in different cellular domains in pyramidal cortical neurons. J Neurosci. 2010;30:6143–6151. doi: 10.1523/JNEUROSCI.5851-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo M, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Porter J, Tsuzuki K, Lambolez B, Rossier J, Quenet B, Audinat E. Classification of fusiform neocortical interneurons based on unsupervised clustering. Proc Natl Acad Sci U S A. 2000;97:6144–6149. doi: 10.1073/pnas.97.11.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers D, Lovenberg T, De Souza E. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Costa L, Santangelo F, Li Volsi G, Ciranna L. Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus. Hippocampus. 2009;19:99–109. doi: 10.1002/hipo.20488. [DOI] [PubMed] [Google Scholar]
- Danik M, Cassoly E, Manseau F, Sotty F, Mouginot D, Williams S. Frequent coexpression of the vesicular glutamate transporter 1 and 2 genes, as well as coexpression with genes for choline acetyltransferase or glutamic acid decarboxylase in neurons of rat brain. J Neurosci Res. 2005;81:506–521. doi: 10.1002/jnr.20500. [DOI] [PubMed] [Google Scholar]
- Dautzenberg F, Hauger R. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Neocortical neuronal diversity: chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb Cortex. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- DiPilato L, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobac E, Tricoire L, Chaffotte AF, Guiot E, Lambolez B. Calcium imaging in single neurons from brain slices using bioluminescent reporters. J Neurosci Res. 2010;88:695–711. doi: 10.1002/jnr.22249. [DOI] [PubMed] [Google Scholar]
- Eiden LE, Samal B, Gerdin MJ, Mustafa T, Vaudry D, Stroth N. Discovery of pituitary adenylate cyclase-activating polypeptide-regulated genes through microarray analyses in cell culture and in vivo. Ann N Y Acad Sci. 2008;1144:6–20. doi: 10.1196/annals.1418.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Hill E, Cauli B, Gibelin N, Kaneko T, Rossier J, Lambolez B. Extensive overlap of mu-opioid and nicotinic sensitivity in cortical interneurons. Cereb Cortex. 2007;17:1948–1951. doi: 10.1093/cercor/bhl104. [DOI] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Tomioka R, Taki K, Nakamura K, Tamamaki N, Kaneko T. In vivo transduction of central neurons using recombinant Sindbis virus: Golgi-like labeling of dendrites and axons with membrane-targeted fluorescent proteins. J Histochem Cytochem. 2001;49:1497–1507. doi: 10.1177/002215540104901203. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Geoffroy H, Rossier J, Lambolez B. Cortical sources of CRF, NKB, and CCK and their effects on pyramidal cells in the neocortex. Cereb Cortex. 2006;16:1440–1452. doi: 10.1093/cercor/bhj081. [DOI] [PubMed] [Google Scholar]
- Gervasi N, Hepp R, Tricoire L, Zhang J, Lambolez B, Paupardin-Tritsch D, Vincent P. Dynamics of protein kinase A signaling at the membrane, in the cytosol, and in the nucleus of neurons in mouse brain slices. J Neurosci. 2007;27:2744–2750. doi: 10.1523/JNEUROSCI.5352-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard J, Vincent P. Serotonin suppresses the slow afterhyperpolarization in rat intralaminar and midline thalamic neurones by activating 5-HT(7) receptors. J Physiol. 2002;541:453–465. doi: 10.1113/jphysiol.2001.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, et al. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Haug T, Storm J. Protein kinase A mediates the modulation of the slow Ca(2+)-dependent K(+) current, I(sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2071–2079. doi: 10.1152/jn.2000.83.4.2071. [DOI] [PubMed] [Google Scholar]
- Hauger R, Smith R, Braun S, Dautzenberg F, Catt K. Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: a potential mechanism for homologous desensitization. Biochem Biophys Res Commun. 2000;268:572–576. doi: 10.1006/bbrc.2000.2183. [DOI] [PubMed] [Google Scholar]
- Hepp R, Tricoire L, Hu E, Gervasi N, Paupardin-Tritsch D, Lambolez B, Vincent P. Phosphodiesterase type 2 and the homeostasis of cyclic GMP in living thalamic neurons. J Neurochem. 2007;102:1875–1886. doi: 10.1111/j.1471-4159.2007.04657.x. [DOI] [PubMed] [Google Scholar]
- Jarnaess E, Tasken K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans. 2007;35:931–937. doi: 10.1042/BST0350931. [DOI] [PubMed] [Google Scholar]
- Jolivel V, Basille M, Aubert N, de Jouffrey S, Ancian P, Le Bigot J, Noack P, Massonneau M, Fournier A, Vaudry H, et al. Distribution and functional characterization of pituitary adenylate cyclase-activating polypeptide receptors in the brain of non-human primates. Neuroscience. 2009;160:434–451. doi: 10.1016/j.neuroscience.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Karagiannis A, Gallopin T, David C, Battaglia D, Geoffroy H, Rossier J, Hillman E, Staiger J, Cauli B. Classification of NPY-expressing neocortical interneurons. J Neurosci. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Koves K, Gorcs T, Kausz M, Arimura A. Present status of knowledge about the distribution and colocalization of PACAP in the forebrain. Acta Biol Hung. 1994;45:297–321. [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Marie J. VPAC receptors for VIP and PACAP. Recept Channels. 2002;8:137–153. [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides. 2007;28:1631–1639. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Lambolez B, Audinat E, Bochet P, Crepel F, Rossier J. AMPA receptor subunits expressed by single Purkinje cells. Neuron. 1992;9:247–258. doi: 10.1016/0896-6273(92)90164-9. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Batchelor A. Novel action of BAPTA series chelators on intrinsic K+ currents in rat hippocampal neurones. J Physiol. 2000;522:231–246. doi: 10.1111/j.1469-7793.2000.t01-1-00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Hu H, Gibb B, Storm JF. Kinetics of ion channel modulation by cAMP in rat hippocampal neurones. J Physiol. 2006;576:403–417. doi: 10.1113/jphysiol.2006.115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Stern EA, Chen B, Svoboda K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature. 2000;404:876–881. doi: 10.1038/35009107. [DOI] [PubMed] [Google Scholar]
- Lorenzon NM, Foehring RC. The ontogeny of repetitive firing and its modulation by norepinephrine in rat neocortical neurons. Brain Res Dev Brain Res. 1993;73:213–223. doi: 10.1016/0165-3806(93)90141-v. [DOI] [PubMed] [Google Scholar]
- Martin AC, Cooper DM. Layers of organization of cAMP microdomains in a simple cell. Biochem Soc Trans. 2006;34:480–483. doi: 10.1042/BST0340480. [DOI] [PubMed] [Google Scholar]
- Martin JL, Gasser D, Magistretti PJ. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide potentiate c-fos expression induced by glutamate in cultured cortical neurons. J Neurochem. 1995;65:1–9. doi: 10.1046/j.1471-4159.1995.65010001.x. [DOI] [PubMed] [Google Scholar]
- McCormick D, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol. 1986;375:169–194. doi: 10.1113/jphysiol.1986.sp016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A. Costorage and coexistence of neuropeptides in the mammalian CNS. Prog Neurobiol. 2002;66:161–190. doi: 10.1016/s0301-0082(01)00031-4. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Dodt H, Zieglgansberger W. Actions of vasoactive intestinal polypeptide (VIP) on neocortical neurons of the rat in vitro. Neurosci Lett. 1992;147:167–170. doi: 10.1016/0304-3940(92)90586-v. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm J. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron. 1993;11:1023–1035. doi: 10.1016/0896-6273(93)90216-e. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm J. Dopamine modulates the slow Ca(2+)-activated K+ current IAHP via cyclic AMP-dependent protein kinase in hippocampal neurons. J Neurophysiol. 1995;74:11716–11720. doi: 10.1152/jn.1995.74.6.2749. [DOI] [PubMed] [Google Scholar]
- Peeters PJ, Gohlmann HW, Van den Wyngaert I, Swagemakers SM, Bijnens L, Kass SU, Steckler T. Transcriptional response to corticotropin-releasing factor in AtT-20 cells. Mol Pharmacol. 2004;66:1083–1092. doi: 10.1124/mol.104.000950. [DOI] [PubMed] [Google Scholar]
- Peters A, Jones E. Cerebral cortex: cellular components of the cerebral cortex. New York: Plenum Press; 1984. [Google Scholar]
- Reglodi D, Borzsei R, Bagoly T, Boronkai A, Racz B, Tamas A, Kiss P, Horvath G, Brubel R, Nemeth J, et al. Agonistic behavior of PACAP6-38 on sensory nerve terminals and cytotrophoblast cells. J Mol Neurosci. 2008;36:270–278. doi: 10.1007/s12031-008-9089-z. [DOI] [PubMed] [Google Scholar]
- Reyes B, Fox K, Valentino R, Van Bockstaele E. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Sassi Y, Lipskaia L, Vandecasteele G, Nikolaev VO, Hatem SN, Cohen Aubart F, Russel FG, Mougenot N, Vrignaud C, Lechat P, Lompré AM, Hulot JS. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheward W, Lutz E, Harmar A. The distribution of vasoactive intestinal peptide2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neuroscience. 1995;67:409–418. doi: 10.1016/0306-4522(95)00048-n. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Prinz V, Endres M, Wu DF, Hollt V. Pituitary adenylate cyclase-activating polypeptide is up-regulated in cortical pyramidal cells after focal ischemia and protects neurons from mild hypoxic/ischemic damage. J Neurochem. 2007;103:1666–1681. doi: 10.1111/j.1471-4159.2007.04895.x. [DOI] [PubMed] [Google Scholar]
- Taki K, Kaneko T, Mizuno N. A group of cortical interneurons expressing mu-opioid receptor-like immunoreactivity: a double immunofluorescence study in the rat cerebral cortex. Neuroscience. 2000;98:221–231. doi: 10.1016/s0306-4522(00)00124-x. [DOI] [PubMed] [Google Scholar]
- Tatsuno I, Uchida D, Tanaka T, Saeki N, Hirai A, Saito Y, Moro O, Tajima M. Maxadilan specifically interacts with PAC1 receptor, which is a dominant form of PACAP/VIP family receptors in cultured rat cortical neurons. Brain Res. 2001;889:138–148. doi: 10.1016/s0006-8993(00)03126-7. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Temporal and spatial properties of local circuits in neocortex. Trends Neurosci. 1994;17:119–126. doi: 10.1016/0166-2236(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Usdin T, Bonner T, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- Ushiyama M, Ikeda R, Sugawara H, Yoshida M, Mori K, Kangawa K, Inoue K, Yamada K, Miyata A. Differential intracellular signaling through PAC1 isoforms as a result of alternative splicing in the first extracellular domain and the third intracellular loop. Mol Pharmacol. 2007;72:103–111. doi: 10.1124/mol.107.035477. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Hayward MD, Le HN, Hartigan DJ, Duman RS, Nestler EJ. Induction of immediate early genes by cyclic AMP in primary cultures of neurons from rat cerebral cortex. Brain Res Mol Brain Res. 1993;19:76–82. doi: 10.1016/0169-328x(93)90151-e. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt J, Chan R, Li H, Arias C, Prins G, Perrin M, Vale W, Sawchenko P. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow B, Hashimoto H, Galas L, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Vincent P, Gervasi N, Zhang J. Real-time monitoring of cyclic nucleotide signaling in neurons using genetically encoded FRET probes. Brain Cell Biol. 2008;36:3–17. doi: 10.1007/s11068-008-9035-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hupfeld C, Taylor S, Olefsky J, Tsien R. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ma Y, Taylor S, Tsien R. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci U S A. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc G. Peptidergic transmission: from morphological correlates to functional implications. Micron. 1996;27:35–91. doi: 10.1016/0968-4328(95)00028-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.