Abstract
Consolidation of fear extinction involves enhancement of N-methyl D aspartate (NMDA) receptor–dependent bursting in the infralimbic region (IL) of the medial prefrontal cortex (mPFC). Previous studies have shown that systemic blockade of metabotropic glutamate receptor type 5 (mGluR5) reduces bursting in the mPFC and mGluR5 agonists enhance NMDA receptor currents in vitro, suggesting that mGluR5 activation in IL may contribute to fear extinction. In the current study, rats injected with the mGluR5 antagonist 2-methyl-6-(phenylethyl)-pyridine (MPEP) systemically, or intra-IL, prior to extinction exhibited normal within-session extinction, but were impaired in their ability to recall extinction the following day. To directly determine whether mGluR5 stimulation enhances the burst firing of IL neurons, we used patch-clamp electrophysiology in prefrontal slices. The mGluR5 agonist, (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), increased intrinsic bursting in IL neurons. Increased bursting was correlated with a reduction in the slow afterhyperpolarizing potential and was prevented by coapplication of MPEP. CHPG did not increase NMDA currents, suggesting that an NMDA receptor–independent enhancement of IL bursting via stimulation of mGluR5 receptors contributes to fear extinction. Therefore, the mGluR5 receptor could be a suitable target for pharmacological adjuncts to extinction-based therapies for anxiety disorders.
Keywords: amygdala, fear expression, mPFC, MPEP, sAHP
Introduction
Deficits in fear extinction are proposed as an underlying factor in the development of anxiety disorders such as posttraumatic stress disorder (PTSD) (Charney and Deutch 1996; Quirk et al. 2006; Rauch et al. 2006). In support of this hypothesis, recent studies found fear extinction deficits in patients with PTSD (Blechert et al. 2007; Milad et al. 2009). Therefore, there is considerable interest in identifying potential targets for pharmacological augmentation of fear extinction (Davis et al. 2006; Hofmann 2008), especially given that extinction-based therapies are used as a treatment (Davis et al. 2006; Foa 2006; Hofmann 2008).
Studies in rodents have shown that the infralimbic subregion (IL) of the medial prefrontal cortex (mPFC) is particularly important for the retrieval of fear extinction memory (Milad et al. 2006; Quirk and Mueller 2008; Sotres-Bayon et al. 2008). For example, lesioning (Morgan and LeDoux 1995; Quirk et al. 2000; Lebron et al. 2004) or pharmacological inactivating (Sierra-Mercado et al. 2006; Laurent and Westbrook 2008) IL disrupts recall of fear extinction. Furthermore, the inhibition of N-methyl D aspartate (NMDA) receptors (Burgos-Robles et al. 2007; Sotres-Bayon et al. 2009), protein kinases (Hugues et al. 2004; Mueller et al. 2008), cannabinoid CB1 receptors (Lin et al. 2009), and protein synthesis (Santini et al. 2004) in IL during or shortly after extinction training also leads to poor recall of fear extinction suggesting that molecular cascades in IL are activated by extinction.
It was previously demonstrated that burst firing in IL shortly after extinction training is important for recalling fear extinction memory (Burgos-Robles et al. 2007). Previous studies show that activation of metabotropic glutamate receptor type 5 (mGluR5) enhances NMDA receptor currents in hippocampus and subcortical structures (Awad et al. 2000; Mannaioni et al. 2001; Pisani et al. 2001) and increases burst firing in mPFC (Homayoun and Moghaddam 2006). Together, these findings suggest that mGluR5 could play a role in fear extinction by enhancing burst firing in IL neurons. In support of this, it was recently shown that knockout mice with deletion of mGluR5 receptors were deficient in fear extinction (Xu et al. 2009). However, the location and the mechanisms of mGluR5 activity important for fear extinction are not known. To test whether local mGluR5 stimulation in IL is important for fear extinction and burst activity, we examined the effect of systemic injection and local IL infusion of the mGluR5 antagonist, 2-methyl-6-(phenylethyl)-pyridine (MPEP), on fear extinction. We also examined the effect of mGluR5 stimulation on IL neuronal excitability using patch-clamp electrophysiology in brain slices.
Methods
Subjects
All procedures involving the use of animals were approved by the Institutional Animal Care and Use Committee of the Ponce School of Medicine in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals. Male Sprague-Dawley rats (300 g) were transported from the Ponce School of Medicine colony to a satellite facility nearby where they were housed in transparent polyethylene cages inside a negative-pressured Biobubble (Colorado Clean Room). Food was restricted until rats reached 85% of their free-feeding weight. Rats were then trained to press a bar for food on a variable interval schedule of reinforcement (VI-60) in order to maintain a constant level of activity against which freezing can be reliably measured (Quirk et al. 2000). Rats were maintained on a 12:12 h light:dark schedule.
Surgery
After bar-press training, rats were anesthetized with ketamine (8.7 mg/100 g) and xylazine (1.3 mg/100 g) and placed in the stereotaxic frame. After anesthesia, the skin was retracted and holes were drilled in the skull. Rats were implanted with a single 26 gauge stainless-steel guide cannula (Plastics One) in the mPFC as described previously (Santini et al. 2004). Stereotaxic coordinates were 2.8 mm anterior, 1.0 mm lateral, and 4.1 mm ventral from bregma (Paxinos and Watson 1986), with the cannula angled 11° toward the midline in the coronal plane. Rats were allowed 7 days to recover from surgery, after which training was resumed.
MPEP Infusions
For the infusions of the selective mGluR5 blocker, MPEP, cannula dummies were removed from guide cannulas and replaced with 33 gauge injectors, which were connected by polyethylene tubing (PE-20; Small Parts Inc.) to 5-μL syringes mounted in an infusion pump (Harvard Apparatus). Saline (vehicle) or MPEP (1.5 μg) was infused 30 min prior to extinction training at a rate of 0.5 μL/min for 1 min.
Behavioral Protocol
After successful completion of bar-press training, rats underwent fear conditioning and extinction procedures in chambers (Coulbourn Instruments) located inside sound-attenuating boxes (Med Associates). Details of the apparatus have been previously described (Quirk et al. 2000). On day 1, rats received 5 habituation trials (4 kHz, 30 s, 75 dB), immediately followed by fear conditioning that consisted of 7 tones that co-terminated with footshocks (0.5 s, 0.43 mA). At the end of day 1, groups were matched for acquisition levels of freezing. On day 2, rats were returned to the same chambers and were given extinction training that consisted of tone-alone trials (20 trials). On day 3, rats were given 15 additional tone-alone trials. Intertrial intervals were varied with an average intertrial interval of 3 min.
mPFC Slice Preparation
Slice recording methods were similar to our previously described studies (Mueller et al. 2008; Santini et al. 2008). Juvenile (P19–30) and adult (P57–62) naive rats were deeply anesthetized with pentobarbital (150 mg/kg) and decapitated. The majority of electrophysiology experiments were done using slices from the juvenile rats due to better slice viability and more uniform space clamp for measuring the afterhyperpolarization currents. To verify that the results from the juvenile rats also apply to adult rats that were used for the behavioral experiments, some experiments were also performed on brain slices from adult rats. Brains were quickly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) NaCl (126), KCl (3), NaH2PO4 (1.25), MgSO4 (1), NaHCO3 (26), glucose (20), and CaCl2 (2) and bubbled with 95% O2 and 5% CO2. Coronal slices including the mPFC were cut at a thickness of 300 μm with a Vibratome 1000 Plus (Vibratome). Slices were incubated in room temperature ACSF for at least an hour prior to experiments. After incubation, slices were transferred to a submersion recording chamber mounted on a microscope stage and perfused at 2–3 mL/min with room temperature ACSF. Neurons were visualized with infrared video microscopy using a ×40 water immersion objective on an upright E600FN microscope (Nikon Instruments). Whole-cell recordings were done with glass micropipettes pulled on a Flaming/Brown micropipette puller (Sutter Instruments) with a resistance of 3–5 MΩ when filled. The internal solution for patch-clamp recordings was prepared containing (in mM) KCl (12), Kgluconate (130), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (10), sodium phosphocreatine (10), biocytin (5), adenosine triphosphate (2), and guanosine triphosphate (0.3); pH was adjusted to 7.3 with KOH and sucrose was added to adjust osmolarity to 300 mOsm. For recordings from adult rats, 0.5-mM ethyleneglycol-bis(2-aminoethylether)-N,N,N′,N′-tetra acetic acid (EGTA) was included in the internal solution, since we found in preliminary experiments on neurons from adult rats that the number of spikes evoked by a depolarizing pulse reduced with time in the absence of EGTA.
Electrophysiology
After incubation, pyramidal neurons were recorded in the whole-cell mode. The changes in intrinsic excitability were assessed with a patch-clamp amplifier (MultiClamp 700A, Axon Instruments). Single neurons were visually identified using a video camera (Dage MTI) connected to the Nikon E600 microscope. Resting membrane potential was measured after achieving whole-cell configuration, and cells with a membrane potential more depolarized than −50 mV were discarded. Recordings were filtered at 4 kHz, digitized at 10 kHz, and saved to a computer using pCLAMP9 software (Axon Instruments). Membrane potentials were not corrected for the junction potential. After establishing a whole-cell current-clamp recording, 800-ms depolarizing current pulses were injected every 15 s and adjusted to evoke 3–4 spikes. After a stable 5-min baseline, the selective mGluR5 agonist, (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), at concentrations of 100 or 500 μM was bath-applied for 5 min. The number and frequency of action potentials evoked by each pulse were measured. The slow afterhyperpolarizing potential (sAHP) was measured as the average potential during a 50-ms period beginning 280 ms after the end of the 800-ms depolarizing pulse (Santini et al. 2008). AHP currents were evoked by giving 800-ms depolarizing steps from −50 to 0 mV and measured as the area under the curve during a 1-s period beginning at the peak of the AHP current. Voltage-clamp recordings were not compensated for series resistance, but changes in series resistance were continuously monitored and recordings were eliminated from analysis if the series resistance changed by more than 15%. Input resistance was measured as the difference between baseline and the average voltage response during a 100-ms period beginning 700 ms after the beginning of a 1-s hyperpolarizing step.
To record NMDA receptor–mediated excitatory postsynaptic currents (EPSCs) and excitatory postsynaptic potentials (EPSPs), slices were perfused with ACSF in which the MgSO4 concentration was reduced to 0.1 mM to relieve the Mg2+ block of the NMDA receptors. We added 100-μM picrotoxin and 10-μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to block γ-aminobutyric acid (GABAA) and α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate receptors, respectively. A monopolar electrode was used to stimulate glutamatergic fibers impinging on the apical dendrites of layer V pyramidal neurons in IL every 15 s. NMDA EPSCs were recorded in neurons voltage-clamped at −60 mV. NMDA EPSPs were recorded in neurons manually held at −60 mV by injecting negative current.
MPEP was purchased from Tocris, apamin was purchased from Sigma-Aldrich, and CHPG was purchased from Ascent Scientific.
Statistical Analysis
To quantify conditioned fear, the total time spent freezing during the 30-s tone was measured and converted to percent freezing. The majority of the experiments were analyzed from digitized videos using commercial software (FreezeScan, Clever Systems). The last infusion experiment was hand-scored by a blinded observer, due to a 1-year lapse in Freezescan availability when Dr Quirk's laboratory moved to another institution. Data were averaged in blocks of 2 trials. Group comparisons were made between the averages of the first 4 trials of extinction recall on day 3. Electrophysiological data were analyzed using Clampfit 9.2 (Axon Instruments). Traces illustrating the number of spikes evoked by depolarizing pulses are single traces. All other data were analyzed from averages of 5 consecutive measurements. Data were compared using Student's t-test, paired t-test, or repeated-measures analysis of variance (ANOVA) (Statistica, Statsoft). After a significant main effect, post hoc comparisons were done using Tukey's test. Values are reported as the mean ± the standard error of the mean.
Results
Systemic Blockade of mGluR5 Impairs Recall of Fear Extinction
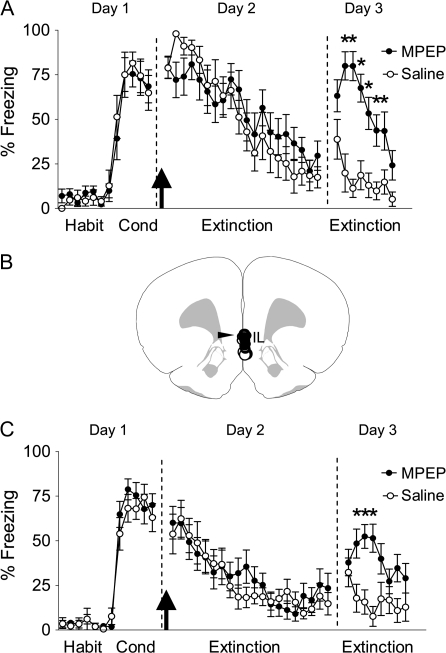
First, we examined whether activation of mGluR5 is necessary for fear extinction. On day 1, rats were conditioned with 7 tone-shock pairings. The next day rats received intraperitoneal injections of MPEP (10 mg/kg) or saline 30 min prior to extinction training that consisted of 20 tone-alone trials. This dose of MPEP blocks mPFC burst firing (Homayoun and Moghaddam 2006) and occupies about 90% of mGluR5 binding sites in vivo (Anderson et al. 2002). MPEP neither affect fear expression or extinction learning (Fig. 1) nor affect the rate of bar-pressing for food (14 presses/min, MPEP; 12 presses/min, saline; t = −0.495; P = 0.312). The following day, however, MPEP-injected rats were severely impaired in their ability to recall extinction compared with saline-injected rats. Repeated-measures ANOVA across the 2 groups revealed a main effect of group (F1,20 = 22.97; P = 0.0001), of trial (F14,280 = 18.44; P < 0.0001), and a significant trial by group interaction (F14,280 = 5.95; P < 0.001). Post hoc analysis confirmed that the MPEP-injected group froze more than saline controls in trials 2–5 (values of P < 0.01). These results suggest that the endogenous activation of mGluR5 during extinction learning is essential for consolidation of fear extinction.
Figure 1.
Systemic or IL blockade of mGluR5 impairs recall of fear extinction. (A) Percent freezing to the tone for saline-injected rats (n = 10) and rats injected intraperitoneally with 10 mg/kg MPEP (n = 12). MPEP-treated rats showed more freezing on day 3; *P < 0.001. (B) A coronal drawing (bregma, 3.20 mm) showing placements of injector tips for all rats that received IL infusions. (C) Percent freezing to the tone for saline-infused rats (n = 12) and rats infused with 1.5 μg in 0.5-μL MPEP into IL (n = 16); *P < 0.01. Arrow indicates the time of the injection.
Local Blockade of mGluR5 in IL Impairs Recall of Fear Extinction
MPEP's disruption of extinction recall resembles the pattern of effects of IL blockade of NMDA receptors (Burgos-Robles et al. 2007; Sotres-Bayon et al. 2009) or beta adrenergic receptors (Mueller et al. 2008). Therefore, to determine whether mGluR5 stimulation in IL is necessary for recall of fear extinction, we infused MPEP (1.5 μg) or saline locally into IL at the same time point as the systemic experiment (30 min prior to extinction; Fig. 1B,C). When infused into the amygdala, this dose of MPEP was previously shown to disrupt fear conditioning (Rodrigues et al. 2002) and synaptic plasticity (Zheng et al. 2008). Similar to our systemic finding, MPEP-infused rats showed normal extinction learning, but were impaired in their recall of extinction memory the following day. Repeated-measures ANOVA on day 3 revealed a main effect of group (F1,26 = 2.92; P = 0.009), of trial (F7,182 = 2.24; P = 0.03), and a significant trial by group interaction (F7,182 = 2.92; P = 0.006). Post hoc analysis confirmed that the rats receiving MPEP infusions froze more than saline controls in trials 2–4 (values of P < 0.01). These results indicate that mGluR5 activation in IL during extinction training is necessary for consolidation of fear extinction. Note that local infusion of MPEP resulted in a smaller impairment of extinction than systemic administration, suggesting that mGluR5 activation in brain areas other than IL may also be necessary for consolidating the extinction memory.
Activation of mGluR5 Enhances the Intrinsic Excitability of IL Neurons from Juvenile Rats
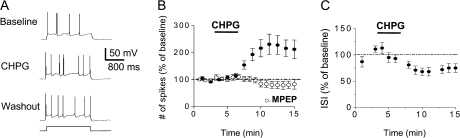
To examine whether mGluR5 activation increases the intrinsic excitability of IL neurons, we measured the number of action potentials elicited by a depolarizing current pulse using whole-cell patch-clamp recordings in mPFC slices. Due to better slice viability, we initially examined IL neurons from P19–23 rats. After obtaining a stable baseline of 3–4 spikes, we applied the selective mGluR5 agonist CHPG (Fig. 2A,B). Perfusion of 500-μM CHPG significantly increased the number of spikes (216 ± 32% of baseline, t = 3.02; degrees of freedom [df] 7; P < 0.01). The effect of CHPG was concentration-dependent as 100-μM CHPG produced a significant but smaller increase in the number of spikes (162 ± 9% of baseline, t = 6.93; df 6; P < 0.001). As shown in Figure 2B, the CHPG-induced enhancement in spikes was prevented by pretreatment with 100 μM of the selective mGluR5 antagonist MPEP (94 ± 8% of MPEP alone; t = 1.02; df 4; P = 0.18), indicating that the enhancement was mediated through mGluR5 receptors. As an in vitro measure of the ability of IL neurons to fire bursts of action potentials in vivo, we measured the duration of the interval separating the first 2 spikes (Santini et al. 2008). As shown in Figure 2C, application of CHPG significantly decreased the interspike interval (68 ± 7% of baseline, t = 2.36; df 7; P = 0.008). These results suggest that selective activation of mGluR5 increases the intrinsic excitability of IL neurons and enhances their ability to fire bursts of action potentials.
Figure 2.
Activation of mGluR5 increases the intrinsic excitability of IL pyramidal neurons in juvenile rats. (A) Traces showing the number of spikes evoked by a current pulse during baseline, perfusion with the mGluR5 agonist, CHPG (500 μM), and washout in an IL neuron from a P19 rat. (B) Time course showing that CHPG (bar) increased the number of spikes (n = 8). The increase in spikes was blocked by the mGluR5 antagonist MPEP (100 μM, unfilled circles). (C) Time course demonstrating that CHPG also decreased the first interspike interval.
As indicated in Table 1, CHPG did not significantly increase input resistance (110 ± 5% of baseline, t = 2.02; df 7; P = 0.09), but did depolarize the IL neurons from a baseline of −56 ± 1 to −53 ± 1 mV (t = 6.24; df 7; P = 0.0004).
Table 1.
Electrophysiological properties of IL neurons
| Input resistance (% of baseline) |
Membrane potential (mV) |
|||
| Pre-drug | CHPG | Pre-drug | CHPG | |
| Juvenile | 100 ± 0 | 110 ± 5 | –56 ± 1 | –53 ± 1* |
| Adult | 98 ± 1 | 112 ± 3* | –61 ± 1 | –57 ± 2* |
*P < 0.05.
mGluR5 Decreases the Slow AHP
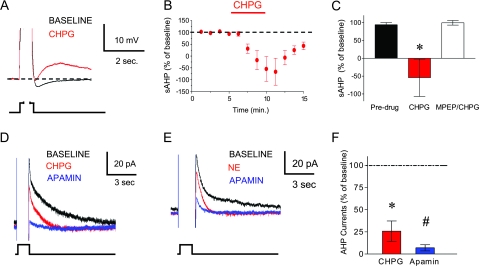
In the hippocampus and sensorimotor cortex, it has been shown that activation of mGluR5 increases intrinsic excitability through a reduction in Ca2+-dependent K+ currents underlying the AHP (Mannaioni et al. 2001; Sourdet et al. 2003). In IL neurons, we measured the AHP as the average potential during a 50-ms period 280 ms after an 800-ms depolarizing pulse (Santini et al. 2008). Figure 3A–C shows that the AHP was significantly reduced during CHPG perfusion (−76 ± 64% of baseline, t = 2.83; df 6; P = 0.015). In fact, the AHP was converted to an afterdepolarizing potential in 3 of 7 neurons. As indicated in Figure 3C, the CHPG-induced reduction in the AHP was prevented by pretreatment with 10-μM MPEP (92 ± 3% of MPEP alone; t = 0.42; df 4; P = 0.35).
Figure 3.
Activation of mGluR5 decreases the sAHP. (A) Average traces of the sAHP evoked by an 800-ms depolarizing pulse during baseline (black) and CHPG (red). Traces are truncated to emphasize the AHP. (B–C) Time course and bar graph showing that CHPG perfusion (500 μM; red bar) decreased the sAHP. MPEP prevented the CHPG-mediated reduction in the sAHP. (D) Average traces of AHP currents evoked by 800-ms step from −50 to 0 mV. CHPG blocked the slower component leaving a faster component that was blocked by apamin (100 nM). (E) This current was also blocked by norepinephrine (NE, 100 μM), a known blocker of the IsAHP. (F) Bar graph summarizing the effects of CHPG and apamin on the AHP currents. *P < 0.05 compared with baseline. #P < 0.05 compared with CHPG.
Evoking AHPs by prolonged depolarizing pulses produces 2 underlying currents. One is the ImAHP that is blocked by the small conductance (SK) K+ channel antagonist apamin, and the other is the IsAHP that is slower, apamin-insensitive, and blocked by norepinephrine (Sah and Faber 2002). The stimulation of mGluR5 can reduce both the ImAHP (Sourdet et al. 2003) and the IsAHP (Mannaioni et al. 2001; Sah and Faber 2002), leaving in doubt the exact mechanism. To compare the effect of CHPG on these 2 currents, we measured current directly in voltage-clamp mode to reveal the time course of the underlying currents. Consistent with blockade of IsAHP, CHPG perfusion reduced the slower component of the AHP currents (26 ± 11% of baseline, t = 6.45; df 2; P = 0.02), but left a faster component unblocked (Fig. 3D–F). The slow component was also blocked by 100-μM norepinephrine (68 ± 15% of baseline, t = 2.24; df 4; P = 0.04, Fig. 3E). In both cases, adding 100-nM apamin blocked the remaining faster component. These results suggest that mGluR5 stimulation increases the number of evoked spikes and enhances burst firing in IL by closing the Ca2+-dependent K+ channels underlying the sAHP.
mGluR5 Increases the Intrinsic Excitability of IL Neurons from Adult Rats
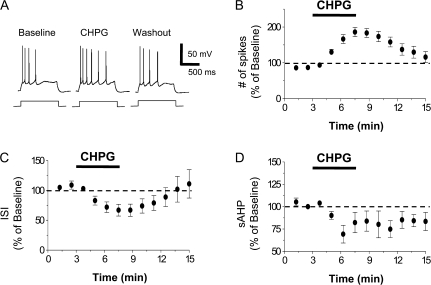
Since our behavioral data were from adult rats (∼90 days old) and the electrophysiology experiments used slices from juvenile rats less than 24 days old, we examined whether mGluR5 also increases the intrinsic excitability of IL neurons from adult rats (P57–62). Figure 4 shows that CHPG increased the number of spikes (186 ± 12% of baseline, t = 7.22; df 6; P < 0.001), reduced the first interspike interval (67 ± 10% of baseline, t = 3.87; df 6; P = 0.008), and reduced the sAHP (69 ± 10% of baseline, t = 3.41; df 6; P = 0.01) in layer 5 IL pyramidal neurons from adult rats. As shown in Table 1, CHPG also increased the input resistance (112 ± 3% of baseline, t = 4.53; df 6; P = 0.003) and depolarized the IL neurons (−61 ± 1 mV [baseline]; −57 ± 1 mV [CHPG]; t = 4.98; df 6; P = 0.002). Thus, mGluR5 similarly increases the intrinsic excitability of IL neurons from both juvenile and adult rats.
Figure 4.
CHPG also increases the intrinsic excitability of IL pyramidal neurons from adult rats. (A) Traces showing the number of spikes evoked by a current pulse during baseline, perfusion with CHPG (500 μM), and washout in a pyramidal neuron from a P60 rat. (B) Time course demonstrating that CHPG (bar, n = 7) increased the number of spikes. (C) Time course showing that CHPG reduced the first interspike interval. (D) Time course showing that CHPG reduced the sAHP.
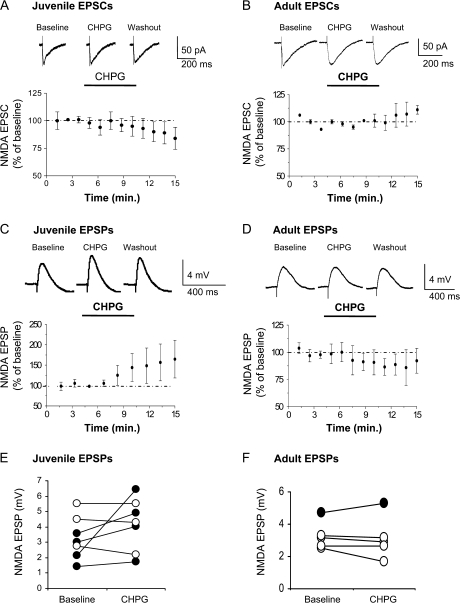
mGluR5 Does Not Increase NMDA Receptor Synaptic Currents in IL
Given the importance of NMDA receptor activity in IL for consolidation of the extinction memory (Burgos-Robles et al. 2007) and the documented enhancement of NMDA receptor currents by mGluR5 agonists (Awad et al. 2000; Mannaioni et al. 2001; Benquet et al. 2002; Huang and van den Pol 2007), we examined the effect of CHPG on NMDA receptor–mediated EPSCs in IL pyramidal neurons from juvenile (P28–30) and adult (P57–62) rats, in response to stimulation of layer 2. NMDA receptor–mediated EPSCs were isolated by blocking GABAA and AMPA/kainate receptors with picrotoxin and CNQX in the bath, respectively. As shown in Figure 5A,B, 500-μM CHPG did not enhance the NMDA receptor–mediated EPSCs in either juvenile (97 ± 5% of baseline, t = 0.658; df 8; P = 0.53) or adult (95 ± 1.6% of baseline, t = 0.87; df 6; P = 0.42) rats. Therefore, mGluR5 receptor stimulation does not directly enhance NMDA receptor currents in IL neurons. Although mGluR5 stimulation did not directly enhance the NMDA receptor currents, the increase in intrinsic excitability produced by mGluR5 stimulation could still enhance NMDA receptor–mediated depolarization (Doherty et al. 1997; Ugolini et al. 1999; Pisani et al. 2001). To test this possibility, we examined the effect of CHPG on NMDA receptor–mediated EPSPs. On average, CHPG did not significantly enhance the NMDA receptor–mediated EPSPs in juvenile (137 ± 34% of baseline, t = −1.35, df 6, P = 0.23) or adult (92 ± 13.4% of baseline; t = 0.36; df 8; P = 0.715) rats (Fig. 5C–F). However, testing cells individually showed that CHPG significantly increased NMDA EPSPs in 4 of 7 neurons from the juvenile rats and in 1 of 5 neurons from the adult rats (paired t-test, P < 0.01). Thus, although mGluR5 stimulation does not enhance NMDA receptor–mediated currents or depolarization in the entire population of IL neurons, mGluR5 stimulation did enhance NMDA receptor–mediated depolarization in a subset of IL neurons, especially in juveniles.
Figure 5.
mGluR5 effects on NMDA receptor–mediated EPSCs or EPSPs in IL slices from juvenile and adult rats. (A,B) Time courses showing NMDA EPSC amplitudes during baseline, application of CHPG (500 μM; bar), and washout. Traces show average EPSCs in IL neurons from a P28 (A) and a P60 (B) rat. (C,D) Time courses showing NMDA EPSP amplitudes during baseline, application of CHPG, and washout. Traces show average EPSPs in IL neurons from a P28 (C) and a P60 (D) rat. (E,F) NMDA EPSP amplitudes of all neurons from the juvenile and adult rats during baseline and perfusion of CHPG. Black filled circles indicate EPSPs that were increased by CHPG (P < 0.01).
Taken together, our results suggest that mGluR5 agonists may be useful for enhancing fear extinction. However, the development of better mGluR5 agonists may be necessary since simply infusing 500-μM CHPG into IL prior to fear extinction did not enhance acquisition or recall of fear extinction (Supplementary Fig. 1).
Discussion
In this study, we examined the role of mGluR5 receptors in extinction of conditioned fear and IL excitability. Our main findings are 1) systemic inhibition of mGluR5 prior to extinction learning prevented the recall of the extinction memory 24 h later without affecting fear expression or extinction learning, 2) localized infusion of the mGluR5 blocker into IL produced a similar effect, 3) pharmacological activation of mGluR5 increased the ability of IL pyramidal neurons to fire bursts through a reduction in their sAHP currents, and 4) mGluR5 agonists did not enhance NMDA receptor–mediated currents or depolarization in adult IL neurons. Our behavioral and electrophysiological results suggest that mGluR5-mediated enhancement of IL burst firing through reductions in the sAHP is necessary for recall of fear extinction.
Our results are in agreement with a recent transgenic study demonstrating that knockout of mGluR5 prevents fear extinction across days (Xu et al. 2009). We extend their findings by showing that the extinction deficits were unlikely to be due to developmental changes caused by the absence of mGluR5 receptors (Hannan et al. 2001; Wijetunge et al. 2008). We also demonstrate that mGluR5 appears to be important for consolidation rather than initial acquisition of fear extinction. The lack of effect of MPEP on freezing during acquisition of fear extinction is consistent with previous work showing that mGluR5 blockade does not affect fear expression (Rodrigues et al. 2002). Furthermore, we also provide evidence that the modulation of extinction by mGluR5 occurs locally in IL.
Different subtypes of mGluRs modulate different aspects of fear extinction. Local blockade of mGluR1 receptors, a group I mGluR subtype, in the amygdala impairs acquisition of fear extinction suggesting that mGluR1 stimulation induces plasticity critical for extinction learning in the amygdala (Kim et al. 2007). Consistent with this, mGluR1 receptors appear to mediate extinction-induced depotentiation of thalamic inputs to the lateral amygdala (Kim et al. 2007). In contrast, our results suggest that the other group I mGluR subtype, mGluR5, modulates plasticity required for recall of fear extinction in IL. In addition to group I mGluRs, group II and III mGluRs also modulate fear extinction. Group II mGluR stimulation in the lateral amygdala depotentiates cortical inputs and mimics extinction of fear potentiated startle (Lin et al. 2005) suggesting a role in extinction learning plasticity. Stimulation of the group III mGluR subtype, mGluR7, enhances fear extinction (Fendt et al. 2008), but the location and mechanism of this modulation are unknown. Thus, all 3 groups of mGluRs modulate fear extinction with mGluR1 and group II mGluRs modulating extinction learning in the amygdala while mGluR5 mediates extinction plasticity in IL.
Numerous studies indicate that while IL is not important for the acquisition of fear extinction, it is critical for recall of the extinction memory (Quirk and Mueller 2008). For proper recall of extinction, various events must occur in IL including activation of NMDA receptors (Santini et al. 2001; Burgos-Robles et al. 2007), the mitogen-activated protein kinase pathway (Hugues et al. 2004), protein kinase A (Mueller et al. 2008), and protein synthesis (Santini et al. 2004). Our current findings show that recall of fear extinction also requires IL activation of mGluR5, which increases the intrinsic excitability of IL neurons. This suggests that the consolidation process requires receptor-mediated enhancement of IL intrinsic excitability, which could be mediated by mGluR5 or noradrenergic beta receptors (Mueller et al. 2008). Indeed, we previously observed that extinction increases intrinsic excitability in IL (Santini et al. 2008).
The increase in intrinsic excitability induced by mGluR5 stimulation could enhance consolidation of the extinction memory by increasing IL bursting. Consistent with previous studies in other structures (Mannaioni et al. 2001; Ireland and Abraham 2002; Young et al. 2008), we observed that mGluR5 stimulation in IL reduces the IsAHP suggesting that stimulation of mGluR5 closes Ca2+-dependent K+ channels that underlie the sAHP (Lancaster and Adams 1986; Sah and Faber 2002). The mGluR5-mediated reduction in the sAHP enhanced the intrinsic ability of IL neurons to fire bursts of high-frequency action potentials. Therefore, mGluR5 blockade may have disrupted fear extinction by reducing bursting in IL that is required for consolidation of fear extinction (Burgos-Robles et al. 2007). In support of this possibility, blocking mGluR5 reduces burst firing of mPFC neurons in vivo (Homayoun and Moghaddam 2006).
In contrast to our findings, several studies have shown that the stimulation of mGluR5 receptors enhances NMDA receptor–mediated currents and depolarization (Awad et al. 2000; Mannaioni et al. 2001; Pisani et al. 2001; Benquet et al. 2002; Huang and van den Pol 2007), while we observed no effect on NMDA currents. This discrepancy could be due to developmental differences, as many of the previous studies used rats and mice less than 21 days old (Ugolini et al. 1999; Awad et al. 2000; Mannaioni et al. 2001; Benquet et al. 2002; Huang and van den Pol 2007). Consistent with this, NMDA receptor–mediated EPSCs in nucleus accumbens slices from juvenile Spraque-Dawley rats (100–170 g; ∼30–40 days postnatal) were not affected by agonists that stimulate mGluR5 receptors (Martin et al. 1997). Our finding that CHPG enhanced NMDA receptor EPSPs more often in juvenile than adult rats supports a developmental loss of mGluR5-mediated enhancement of NMDA receptor activity. In addition, many of the previous studies examined the effects of mGluR5 agonists on responses to application of NMDA (Ugolini et al. 1999; Awad et al. 2000; Mannaioni et al. 2001; Pisani et al. 2001; Benquet et al. 2002) rather than synaptic activation of NMDA receptors, raising the possibility that NMDA receptors located outside the synapses may be more sensitive to potentiation by mGluR5 stimulation as previously suggested (Ireland and Abraham 2009). It is also important to note that mGluR5 receptor activation may enhance NMDA receptor intracellular signaling, even without increasing NMDA currents. Co-activation of mGluR5 and NMDA receptors in striatal neurons produces a synergistic increase in phosphorylated ERK that requires interactions with the intracellular scaffolding proteins, PSD-95 and Homer1b/c, which lead to phosphorylation of transcription factors, Elk-1 and CREB (Yang et al. 2004). Consistent with this, extinction of tone fear conditioning activates ERK in IL (Kim et al. 2009) and infusion of ERK inhibitors into IL disrupts the recall of fear extinction (Hugues et al. 2004). In addition, synaptic plasticity occurs at lower stimulation levels during co-activation of mGluR5 and NMDA receptors due to a synergistic activation of protein kinase Cγ (Codazzi et al. 2006).
Based on our results, we propose the following model of extinction-induced plasticity in IL. During extinction learning, glutamatergic inputs from the amygdala, hippocampus, and medial dorsal thalamus release glutamate that stimulates mGluR5 in IL. Concurrently, norepinephrine, released from the locus coereleus during extinction (Hugues et al. 2007), stimulates beta adrenergic receptors in IL (Mueller et al. 2008). The stimulation of mGluR5 and beta adrenergic receptors leads to a prolonged reduction of the sAHP (Gereau and Conn, 1994) that enhances burst firing. The bursts of action potentials could strengthen synapses from IL neurons onto their downstream targets, such as the intercalated neurons in the amygdala (Quirk et al. 2003; Likhtik et al. 2008), and could also enhance synapses onto IL neurons via back propagating spikes (Kampa et al. 2007). During extinction recall, the IL neurons would more strongly activate their targets to inhibit fear expression.
It should be noted that mGluR5 may produce other effects in IL that are important for the plasticity required for recall of fear extinction. For example, the synaptic stimulation of mGluR5 receptors in mPFC slices induces the production of endocannabinoids that stimulate cannabinoid CB1 receptors (Lafourcade et al. 2007). Since recall of fear extinction is disrupted by cannabinoid CB1 receptor blockade in IL (Lin et al. 2009), it is possible that mGluR5 enhances fear extinction recall via indirect stimulation of cannabinoid CB1 receptors.
Although infusion of MPEP into IL disrupted extinction recall, this does not exclude the possibility that mGluR5 receptors in other structures are important for fear extinction. Consistent with this possibility, we found a larger impairment with systemic mGluR5 blockade than with IL infusions. MPEP-infused into the amygdala disrupts fear conditioning (Rodrigues et al. 2002); therefore, it is possible that amygdalar mGluR5 receptors are also important for fear extinction. In addition, mGluR5 receptors in the ventral tegmental area (Renoldi et al. 2007) or locus coeruleus (Romano et al. 1995) could be involved. The activation of mGluR5 in the ventral tegmental area induces the release of dopamine in the mPFC (Renoldi et al. 2007) and systemic MPEP reduces cortical norepinephrine release (Page et al. 2005). Since blocking either D4 dopamine receptors (Pfeiffer and Fendt 2006) or beta adrenergic receptors (Mueller et al. 2008) in the mPFC disrupts fear extinction recall, the enhanced effect of systemic MPEP could be due to blockade of mGluR5 in the ventral tegmental area or locus coeruleus.
One possible implication of our findings is that mGluR5 agonists could be used for the treatment of anxiety disorders, particularly PTSD. Currently, the treatment for PTSD includes extinction-based exposure therapy (Foa 2006). Since PTSD has been correlated with hypofunctioning of the mPFC (Rauch et al. 2006), activation of the mPFC with mGluR5 agonists combined with behavioral therapy could lead to better retention of the extinction memory. Positive allosteric modulators of mGluR5 receptors shown to enhance extinction of conditioned place preference (Gass and Olive 2009) could serve this role.
Supplementary Material
Supplementary figure can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institutes of Health (F31-GM075489 to D.F.N.; R01-MH058883 and R01-MH081975 to G.J.Q.); National Science Foundation (IOS 0842159 to J.T.P.).
Acknowledgments
We thank Ana Veronica Lopez and Eliezer Ruiz for assistance with the behavioral studies and Dr Etienne Audinat for comments on the manuscript. Conflict of Interest: None declared.
References
- Anderson JJ, Rao SP, Rowe B, Giracello DR, Holtz G, Chapman DF, Tehrani L, Bradbury MJ, Cosford NDP, Varney MA. [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J Pharmacol Exp Ther. 2002;303:1044–1051. doi: 10.1124/jpet.102.040618. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Charney DS, Deutch A. A functional neuroanatomy of anxiety and fear: implications for the pathophysiology and treatment of anxiety disorders. Crit Rev Neurobiol. 1996;10:419–446. doi: 10.1615/critrevneurobiol.v10.i3-4.70. [DOI] [PubMed] [Google Scholar]
- Codazzi F, Di Cesare A, Chiulli N, Albanese A, Meyer T, Zacchetti D, Grohovaz F. Synergistic control of protein kinase Cgamma activity by ionotropic and metabotropic glutamate receptor inputs in hippocampal neurons. J Neurosci. 2006;26:3404–3411. doi: 10.1523/JNEUROSCI.0478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Myers KM, Chhatwal J, Ressler KJ. Pharmacological treatments that facilitate extinction of fear: relevance to psychotherapy. NeuroRx. 2006;3:82–96. doi: 10.1016/j.nurx.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Henley JM, Collingridge GL, Jane DE. (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) activates mGlu5, but not mGlu1 receptors expressed in CHO cells and potentiates NMDA responses in the hippocampus. Neuropharmacology. 1997;36:265–267. doi: 10.1016/s0028-3908(97)00001-4. [DOI] [PubMed] [Google Scholar]
- Fendt M, Schmid S, Thakker DR, Jacobson LH, Yamamoto R, Mitsukawa K, Maier R, Natt F, Husken D, Kelly PH, et al. mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Mol Psychiatry. 2008;13:970–979. doi: 10.1038/sj.mp.4002073. [DOI] [PubMed] [Google Scholar]
- Foa EB. Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry. 2006;67(Suppl 2):40–45. [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereau RW, 4th, Conn PJ. A cyclic AMP-dependent form of associative synaptic plasticity induced by coactivation of beta-adrenergic receptors and metabotropic glutamate receptors in rat hippocampus. J Neurosci. 1994;14:3310–3318. doi: 10.1523/JNEUROSCI.14-05-03310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan AJ, Blakemore C, Katsnelson A, Vitalis T, Huber KM, Bear M, Roder J, Kim D, Shin HS, Kind PC. PLC-beta1, activated via mGluRs, mediates activity-dependent differentiation in cerebral cortex. Nat Neurosci. 2001;4:282–288. doi: 10.1038/85132. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006;16:93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- Huang H, van den Pol AN. Rapid direct excitation and long-lasting enhancement of NMDA response by group I metabotropic glutamate receptor activation of hypothalamic melanin-concentrating hormone neurons. J Neurosci. 2007;27:11560–11572. doi: 10.1523/JNEUROSCI.2147-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R, Lena I. Time course of extracellular catecholamine and glutamate levels in the rat medial prefrontal cortex during and after extinction of conditioned fear. Synapse. 2007;61:933–937. doi: 10.1002/syn.20448. [DOI] [PubMed] [Google Scholar]
- Ireland DR, Abraham WC. Group I mGluRs increase excitability of hippocampal CA1 pyramidal neurons by a PLC-independent mechanism. J Neurophysiol. 2002;88:107–116. doi: 10.1152/jn.2002.88.1.107. [DOI] [PubMed] [Google Scholar]
- Ireland DR, Abraham WC. Mechanisms of group I mGluR-dependent long-term depression of NMDA receptor-mediated transmission at Schaffer collateral-CA1 synapses. J Neurophysiol. 2009;101:1375–1385. doi: 10.1152/jn.90643.2008. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Dendritic mechanisms controlling spike-timing-dependent synaptic plasticity. Trends Neurosci. 2007;30:456–463. doi: 10.1016/j.tins.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park H, Song B, Hong I, Geum D, Shin K, Choi S. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem Biophys Res Commun. 2007;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, et al. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci U S A. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hamlin AS, Richardson R. Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci. 2009;29:10802–10808. doi: 10.1523/JNEUROSCI.0596-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Huang YC, Wang SJ, Gean PW. Activation of group II metabotropic glutamate receptors induces depotentiation in amygdala slices and reduces fear-potentiated startle in rats. Learn Mem. 2005;12:130–137. doi: 10.1101/lm.85304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex. 2009;19:165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Nie Z, Siggins GR. Metabotropic glutamate receptors regulate N-methyl-D-aspartate-mediated synaptic transmission in nucleus accumbens. J Neurophysiol. 1997;78:3028–3038. doi: 10.1152/jn.1997.78.6.3028. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Szeliga P, Gasparini F, Cryan JF. Blockade of the mGlu5 receptor decreases basal and stress-induced cortical norepinephrine in rodents. Psychopharmacology (Berl) 2005;179:240–246. doi: 10.1007/s00213-005-2142-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego (CA): Academic Press; 1986. [Google Scholar]
- Pfeiffer UJ, Fendt M. Prefrontal dopamine D4 receptors are involved in encoding fear extinction. Neuroreport. 2006;17:847–850. doi: 10.1097/01.wnr.0000220142.29413.6f. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/s0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Renoldi G, Calcagno E, Borsini F, Invernizzi RW. Stimulation of group I mGlu receptors in the ventrotegmental area enhances extracellular dopamine in the rat medial prefrontal cortex. J Neurochem. 2007;100:1658–1666. doi: 10.1111/j.1471-4159.2006.04317.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Corcoran KA, Peters J, Sierra-Mercado D. Neural correlates of individual variability in fear extinction. J Neurosci. 2008;28:12147–12149. doi: 10.1523/JNEUROSCI.4373-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex. 2009;19:474–482. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci. 2003;23:10238–10248. doi: 10.1523/JNEUROSCI.23-32-10238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by the specific mGluR5 agonist CHPG in spinal cord motoneurons. Neuropharmacology. 1999;38:1569–1576. doi: 10.1016/s0028-3908(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Wijetunge LS, Till SM, Gillingwater TH, Ingham CA, Kind PC. mGluR5 regulates glutamate-dependent development of the mouse somatosensory cortex. J Neurosci. 2008;28:13028–13037. doi: 10.1523/JNEUROSCI.2600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29:3676–3684. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Mao L, Tang Q, Samdani S, Liu Z, Wang JQ. A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. J Neurosci. 2004;24:10846–10857. doi: 10.1523/JNEUROSCI.2496-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SR, Bianchi R, Wong RK. Signaling mechanisms underlying group I mGluR-induced persistent AHP suppression in CA3 hippocampal neurons. J Neurophysiol. 2008;99:1105–1118. doi: 10.1152/jn.00435.2007. [DOI] [PubMed] [Google Scholar]
- Zheng J, Wu X, Li L. Metabotropic glutamate receptors subtype 5 are necessary for the enhancement of auditory evoked potentials in the lateral nucleus of the amygdala by tetanic stimulation of the auditory thalamus. Neuroscience. 2008;152:254–264. doi: 10.1016/j.neuroscience.2007.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.