Abstract
Notch signaling plays a crucial role in the development of colon cancer; targeting the Notch pathway may sensitize colon cancers to various adjuvant agents. The focus of our current study is to identify natural compounds that target Notch signaling and that might be beneficial for the prevention and treatment of colon cancer. Withaferin-A (WA) is a bioactive compound derived from Withania somnifera, which inhibits Notch-1 signaling and downregulates pro-survival pathways, such as Akt/NF-κB/Bcl-2, in three colon cancer cell lines (HCT-116, SW-480 and SW-620). In addition, WA downregulated expression of mTOR signaling components, pS6K and p4E-BP1 and activated JNK-mediated apoptosis in colon cancer cells. We also established the molecular link between Notch/Akt/mTOR signaling by complementary approaches (i.e., overexpression of Notch-1 or inhibition of Notch-1 by siRNA). Our results suggest that WA inhibits Notch-mediated pro-survival signaling, which facilitates JNK mediated apoptosis in colon cancer cell lines. These results underscore the anti-cancer activity of WA, which exhibits potential for further development for targeted chemotherapy and/or chemoprevention strategies in the context of colon cancer.
Introduction
Over the past several years, therapeutic options for patients with colon cancer have increased substantially due to earlier diagnosis and more effective chemotherapeutic agents. However, efforts to better understand the biological basis for colon cancer progression and identifying novel agents which target specific signaling pathways may provide a better therapeutic option for patients with colon cancer.
Notch signaling has also been considered as an oncogene involved in the pathogenesis of colorectal cancer (1, 2). Previous studies revealed deregulated Notch overexpression in a number of solid human tumors (3), including colon cancers (1, 2). So far, four Notch genes have been identified (Notch-1, Notch-2, Notch-3, and Notch-4) and five Notch ligands (Dll-1, Dll-3, Dll-4, Jagged-1, and Jagged-2) have been found in mammals. These molecules play important roles in regulating cell fate decisions (4). Activation of the Notch pathway occurs when specific ligands like Jagged-1 (JAG-1) or Delta-like-3 (DLL3) bind to four related transmembrane Notch receptors, which bind and activate the γ-secretase protein complex. This complex cleaves the Notch-1 receptor in the transmembrane domain to release the cytoplasmic portion, known as the Notch-1 intracellular domain (NICD/Truncated/activated-Notch) (5–8). Gamma-secretase is a complex of proteins which has not yet been fully characterized (9) but minimally consists of four subunits: Presenilin-1, Preselin-2, Nicastrin and APH-1 (Anterior Pharynx-defective-1) (10). Presenilin-1 and Presenilin-2 catalyze the intramembrane cleavage of integral membrane proteins such as Notch receptors, but the other members of the γ-secretase complex are required for protease activity (9, 10). Activated Notch-1 translocates to the nucleus and forms a ternary complex with a highly conserved transcription factor, CSL (CBF1/Suppressor of Hairless/ Lag1) and co-activators of the mastermind-like (MAML) family (5). This complex activates target gene transcription, including Hes-1 and Hey-1 (11, 12). Multiple oncogenic pathways, such as MAP Kinase, Akt, NF-κB, matrix metalloproteinases (MMP), and mammalian target of rapamycin (mTOR) signaling have been reported to engage in cross-talk with Notch signaling. Therefore it is believed that this signaling collectively plays an important role in tumor aggressiveness in colon cancer (13–15).
Withaferin-A (WA) is a bioactive compound isolated from the medicinal plant Withania somnifera, which has been safely used for centuries in the practice of Indian Ayurvedic medicine for the treatment of various ailments, including anti-cancer and anti-inflammatory uses (16–18). Since Notch-1 is one of the major causative factors of inflammatory diseases (19) and also Withania somnifera is a widely used for anti-inflammatory, we investigated whether WA inhibits Notch and its associated signaling, which might cause growth arrest in colon cancer cells. This study provides the evidence that WA exerts anticancer effects by downregulating Notch and its cross-talk signaling (Akt/NF-κB/mTOR), which resulted in the inhibition of colon cancer survival. On the other hand, WA induces JNK-mediated apoptosis in the colon cancer cells without any significant effect on normal colon epithelial (FHC) cells.
Materials and methods
Cell Lines and Reagents
Human colon cancer cell lines (HCT-116, SW-480 and SW-620) and a normal colon epithelial cell line (FHC) were purchased from the American Type Culture Collection (Manassas, VA). HCT-116 and SW-480 cell lines were grown in DMEM supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, and antibiotics in the presence of 5% CO2 at 37°C in an incubator. SW-620 cells were grown in Leibovitz’s L-15 Medium (ATCC, Manassas, VA) in the absence of CO2 (tightly capped) at 37°C in the incubator. The FHC cells were grown in Ham’s F12 medium (45%) and Dulbecco’s modified Eagle’s medium (45%), which contains 25 mM HEPES; 10 ng/ml cholera toxin; 0.005 mg/ml insulin; 0.005 mg/ml transferrin; 100 ng/ml hydrocortisone; and 10% fetal bovine serum. Commercially available HPLC-grade WA was purchased from the Chromadex (Irvine, CA).
Western Blot Analysis
Colon cancer cells were treated with WA for various time intervals. Whole cell lysates were obtained and subjected to western blot analysis using the following antibodies: Presenelin-1, Presenelin-2, and Nicastrin (purchased from GeneScript [Piscataway, NJ]), Notch-1 (Cleaved or NID), Hes-1, Hey-1, Akt, pAkt (Ser473), S6K, pS6K (Thr398), 4E-BP1, p4E-BP1 (Thr70), c-Jun, p-c-Jun, JNK-1, pMEK-3/6, ERK, pERK, IKK-α, IκB, Bcl-2, pHistone H3, p65-NF-κB (from Santa Cruz Biotechnology [Santa Cruz, CA]); PARP and cleaved Caspase-3 were from Cell Signaling Technology (Danvers, MA). GAPDH, β-actin, Histone H3, anti-mouse and anti-rabbit secondary antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Viability and Apoptotic Assays
Colon cancer cells (HCT-116, SW-480 and SW-620) were treated with WA or with a vehicle (DMSO) for 24h. Trypan blue dye exclusion or MTT assays for cell viability (20) and apoptotic assay (Annexin V-FITC) were performed on HCT-116, SW-480 and SW-620 cell lines as described earlier (21, 22).
Statistical Analysis
All the experiments were performed three times to ascertain the reproducibility of the results. The data shown are representative of three experiments. The ANOVA was used to calculate statistical significance between samples.
Results
WA negatively regulates Notch-1 activation in colon cancer cells
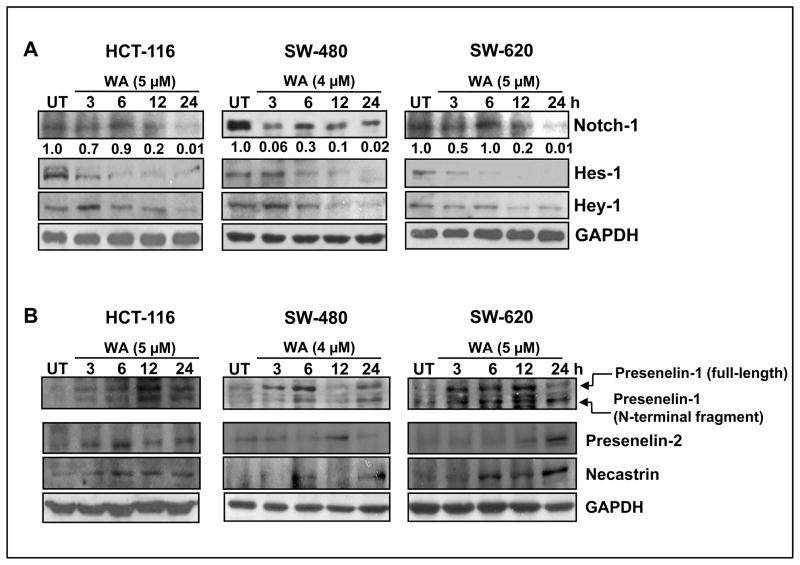
Notch signaling is known to suppress apoptosis and promote cell proliferation/survival pathways in colon cancer cells (15, 23). We explored whether WA targets Notch-1 signaling in colon cancer cells (HCT-116, SW-480 and SW-620). As depicted in Figure 1A, we observed a gradual time-dependent decrease of cleaved Notch-1 expression in HCT-116 and SW-620 cells, whereas in SW-480 cells, cleaved Notch-1 was drastically reduced after 3 h of treatment with WA (4 μM). Next we investigated whether inhibition of Notch-1 affects downstream targets Hes-1 and Hey-1, which were also downregulated after 3 h of treatment with WA in all three colon cancer cell lines (Figure 1A). These results suggest that WA significantly inhibits Notch signaling in colon cancer cells. Next we investigated whether WA inhibits γ-secretase (an activator of Notch-1) which in turn downregulates Notch signaling in colon cancer cells. We analyzed the expression of γ-secretase subunits Presenilin-1, Presenilin-2 and Nicastrin in WA-treated colon cancer cell lines. Our results suggest that WA fails to inhibit γ-secretase subunits in all three cell lines, implying that WA may directly inhibit Notch signaling in colon cancer cells (Figure 1B). In order to determine the transcriptional regulation of Notch-1 by WA, we performed reverse transcription-PCR analysis. Our results suggest that WA downregulates Notch-1 mRNA expression in all three cell lines in a time-dependent manner (data not shown).
Figure 1. Withaferin-A inhibits Notch signaling in colon cancer cells.
HCT-116, SW-480, and SW-620 cells were treated with either vehicle control (DMSO) or WA for varying time intervals. Cell lysates were subjected to western blot analysis using (A) Notch-1 (cleaved), Hey-1 and Hes-1 antibodies, and (B) gamma-secretase components. GAPDH was used as the internal loading control.
Inhibition of Akt signaling by WA in colon cancer cells
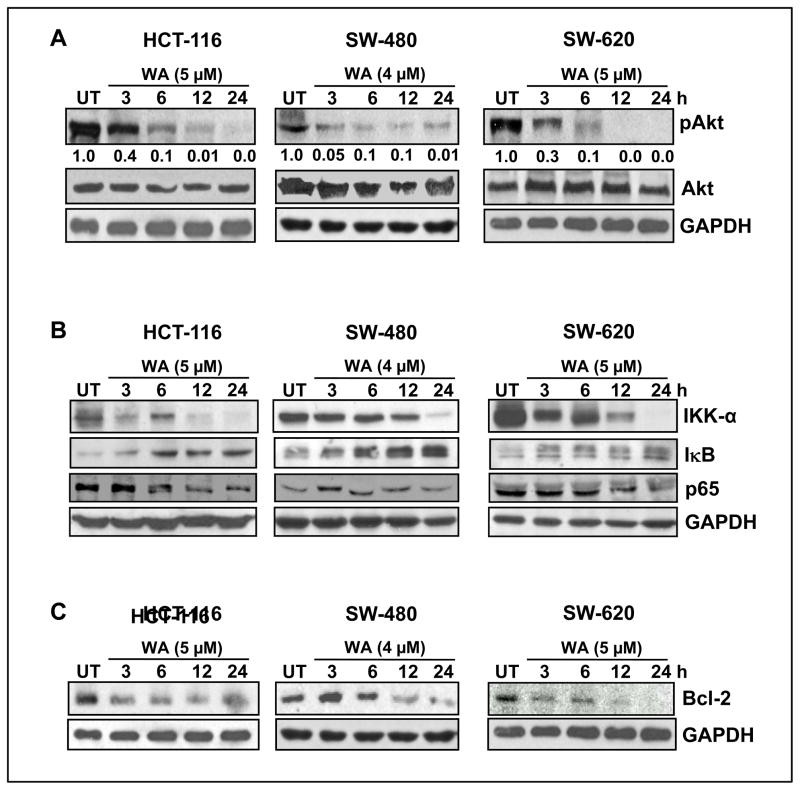
It has been shown that activated Notch induces Akt expression in many cancer cell types (24); hence we investigated whether WA inhibits Akt phosphorylation in colon cancer cells. We noted a time-dependent inhibition of pAkt, yet did not observe any alterations in total Akt protein levels in HCT-116, SW-480 or SW-620 cells (Figure 2A). Activated Akt regulates NF-κB signaling (25); on the other hand, the cross-talk during activation of Notch and NF-κB in colon cancer cells is well established (26). Since WA inhibits Notch and Akt activation, we examined whether inhibition of both signaling pathways negates NF-κB activation in colon cancer cells. We observed that expression of the NF-κB p65 subunit was downregulated by WA in a time-dependent manner in HCT-116, SW-480 and SW-620 cells (Figure 2B). On the other hand, total IκB-α protein level increased in a time-dependent manner with WA treatment (Figure 2B), suggesting that WA is capable of maintaining IκB-α in the unphosphorylated form, thereby retaining the active NF-κB dimers. Furthermore, when WA modulates upstream IKK-α activity, this phosphorylates IκB-α and releases NF-κB subunits. As depicted in Figure 2B, treatment with WA downregulated IKK-α in all three colon cancer cell lines. Finally, we examined whether inhibition of Notch-1, Akt and NF-κB affects major survival factor Bcl-2 in colon cancer cells. As expected, WA downregulated Bcl-2 levels (Figure 2C), suggesting that WA significantly inhibits pro-survival molecules in colon cancer cells.
Figure 2. Withaferin-A Inhibits Akt/ NF-κB/Bcl-2 signaling in colon cancer cells.
(A, B and C) HCT-116, SW-480, and SW-620 cells were treated with either vehicle control (DMSO) or WA for varying time intervals, and cell lysates were subjected to western blot analysis using the indicated antibodies. GAPDH was used as the internal loading control.
Effect of WA on mTOR signaling in colon cancer cells
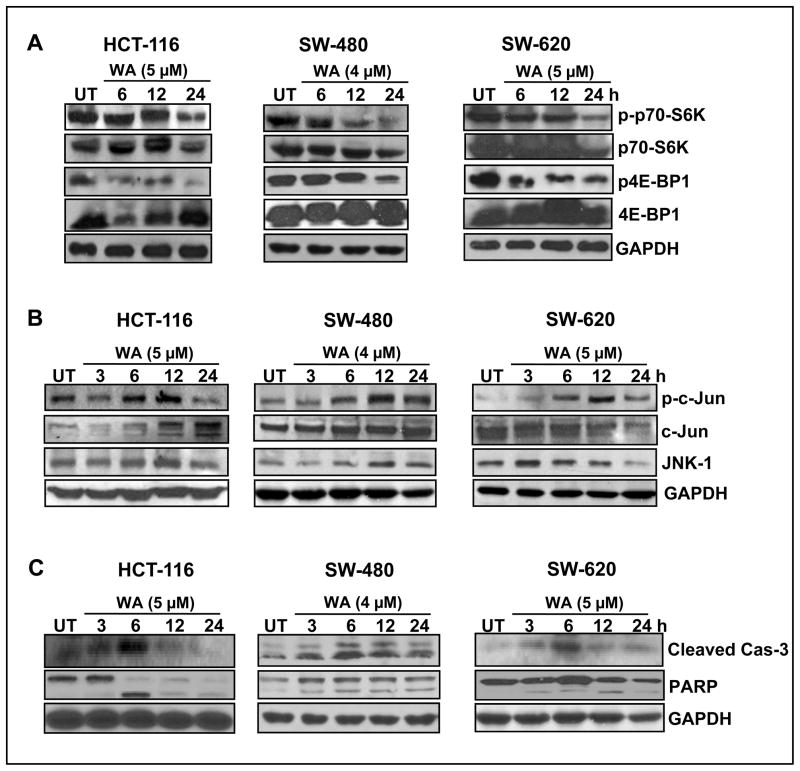
Interestingly, mTOR has recently been shown to mediate Notch in survival signaling (27). The relationship between Notch and mTOR signaling has not yet been fully established. On the other hand, Akt-mediated mTOR regulation in colon cancer cells is well established (28). We noted that treatment with WA downregulated the phosphorylation of p70-S6K in SW-480 cells when compared to HCT-116 and SW-620 cells (Figure 3A), however, WA significantly downregulated phosphorylation of 4E-BP1 in HCT-116 and SW-620 when compared to SW480 (Figure 3A). These results suggest that WA inhibits Notch and its cross-talk with mTOR signaling in colon cancer cells.
Figure 3. Withaferin-A regulates mTOR, ERK and JNK pathways in colon cancer cells, leading to the induction of apoptosis.
(A, B, and C) HCT-116, SW-480 and SW-620 cells were treated with either vehicle control (DMSO) or WA for varying time intervals, and cell lysates were subjected to western blot analysis using the indicated antibodies. GAPDH was used as loading control.
Activation of c-Jun and JNK signaling in colon cancer cells
Cross-talk among intracellular signaling pathways is important for the regulation of cell fate decisions and cellular responses to extracellular signals. Both Notch and MAPK pathways play important roles in many biological processes, and the Notch pathway has been shown to interact with the ERK and JNK pathways (29, 30). Activated Notch-1 negatively regulates c-Jun and JNK in many cancer types, thereby inhibiting apoptosis (30). Therefore we sought to determine the role of WA in JNK signaling in WA-treated colon cancer cells. As seen in Figure 3B, significant increases in phosphorylated c-Jun (active) and JNK were observed in HCT-116, SW-480 and SW-620 cells. Next, we determined the effect of WA on ERK signaling in colon cancer cells. Phosphorylated ERK1/2 was upregulated in a timely manner in all three cell lines, without alterations to total ERK protein levels (data not shown). We also observed an induction of apoptotic markers, such as caspase-3 and PARP cleavage in HCT-116, SW-480 and SW-620 cells, suggesting JNK activation would have induced apoptosis in colon cancer cells (Figure 3C).
Notch-1 regulates Akt/mTOR signaling in colon cancer cells
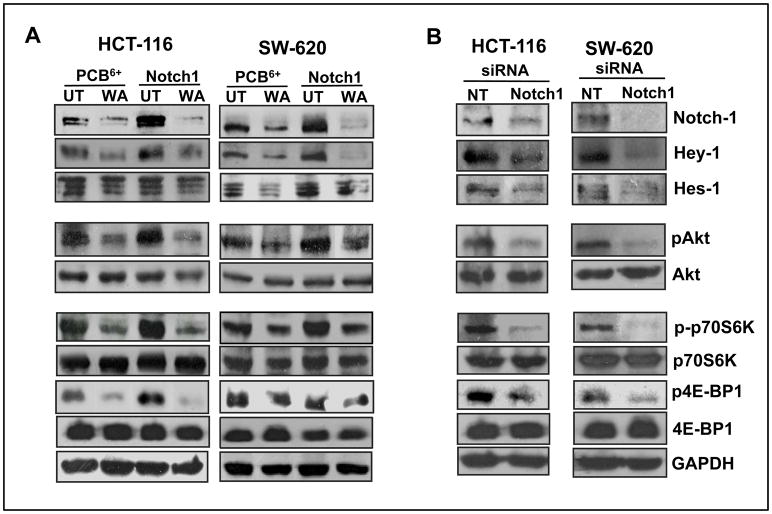
To establish whether Notch-1 regulates Akt and mTOR signaling in colon cancer cells, we either overexpressed full-length Notch-1 or specifically inhibited Notch-1 by siRNA in colon cancer cells. Our results showed that an overexpression of Notch-1 increased the expression levels of its downstream targets, Hey-1 and Hes-1 in both colon cancer cell lines (Figure 4A). In addition, there was also an increase in the expression of pAkt and mTOR (pp70S6K and p-4E-BP1) in both HCT-116 and SW-620 (Figure 4A). On the other hand, inhibition of Notch using siRNA Notch-1 significantly reduced expression of Hey-1, Hes-1, pAkt and mTOR (p-p70-S6K and p4E-BP1) in both HCT-116 and SW-620 cells (Figure 4B). These results imply that Notch-1 regulates Akt/mTOR signaling in colon cancer cells. As seen in Figure 4A, we also observed that WA overcomes Notch-mediated resistance by downregulating the expression of pAkt, p-p70-S6K and p4E-BP1 in colon cancer cells over expressing Notch-1. These results establish the link between Notch-Akt-mTOR signaling in colon cancer cells.
Figure 4. Knockdown or overexpression of Notch-1 significantly modulates Akt/mTOR signaling in colon cancer cells.
(A) Full-length Notch-1 was overexpressed in HCT-116 and SW-620 cells followed by treatment with vehicle or DMSO, and cell lysates were subjected to western blot analysis using the indicated antibodies. (B) HCT-116 and SW-620 cells were transfected with siRNA and cell lysates were subjected to western blot analysis; GAPDH was used as a loading control.
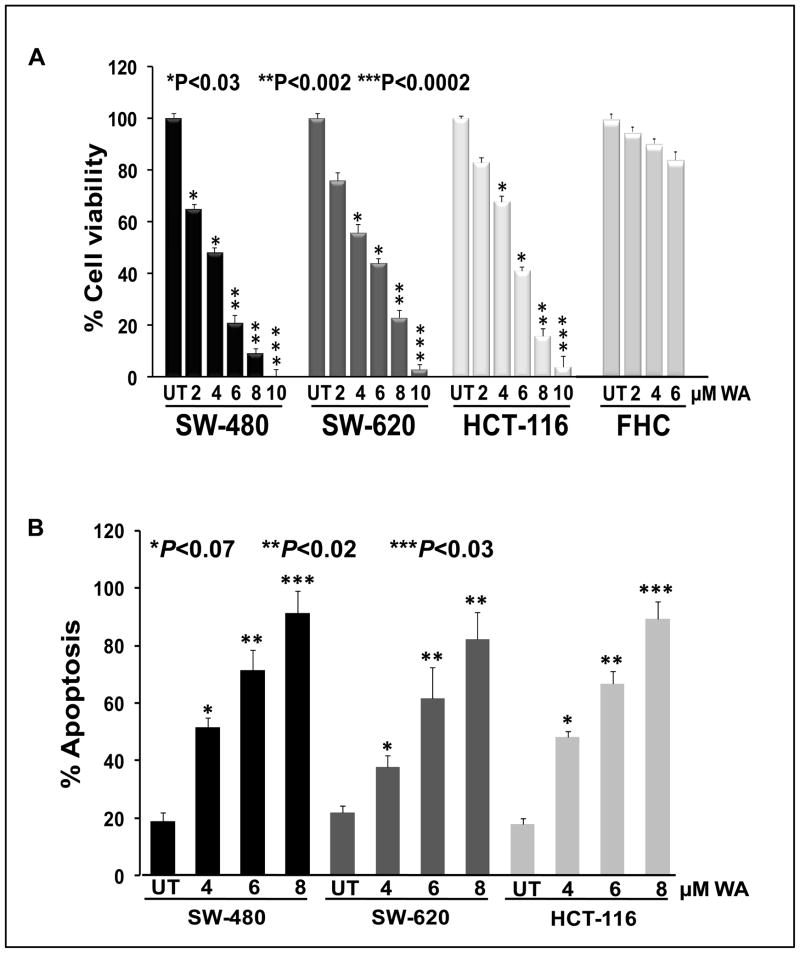
WA inhibits cell viability and induces apoptosis in colon cancer cells in vitro
Molecular kinetic studies revealed that WA downregulates cell proliferation/survival signaling; therefore we determined the effect of WA treatment on colon cancer cell survival/proliferation and apoptosis induction. The colon cancer cells were treated with various (2–10 μM) concentrations of WA for 24 h, and cell viability was quantified by Trypan Blue Exclusion/MTT assays. Our results indicate that WA inhibited cell viability in all three colon cancer cell lines in a dose-dependent manner. However, SW-480 (IC50: 3.56 μM) cells were more sensitive to WA treatment when compared to SW-620 (IC50: 5.0 μM) and HCT-116 (IC50: 5.33 μM) cell lines and we also tested the toxicity of WA on normal colon epithelial cells (FHC), which shows no significant effect on FHC cells (Figure 5A). Similarly, apoptotic assays (using Annexin V-FITC by flow cytometric analysis) were performed in colon cancer cell lines following treatment with WA (4, 6 and 8 μM). Our results suggest that WA induced a significant amount of apoptosis in all three colon cancer cell lines (Figure 5B). These results suggest that WA is a potent anticancer drug, which we can use as a therapeutic option for the treatment of colon cancer.
Figure 5. Withaferin-A inhibits cell viability and induces apoptosis in colon cancer cells.
(A) HCT-116, SW-480, SW-620, and FHC cells were treated with varying concentrations of WA for 24h. Trypan Blue Exclusion assay was performed. Each data point represents the mean of four wells from three independent experiments (mean ± SE). (B) Apoptotic assays were performed using Annexin V-FITC staining. The data shown are representative of the combined means from three independent experiments, and the error bars represent the standard error.
Discussion
Notch-1is overexpressed during colon cancer progression and governs many important functions including cell proliferation/differentiation and survival. A recent study reported the overexpression of Notch-1 (77%) and its downstream targets Hey-1 and Hes-1 in human colon cancer (31, 32). There are a number of groups working on γ-secretase inhibitors (GSI) that inhibit Notch-1 activation, which represents an effective treatment for sarcoma (33), medulloblastoma (34), breast cancer (35) and colon cancer (5). In our studies, we show for the first time that WA, a dietary compound, targets and inhibits Notch signaling without altering upstream events, such as activity mediated by the γ-secretase family of proteins (Presenilin-1, Presenilin-2 and Nicastrin), which induces apoptosis in three colon cancer cell lines. Our results demonstrated that WA Inhibits Notch cleavage and downstream activation mediated by Hey-1 and Hes-1 in HCT-116, SW 480 and SW-620 cell lines. However, it is not clear whether WA binds to the Notch receptor, which in turn reduces γ-secretase binding efficiency in colon cancer cells. More studies are required to dissect these molecular events in colon cancer cells.
Activation of Akt by Notch signaling has been demonstrated in many cancer models (27, 36, 37), and Akt activation plays a crucial role in the initiation and progression of colon cancer metastasis (38). In the present study, WA inhibited Akt activation in colon cancer cells, suggesting it might be due to inhibition of Notch-1. To establish the link between Notch and Akt, we either overexpressed Notch-1 or inhibited Notch-1 using Notch siRNA in colon cancer cells. Ectopic expression of Notch-1 induced pAkt expression, on the other hand, inhibition of Notch-1 downregulates Akt activation, which implies that Notch-1 regulates Akt activation in colon cancer cells. Recently, Meurette et al (39) demonstrated that Notch induced an autocrine signaling loop that activated Akt in breast epithelial cells. Inhibition of Notch-1 caused Akt inhibition, resulting in the induction of apoptosis in breast epithelial cells (39). These results corroborate with our results that Notch-1 may regulate Akt activation and inhibition of Notch-1 may also inhibit Akt-mediated signaling in colon cancer cells.
The transcription factor NF-κB, downstream of Akt, has been shown to be activated in colon cancer cells. Inhibition of NF-κB markedly sensitizes colon cancer cells to apoptosis (40). On the other hand, Notch inhibits NF-κB activation by modulating recombination signal binding protein Jκ (RBP-Jκ) (41). So, inhibition of both Notch-1 and Akt may inhibit NF-κB signaling in colon cancer cells. To confirm this hypothesis, we examined NF-κB signaling. Our results suggest inhibition of IKK-α and upregulation of IκB result in reduced nuclear translocation of p65 protein in colon cancer cells. In addition, downregulation of Bcl-2 expression in all three colon cancer cell lines and the consequent over-expression imparts survival advantages to cancer cells (42). Downregulation of Bcl-2 may increase the sensitivity of the cell to chemotherapeutic drugs and radiation (43–45). Thus, therapeutic strategies directed towards inhibition of NF-κB and Bcl-2 activation may have great clinical importance. We found that WA downregulates Notch-1/Akt/NF-κB/Bcl-2 protein expression in all three colon cancer cell lines, suggesting that WA may prove to be a potent therapeutic agent for colon cancer.
We observed inhibition of mTOR components pS6K and p4E-BP1 in all three colon cancer cell lines. Although the molecular link between Notch and mTOR remains to be clarified, few published reports suggest that Notch regulates mTOR in both an Akt-dependent and -independent manner in T-ALL. For example, γ-secretase treatment effectively suppresses mTOR activation in T-ALL, suggesting a possible molecular link between these two pathways (46). WA inactivates Notch-1 and Akt, which might result in the inhibition of mTOR in colon cancer cells. The expression pattern of mTOR in colon cancer specimens is not known, however, it is well established that mTOR is overexpressed or is aberrant in other cancer types. Furthermore, inhibition of mTOR by rapamycin significantly inhibited tumor growth in prostate, breast and T-ALL (47–49). The chemotherapeutic efficacy of rapamycin is controversial, as there are reports that rapamycin-treated cells showed increased Akt expression, as mediated by a feedback mechanism. Thus, some studies have concluded that simultaneous inhibition of both Akt and mTOR is essential for an effective therapeutic strategy (22, 50, 51). We believe WA could be a better therapeutic compound because it inhibits Notch/Akt/NF-κB/mTOR-mediated pro-survival signaling in colon cancer cells.
To elucidate the mechanisms by which WA induces apoptosis, we examined the potential contributions of ERK and JNK signaling in colon cancer cells. ERKs and JNKs (stress-activated protein kinases) are members of the MAP kinase super family. In general, JNKs are activated by stress and inflammatory signals, which induce apoptosis and inhibit cell growth (52). Our results revealed an induction of pJNK and pc-Jun in all three colon cancer cell lines, suggesting the possible role of JNK-mediated pro-apoptotic signaling induced by WA. Also, apoptotic markers such as cleaved caspase-3 and PARP cleavage confirmed induction of WA-mediated apoptosis in our cell lines. Furthermore, our cell viability and apoptotic studies suggest that WA exerts a potent chemotherapeutic effect on colon cancer cells. Interestingly, no significant toxicities were observed in normal colon epithelial cells (FHC), which might suggest that WA targets only cancer cells. We believe that inhibition of Notch-mediated pro-survival signaling could play a major role in the induction of apoptosis in colon cancer cells in response to WA treatment.
In summary, this study demonstrates that direct modulation of Notch/Akt/NF-κB signaling activity by WA could provide the molecular basis for apoptosis induction in colon cancer cells. Considering the pivotal role of Notch/Akt signaling in the pathogenesis of human colon cancer, these findings may have significant clinical relevance, and WA could be developed as an agent for the management of colon cancer. However, further studies are warranted to fully dissect the mechanism of action of WA in colon cancer models, as well as to validate our in vitro findings in an in vivo xenograft model.
References
- 1.Katoh M. Notch signaling in gastrointestinal tract (review) Int J Oncol. 2007;30:247–51. [PubMed] [Google Scholar]
- 2.Leow CC, Romero MS, Ross S, Polakis P, Gao WQ. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004;64:6050–7. doi: 10.1158/0008-5472.CAN-04-0290. [DOI] [PubMed] [Google Scholar]
- 3.Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–33. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- 4.Li JL, Harris AL. Notch signaling from tumor cells: a new mechanism of angiogenesis. Cancer Cell. 2005;8:1–3. doi: 10.1016/j.ccr.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Meng RD, Shelton CC, Li Y-M, et al. {gamma}-Secretase Inhibitors Abrogate Oxaliplatin-Induced Activation of the Notch-1 Signaling Pathway in Colon Cancer Cells Resulting in Enhanced Chemosensitivity. Cancer Res. 2009;69:573–82. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–6. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 7.Mumm JS, Schroeter EH, Saxena MT, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 8.Rodilla V, Villanueva A, Obrador-Hevia A, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci U S A. 2009;106:6315–20. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Hasegawa H, Schmitt-Ulms G, et al. TMP21 is a presenilin complex component that modulates [gamma]-secretase but not [epsiv]-secretase activity. Nature. 2006;440:1208–12. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 10.Kaether C, Haass C, Steiner H. Assembly, trafficking and function of gamma-secretase. Neurodegener Dis. 2006;3:275–83. doi: 10.1159/000095267. [DOI] [PubMed] [Google Scholar]
- 11.Bhanot U, Kohntop R, Hasel C, Moller P. Evidence of Notch pathway activation in the ectatic ducts of chronic pancreatitis. J Pathol. 2008;214:312–9. doi: 10.1002/path.2293. [DOI] [PubMed] [Google Scholar]
- 12.Ehebauer M, Hayward P, Arias AM. Notch, a universal arbiter of cell fate decisions. Science. 2006;314:1414–5. doi: 10.1126/science.1134042. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa L, Santos S, Ingles-Esteve J, Munoz-Canoves P, Bigas A. p65-NF{kappa}B synergizes with Notch to activate transcription by triggering cytoplasmic translocation of the nuclear receptor corepressor N-CoR. J Cell Sci. 2002;115:1295–303. doi: 10.1242/jcs.115.6.1295. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z-J, Xiao M, Balint K, et al. Inhibition of endothelial cell proliferation by Notch1 signaling is mediated by repressing MAPK and PI3K/Akt pathways and requires MAML1. FASEB J. 2006;20:1009–11. doi: 10.1096/fj.05-4880fje. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Banerjee S, Li Y, Rahman KMW, Zhang Y, Sarkar FH. Down-regulation of Notch-1 Inhibits Invasion by Inactivation of Nuclear Factor-{kappa}B, Vascular Endothelial Growth Factor, and Matrix Metalloproteinase-9 in Pancreatic Cancer Cells. Cancer Res. 2006;66:2778–84. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Diwanay S, Patki P, Patwardhan B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J Ethnopharmacol. 1999;67:27–35. doi: 10.1016/s0378-8741(99)00065-3. [DOI] [PubMed] [Google Scholar]
- 17.Rasool M, Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: An in vivo and in vitro study. Vascul Pharmacol. 2006;44:406–10. doi: 10.1016/j.vph.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Stan SD, Hahm E-R, Warin R, Singh SV. Withaferin A Causes FOXO3a- and Bim-Dependent Apoptosis and Inhibits Growth of Human Breast Cancer Cells In vivo. Cancer Res. 2008;68:7661–9. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–68. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 20.Baliga R, Zhang Z, Shah SV. Role of cytochrome P-450 in hydrogen peroxide-induced cytotoxicity to LLC-PK1 cells. Kidney Int. 1996;50:1118–24. doi: 10.1038/ki.1996.418. [DOI] [PubMed] [Google Scholar]
- 21.Sowmyalakshmi S, Nur EAM, Akbarsha MA, Thirugnanam S, Rohr J, Chendil D. Investigation on Semecarpus Lehyam-a Siddha medicine for breast cancer. Planta. 2005;220:910–8. doi: 10.1007/s00425-004-1405-4. [DOI] [PubMed] [Google Scholar]
- 22.Koduru S, Sowmyalakshmi S, Kumar R, Gomathinayagam R, Rohr J, Damodaran C. Identification of a potent herbal molecule for the treatment of breast cancer. BMC Cancer. 2009;9:41. doi: 10.1186/1471-2407-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucio Miele BO. Arbiter of differentiation and death: Notch signaling meets apoptosis. Journal of Cellular Physiology. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZJ, Xiao M, Balint K, et al. Notch1 signaling promotes primary melanoma progression by activating mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt pathways and up-regulating N-cadherin expression. Cancer Res. 2006;66:4182–90. doi: 10.1158/0008-5472.CAN-05-3589. [DOI] [PubMed] [Google Scholar]
- 25.Shant J, Cheng K, Marasa BS, Wang J-Y, Raufman J-P. Akt-dependent NF-[kappa]B activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Experimental Cell Research. 2009;315:432–50. doi: 10.1016/j.yexcr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwee-Luan A, Vinay T. Notch and NFkappaB signaling pathways: Do they collaborate in normal vertebrate brain development and function? BioEssays. 2007;29:1039–47. doi: 10.1002/bies.20647. [DOI] [PubMed] [Google Scholar]
- 27.Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–6. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 28.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cellular Signalling. 2009;21:656–64. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JW, Kim MJ, Kim KJ, et al. Notch interferes with the scaffold function of JNK-interacting protein 1 to inhibit the JNK signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14308–13. doi: 10.1073/pnas.0501600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondoh K, Sunadome K, Nishida E. Notch Signaling Suppresses p38 MAPK Activity via Induction of MKP-1 in Myogenesis. J Biol Chem. 2007;282:3058–65. doi: 10.1074/jbc.M607630200. [DOI] [PubMed] [Google Scholar]
- 31.Veenendaal LM, Kranenburg O, Smakman N, Klomp A, Borel Rinkes IH, van Diest PJ. Differential Notch and TGFbeta signaling in primary colorectal tumors and their corresponding metastases. Cell Oncol. 2008;30:1–11. doi: 10.1155/2008/839076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu D, Wang W, Xie H, et al. Notch1 expression in colorectal carcinoma determines tumor differentiation status. J Gastrointest Surg. 2009;13:253–60. doi: 10.1007/s11605-008-0689-2. [DOI] [PubMed] [Google Scholar]
- 33.Curry CL, Reed LL, Golde TE, Miele L, Nickoloff BJ, Foreman KE. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi’s sarcoma tumor cells. Oncogene. 2005;24:6333–44. doi: 10.1038/sj.onc.1208783. [DOI] [PubMed] [Google Scholar]
- 34.Fan X, Mikolaenko I, Elhassan I, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–93. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 35.Farnie G, Clarke RB, Spence K, et al. Novel cell culture technique for primary ductal carcinoma in situ: role of Notch and epidermal growth factor receptor signaling pathways. J Natl Cancer Inst. 2007;99:616–27. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- 36.Weng AP, Nam Y, Wolfe MS, et al. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23:655–64. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akiyoshi T, Nakamura M, Yanai K, et al. Gamma-secretase inhibitors enhance taxane-induced mitotic arrest and apoptosis in colon cancer cells. Gastroenterology. 2008;134:131–44. doi: 10.1053/j.gastro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Rychahou PG, Kang J, Gulhati P, et al. Akt2 overexpression plays a critical role in the establishment of colorectal cancer metastasis. Proceedings of the National Academy of Sciences. 2008;105:20315–20. doi: 10.1073/pnas.0810715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meurette O, Stylianou S, Rock R, Collu GM, Gilmore AP, Brennan K. Notch Activation Induces Akt Signaling via an Autocrine Loop to Prevent Apoptosis in Breast Epithelial Cells. Cancer Res. 2009;69:5015–22. doi: 10.1158/0008-5472.CAN-08-3478. [DOI] [PubMed] [Google Scholar]
- 40.Jones DR, Broad RM, Madrid LV, Baldwin AS, Jr, Mayo MW. Inhibition of NF-kappaB sensitizes non-small cell lung cancer cells to chemotherapy-induced apoptosis. Ann Thorac Surg. 2000;70:930–6. doi: 10.1016/s0003-4975(00)01635-0. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 41.Oswald F, Liptay S, Adler G, Schmid RM. NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol Cell Biol. 1998;18:2077–88. doi: 10.1128/mcb.18.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol. 2000;165:1743–54. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 43.Koty PP, Tyurina YY, Tyurin VA, Li SX, Kagan VE. Depletion of Bcl-2 by an antisense oligonucleotide induces apoptosis accompanied by oxidation and externalization of phosphatidylserine in NCI-H226 lung carcinoma cells. Mol Cell Biochem. 2002;234–235:125–33. [PubMed] [Google Scholar]
- 44.Fennell DA. Bcl-2 as a target for overcoming chemoresistance in small-cell lung cancer. Clin Lung Cancer. 2003;4:307–13. doi: 10.3816/clc.2003.n.012. [DOI] [PubMed] [Google Scholar]
- 45.Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584–92. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- 46.Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–86. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Z, Guo X, Yang X, et al. PTEN and rapamycin inhibiting the growth of K562 cells through regulating mTOR signaling pathway. Journal of Experimental & Clinical Cancer Research. 2008;27:87. doi: 10.1186/1756-9966-27-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu T, Yacoub R, Graham T, Yang L, Tighiouart M, O’Regan RM. Effect of mTOR inhibition on sensitivity of triple-negative breast cancer cells to epidermal growth factor inhibition. J Clin Oncol (Meeting Abstracts) 2009;27:1055. [Google Scholar]
- 49.Cullion K, Draheim KM, Hermance N, et al. Targeting the Notch1 and mTOR pathways in a mouse T-ALL model. Blood. 2009;113:6172–81. doi: 10.1182/blood-2008-02-136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–53. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 51.Sowmyalakshmi S, Srinivas K, Raj K, Guhan V, Natasha K, Chendil D. Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. International Journal of Cancer. 2009;125:961–7. doi: 10.1002/ijc.24419. [DOI] [PubMed] [Google Scholar]
- 52.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]